Christina Fleckenstein1 BS, K. Nathan Parthasarathy2 MD, Xuezhi Jiang1-3 * MD, Ph.D.
1Drexel College of Medicine, Philadelphia, PA, USA
2Department of Obstetrics and Gynecology, The Reading Hospital of Tower Health, Reading, PA, USA
3Department of Obstetrics and Gynecology, Sidney Kimmel Medical College of Thomas Jefferson University; Philadelphia, PA, USA
*Corresponding Author: Xuezhi Jiang, MD, PhD, Associate Professor of Obstetrics and Gynecology, The Reading Hospital of Tower Health, Department of OBGYN - R1; P.O. Box 16052, Reading, PA 19612-6052., USA
Abstract
Background: Actinomycoses are opportunistic bacteria that can cause invasive, suppurative infections when healthy mucosal barriers are compromised by tissue trauma, surgery, or the presence of a foreign body. The vast majority of pelvic actinomycosis is seen in patients with long-term use of intrauterine devices. Few cases have been published in females without a history of intrauterine contraception, and cases of isolated vaginal actinomycosis are not found in the literature. Abdominopelvic infections present as slow-growing masses that can cross tissue planes, often producing non-specific symptomatology. For these reasons, initial misdiagnosis of malignancy or other diseases is common, which can lead to unnecessary surgery, as most actinomycosis can be managed with antibiotics.
Case Report: We present a case of isolated vaginal actinomycosis in a 66-year-old post-menopausal female without a history of intrauterine contraception, presenting with the chief complaint of vaginal bleeding. This patient had a history of colpoperineorrhaphy with inorganic mesh placement secondary to anterior vaginal prolapse, and a remote history of total abdominal hysterectomy and bilateral salpingectomy secondary to heavy and intermenstrual bleeding. Preoperative biopsy diagnosed actinomycosis and guided a conservative surgical approach. The patient recovered well after surgery with extended antibiotic therapy.
Conclusion: This case is notable for actinomycosis isolated to the vagina, which has not been reported in the literature, and infection in a female without a uterus or concomitant use of an intrauterine device, illustrating a connection between actinomycosis and the persistence of any foreign object in the female genital tract. Actinomycosis should be included on the differential for any female with a slow-growing mass in the genital tract, especially if there is a history of foreign body presence or signs and symptoms atypical for malignancy or another disease. Lastly, preoperative biopsy or—at a minimum—intraoperative frozen section should always be performed to guide care and prevent unnecessarily surgery.
Teaching Points: demonstrate the relationship between genital tract actinomycosis and the foreign body presence; emphasize the importance of pre-therapeutic biopsy in guiding clinical care.
Introduction
Actinomyces are gram-positive, anaerobic coccobacillus that can cause opportunistic infection in the presence of compromised mucosal barriers. The saprophytic bacteria are most often found in soil, but 26 species have been identified as part of the human flora, housed in the oral cavity, the cecum, and the appendix [1-4]. Yet, when mucosal barriers are breached by trauma, surgery, radiation, concomitant infection, or the presence of a foreign body, Actinomyces can produce destructive infections [4-7].
Actinomycosis often presents as a slow-growing mass, granulomatous and suppurative in nature [6]. However, the bacteria are notorious for invading tissue planes, and advanced disease can present with abscesses, fistulae, and sinus tracts [7].
Abdominopelvic infection accounts for 20 % of all Actinomycosis but is itself an exceedingly rare diagnosis, most associated with intrauterine devices (IUDs) [4, 5, 8-10]. While approximately 7 % of females with IUDs have Actinomyces-like organisms (ALOs) detected on Papanicolaou smear, the risk of a female with ALOs developing an infection is less than one in 1,000 [4, 8].
Although pelvic actinomycosis is classically associated with IUDs, there are several reports in non-IUD users [4, 11-13]. Reports from the 1920s describe infection in females using contraceptive pessaries [4]. A 1895 case study details pelvic actinomycosis was secondary to a hairpin in the uterus [4]. Very few cases have occurred in females without obvious foreign bodies or immunocompromising comorbidities [11-13]. These accounts suggest that pelvic actinomycosis is not only associated with IUD use but is more generally associated with persistent foreign bodies in the female genital tract.
Here, we describe a case of vaginal actinomycosis in a postmenopausal female who had no history of IUD use, but who had undergone a colpoperineorrhaphy with vaginal mesh placement 9 years prior to presentation.
