Camila Torres-Vélez1, María Cynthia Fuentes-Lacouture1*, Yurany Duarte-Torres2, Myriam Beatriz Amaya- Bernal2, Jair Figueroa-Emiliani3
1Resident, Clinical Hematology and Oncology. Universidad Militar Nueva Granada, Bogotá, Colombia
2Bacteriologist, morphology. Laboratorio de Referencia en Hemato-Morfología. Bogotá, Colombia
3Hematology department, Hospital Universitario Mayor Méderi, Bogotá, Colombia
*Corresponding Author: María Cynthia Fuentes-Lacouture, Resident, Clinical Hematology and Oncology. Universidad Militar Nueva Granada, Bogotá, Colombia
Abstract
Leishmaniasis is a vector-borne parasitic disease caused by at least 20 species of the Leishmania genus. The most common forms are visceral and mucocutaneous Leishmaniasis. An estimated 0.7-1 million cases are reported annually in almost 100 countries considered endemic. The number of reported cases of Leishmaniasis has decreased substantially in the last decade due to better access to diagnostic methods, which allows the establishment of timely treatment and, as a result, a better vector control. We present the case of an-85-year-old man diagnosed with multiple myeloma. An incidental diagnosis of Leishmaniasis was made in a bone marrow aspirate.
Introduction
Visceral Leishmaniasis is a disseminated protozoan disease caused by Leishmania donovani and infants. It is caused by the bite of a vector (mosquito of the Phlebotomus genus). The prognosis is determined by the parasite's characteristics, the vector's biology, and the host's immunological state, representing the definitive factor for elimination through the immune response mediated by T lymphocytes. A significant decrease in the number of cases has been reported, from 400,000 in 2012 to 90,000 by 2017, in response to vector prevention and control campaigns carried out worldwide [1]. Visceral Leishmaniasis usually occurs in immunosuppressed patients. In this context, the parasite can be found in 2 forms: promastigote (infective form, which is transmitted by the vector) and amastigote, which corresponds to the state phagocytosed by macrophages, and which is generally found in the reticuloendothelial system of the spleen, bone marrow, liver, and lymph nodes [2].
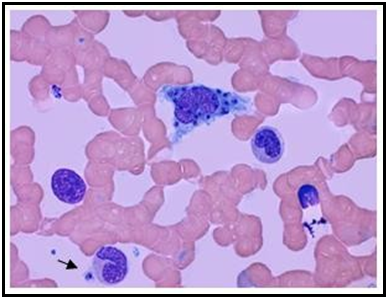
The promastigote, which corresponds to the virulent form, is flagellated and thin. It is released through the sandfly's saliva into the host's dermis. In turn, the amastigote, which corresponds to the phagocytosed form, is blue, with a thin cell membrane and a relatively large and dark nucleus, oval-shaped, 2 to 4 microns in diameter, and has a kinetoplast. This modified mitochondrial structure allows a morphologic differentiation to be made2. (Figure 1)
Figure 1: 100X: Bone marrow aspirate. Wright's stain. Histiocyte phagocytizing amastigotes of Leishmania spp. The arrow points to the kinetoplast in an extracellular parasite.
The severity of the disease is determined by the state of immunocompetence of the host, a factor of vital importance at the time of initiating an immune response. Individuals with an intact cellular response can control infection by forming granulomas. The elimination of Leishmania occurs mainly thanks to the timely action of T helper type 1 lymphocytes, which are responsible for the activation of macrophages. The state of immunosuppression, in the case of patients with HIV or with treatments such as chemotherapy, plays a decisive role in determining the outcome and the risk of relapse [2].
Case Description
An 82-year-old male patient with a history of ischemic heart disease (acute myocardial infarction, with 2 stent implantation), and high blood pressure, consulted consisting of disorientation, profuse sweating, and an episode of fainting for the last 3 days. He also had a history of IgG Kappa multiple myeloma, ISS I (B2 microglobulin
3.3 mg/L, Albumin 4.1), diagnosed in 2015. Until 2018, he had received 2 lines of treatment, showing a second stringent complete remission, and was in regular outpatient follow-up for hematology due to progressive cytopenias but without an increase in M component or development of CRAB. A total blood count showed worsening cytopenia upon admission to the emergency room. Viral deficiency and infectious causes were ruled out by serology. An abdominal ultrasound revealed splenomegaly of 18 cm, which was not clinically evident. Due to past oncological history and hematological involvement in CBC, a bone marrow aspirate and biopsy were performed.
Reported cellularity of 70 % with the representation of the three hematopoietic lines was evidenced, with an additional finding of 6.3% of plasma cells, some atypical and marked rouleaux phenomenon. Additionally, the presence of intracellular microorganisms was striking, which from the morphological point of view at the microscope, were compatible with amastigotes of Leishmania spp. For diagnostic confirmation, a new study in bone marrow was decided to repeat, which shows the