Case Description
Ethical statement: the patient was made aware of the rarity of her diagnosis and consented in writing to publish this case report, including relevant photography.
A 66-year-old female presented to her gynaecologist with 5 months of rust-coloured vaginal discharge and occasionally bright red spotting, most prominent when wiping. She denied pelvic pain but noted dyspareunia and a 6 lb. weight loss.
The patient was status post total abdominal hysterectomy and bilateral salpingectomy for severe heavy and intermenstrual bleeding, pelvic pain, and infertility secondary to endometriosis. In addition, she had a posterior colpoperineorrhaphy with inorganic mesh placement 9 years prior for symptomatic anterior vaginal prolapse. She used a vaginal pessary after this procedure for several years. According to her records, 6 years prior to presentation, a provider noticed “a normal amount of skin erosion … posteriorly without evidence of mesh exposed,” which required no further intervention at that time. The patient had a history of combined oral contraception use but no history of IUD-use.
Her gynaecological history was also significant for several years of perimenopausal hormonal pellet therapy for vasomotor symptoms and current topical vaginal estrogen use for the genitourinary syndrome of menopause; recurrent UTI’s several years prior, adequately treated; and a positive Gardnerella test at the time of presentation, treated with oral Metronidazole.
The patient identified as heterosexual, had fewer than 5-lifetime sexual partners and had no history of sexually transmitted infections. At the presentation, she was sexually active with one male partner and endorsed the current use of latex sex toys. She denied a personal history and a familial history of cancer, including breast, uterine, and ovarian cancers. Her last pap smear 4 years prior, a mammogram 1 year prior, and a colonoscopy 3-year prior were all normal.
The patient had no known immunocompromising illnesses and was up to date with vaccinations and screenings. On examination, a 2 cm by 1 cm polypoid vaginal mass was noted (Image 1), along the posterior midline near the posterior colpoperineorrhaphy suture line, 1 cm proximal to the introitus. It appeared similar to granulation tissue and had no odour or necrosis.
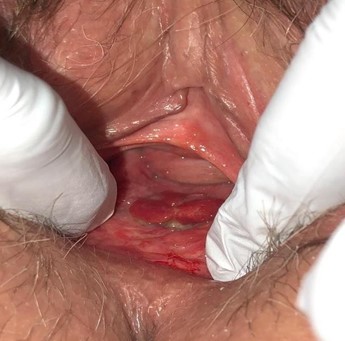
Image 1: 2 cm x 1 cm polypoid mass on posterior vagina, near colpoperineorrhaphy site, 1 cm proximal to the introitus. Granulation-like tissue. Source of patient’s vaginal bleeding.
Ultrasound demonstrated a surgically absent uterus and ovaries. No pelvic free fluid or adnexal cysts or masses were noted. To rule out a vaginal neoplasm, a biopsy was obtained and showed vaginal actinomycosis with marked chronic inflammation.
Laboratory studies revealed a slightly elevated monocytic proportion of 12.3 %, but an otherwise normal WBC count and differential. ESR was normal (6 mm/hour). Haemoglobin and haematocrit were normal (14.7 g/dL, 45.1 %, respectively). HIV 1/2 antibody differentiation, Hepatitis C antibody screening, and vaginal swabs for Candida and Trichomonas species were all negative; Gardnerella species were detected and treated. The basic metabolic panel was unremarkable. Tumour markers were not obtained.
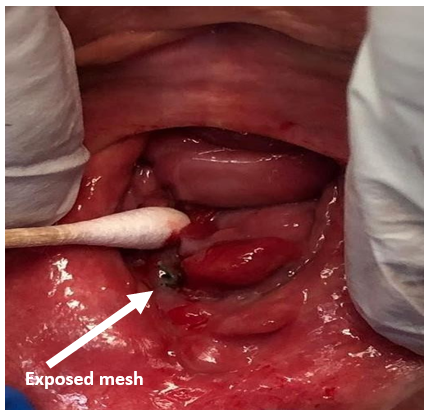
Upon repeat examination, a 2 mm by 2mm portion of the posterior mesh was visible (Images 2, 3). The patient was agreeable to surgical excision and repair, to prevent further erosion.
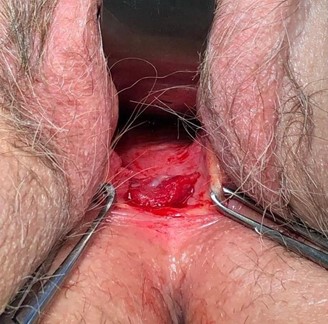
Image 2: beefy, red polypoid mass seen on revisualization.
Image 3: gentle mobilization of the actinomycotic mass revealed black colpoperineorrhaphy mesh visible at lower border of the posterior vaginal wall – patient’s right, viewer’s left.
The patient was cleared for surgery by her family medicine physician and underwent excision of the vaginal lesion and repair via a posterior approach. The rectovaginal septum was dissected, and 3.25 cm x 1.5 cm of tissue was excised, containing the actinomycotic mass and exposed mesh (Image 4, 5). There was no evidence of widespread disease or fistulation. The lateral edges of the healthy vaginal epithelium were vertically reapproximated and inferiorly reapproximated to the vaginal introitus (Image 6). The final histopathological report demonstrated acute and chronic inflammation with granulation tissue and no evidence of malignancy. The patient was treated with 6 weeks of IV ceftriaxone followed by 30 days of oral amoxicillin-clavulanate, per infectious disease’s recommendation.
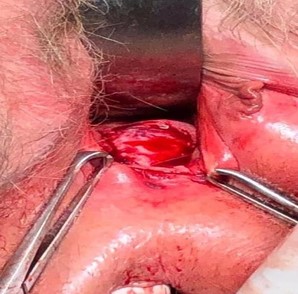
Image 4: posterior vaginal wall after dissection of the rectovaginal septum, excision of the mass and partial excision of mesh. No sinus tracts, fistulization or abscesses found.
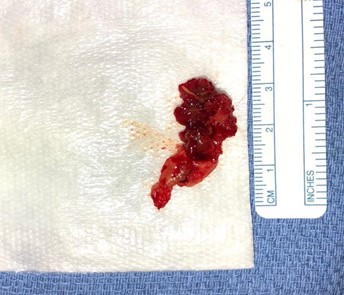
Image 5: 3.25 cm x 1.5 cm tissue excised, containing actinomycotic mass and excised mesh.
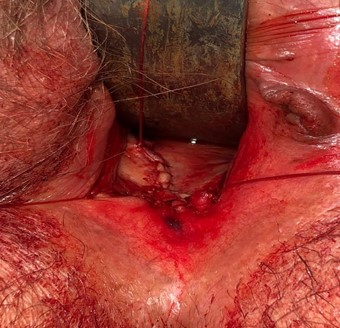
Image 6: vaginal introitus after actinomycotic mass/mesh excision and reapproximation of vaginal mucosa.
Discussion
Actinomycosis is a rare disease, making it difficult to diagnose and study. Females with long-term IUD use, described as 5 years or longer, account for 66 % of those who develop infection [9, 14], but actinomycosis has been documented in females with IUD use as short as 15 months [14]. Furthermore, abdominopelvic actinomycosis can arise secondary to other foreign bodies in the genital tract, as described by Westhoff [4], as well as from the GI tract, such as the appendix or the ileocecum [6, 14-16]. Recent surgery, local trauma, concomitant sexually transmitted infections, bacterial vaginosis (BV), diabetes, or immunocompromising disease may also predispose a female to infection, but none of these risks are predictive [17, 18]. Secondary to its infrequency, actinomycosis is largely studied through case reports.
This case is interesting on several accounts. It demonstrates actinomycosis within the female genital tract not associated with IUD use but in the presence of a persistent foreign body mesh. In addition, the infection was isolated to the vagina in a female without a uterus, which has not been commonly described in the literature.
Early identification of actinomycosis is paramount for minimizing its course. Unfortunately, diagnosis is often made months to years after the infection begins, which is problematic, as infections can spread to organs throughout the body via direct invasion or by circulatory seeding [3]. Genital tract infection can ascend the female genital tract, affecting the uterus, ovaries, fallopian tubes, vulva, and cervix [3]. The extensive disease can burden a patient with a costly and prolonged workup, fear of malignancy, and invasive procedures. Involved organs may be permanently damaged through surgical excision or the infection alone [19]. The case presented here demonstrates actinomycosis isolated to the vagina; providers should become aware of this phenomenon, in order to identify early lesions and prevent ascending or disseminated infection.
Detecting abdominopelvic actinomycosis is also made difficult by its non-specific symptomatology. An infection classically arises as a slow-growing mass, which may appear tumour-like in late stages [6, 19-21]. Patients commonly complain of pelvic pain, abnormal uterine bleeding, altered vaginal discharge, fatigue, weight loss, or rarely, fevers [5, 7, 9, 19, 22]. Thus, abdominopelvic actinomycosis is often misdiagnosed as a pelvic malignancy, Crohn’s disease, disseminated tuberculosis, or even appendicitis [3, 5, 16, 20, 23, 24]. Females may have elevated cancer markers. In one retrospective analysis, 80 % of females with pelvic actinomycosis originally misdiagnosed as ovarian cancer had elevated CA-125 [20]. Infections can masquerade as metastatic disease or carcinomatosis, disseminating from an origin in the uterus or intestines to the adnexa, urinary tract, liver, spleen, lungs, and skin [19, 20-23, 25].
Distinguishing actinomycosis from these conditions is essential for appropriate management. According to a meta-analysis of reports from 1980-2014, a diagnosis of actinomycosis is often made post- operatively, based on histopathology obtained during surgery [5, 6, 15]. Early misdiagnosis often results in exploratory laparotomy or laparoscopy [20, 21, 5, 24] or even neoadjuvant chemotherapy in cases of suspected malignancy [21, 26]. These treatments are unnecessary, as most cases of actinomycosis are cleared with an extended course of high-dose penicillin G or amoxicillin [5, 6, 10, 19, 27]. Surgery is warranted to debride necrotic tissue or facilitate abscess drainage in complex cases [5, 6, 19]. However, there is evidence that pre-operative antibiotic therapy can reduce disease burden even in advanced infection, minimizing the extent of surgical intervention [21].
In the case reported here, there was initially a high suspicion for vaginal malignancy, similar to numerous reports of early abdominopelvic actinomycosis presenting as abdominopelvic malignancy. Preoperative biopsy prevented unnecessary oncology- based interventions and allowed a more conservative surgical approach.
Conclusion
Pelvic actinomycosis is a rare diagnosis, most often presenting in females with long-standing IUD use. Vaginal actinomycosis has not been reported in the literature. Here, we report a case of vaginal actinomycosis in a female who had never used an IUD, who had no uterus at presentation but had a history of a posterior colpoperineorrhaphy with inorganic mesh augmentation and pessary- use. This report strengthens the relationship between foreign bodies in the female genital tract and the development of infection.
Given the diverse presentation of actinomycosis in the female genital tract, this diagnosis should be considered in any female complaining of an abdominal, pelvic, or vaginal mass, changes in discharge, abnormal bleeding, or when a pelvic malignancy is suspected. It should especially be considered if the patient has a history of foreign bodies within the female genital tract, including IUDs, inorganic surgical mesh, pessaries, or other inorganic materials. Obtaining a pre-surgical biopsy, or at minimum, a frozen section biopsy is paramount in guiding the treatment of actinomycosis, in order to minimize the extent of surgery.
Written consent to publish/present both text and images related to the case was obtained from the patient by author Dr K Nathan Parthasarathy
Ethical approval: Approval per patient written consent.
Conflict of interest: None
Financial disclosure: None
References
- Gajdacs M, Urban E, Terhes G (2019) Microbiological and clinical aspects of cervicofacial actinomyces infections: an overview. Dent J 7(3): 85-101.
- Nikolaitchouk N, Hoyles L, Falsen E, Grainger JM, Collins MD (2000) Characterization of actinomyces isolates from samples from the human urogenital tract: description of actinomyces urogenitalis sp. nov. Int J Syst Evol Microbiol. 50(4): 1649-1954.
- Smego RA, Foglia G (1998) Actinomycosis. Clin Infect Dis. 26(6): 1255-1261.
- Westhoff C (2007) IUDs and colonization or infection with Actinomyces. Contraception. 75: S48-S50.
- Garcia-Garcia A, Ramirez-Duran N, Sandoval-Trujillo H, Romero-Figueroa MS (2017) Pelvic Actinomycosis. Canadian J of Inf Dis Med Microbio. 2017: 9428650.
- Moniruddin A, Begum H, Nahar K (2010) Actinomycosis: an update. Medicine Today. 22(1): 43-47.
- Saramago SM, Cominho JC, Procenca SSM, Conde PJC, Nunes FMP (2019) Pelvic actinomycosis mimicking pelvic malignancy. Brazillian J Gynecol Obstet. 41(7): 463-466.
- Merki-Feld GS, Lebeda E, Hogg B, Keller PJ (2000) The incidence of actinomyces-like organisms in Papanicolaou-stained smears of copper- and levonorgestrel- releasing intrauterine devices. Contraception. 61(6): 365-368.
- Perez-Lopez FR, Tobojas JJ, Chedraui P (2010) Female pelvic actinomycosis and intrauterine contraceptive devices. Contraception. 1: 35-38.
- Sehnal B, Benes J, Kolarova Z, Mojhova M, Zikan M (2018) Pelvic actinomycosis and IUD. Ceska Gynekol. 83(5): 386-390.
- Chalageri A, Gupta M, Vijayand M, Srinivas G (2013) Bilateral ovarian actinomycosis masquerading as ovarian malignancy; without any history of intra-uterine contraceptive device. Medical J of Dr. D.Y. Patil University 6(4): 468-471.
- Gonzalez S, Pastrana M, Medina E, Gonzalez A, Martin J (2020) Case study: a rare case of a non-IUD-related chronic endometritis caused by actinomyces in a post-menopausal woman. Hospital San Juan: Poster Sunshine 2020.
- Sharma S, Valiathan M, Roa L, Pai MV (2012) Endometrial actinomycosis in post-menopausal female in the absence of an intrauterine contraceptive device: a rare cause of bleeding per vaginum. J Clinical Diagnostic Res 6(6): 1062-1063.
- Kim YJ, Youm J, Kim HJ, Jee BC (2014) Actinomyces-like organisms in cervical smears: the association with intrauterine device and pelvic inflammatory diseases. Obstet Gynecol Sci. 57(5): 393-396.
- Wong VK, Turmezei TD, Weston VC (2011) Actinomycosis. BMJ 343: d6099.
- Lee SY, Kown HJ, Cho JH, Oh JY, Nam KJ, et al. (2010) Actinomycosis of the appendix mimicking appendiceal tumor: a case report. World J Gastroent. 16(3): 395-397.
- Khadka P, Koirala S (2019) Primary cutaneous actinomycosis: a diagnosis consideration in people living with HIV/AIDS. AIDS Res Therapy 16: 16.
- Kolm I, Aceto L, Homback M, Kamarshev J, Hafner J, et al. (2014) Cervicofacial actinomycosis: a long forgotten infectious complication of immunosuppression – report of a case and review of the literature. Dermatol Online J 20(5): 13.
- Dzupova O, Kulichova J, Benes J (2020) Disseminated pelvic actinomycosis caused by Actinomyces Naeslundii. Antibiotics. 9(11): 748.
- Ertas IE, Gungorduk K, Ozdemir A, Emirdar V, Gokcu M, et al. (2014) Pelvic tuberculosis, echinococcus, and actinomycosis: great imitators of ovarian cancer. Aust and New Zealand J of Obstet Gynecol 54(2): 166-171.
- Yang SS, Im YC (2018) Severe abdominopelvic actinomycosis with colon perforation and hepatic involvement mimicking advanced sigmoid colon cancer with hepatic metastasis: a case study. BMC Surgery 18: 51.
- Lim DR, Hur H, Min BS, Baik SH, Kim NK (2014) Intrauterine contraceptive device-related actinomycosis infection presenting as ovarian cancer with carcinomatosis. Surg Infect 15(6): 826- 828.
- Laios A, Terekh I, Majd HS, Pathiraja P, Manek S, et al. (2014) Differentiating pelvic actinomycosis from advanced ovarian cancer: a report of two cases, management reflections and literature review. Gynecol Oncol Res Pract 1: 5.
- Yeguez JF, Martinez SA, Sands LR, Hellinger MD (2000) Pelvic actinomycosis presenting as malignant large bowel obstruction: a case report and review of the literature. Am Surg. 66(1): 85-90.
- Kim YS, Lee BY, Jung MH (2012) Metastatic hepatic actinomycosis masquerading as distant metastases of ovarian cancer. J Obstet Gynecol Res. 38(3): 601-604.
- Lee YK, Bae JM, Park YJ, Park SY, Jung SY (2008) Pelvic actinomycosis with hydronephrosis and colon stricture simulating an advanced ovarian cancer. J Gynecol Oncol. 19(2): 154-156.
- Committee on Practice Bulletins – Gynecology (2017) Long- acting reversible contraception: implants and intrauterine devices. practice bulletin. accessed on: 05/01/2017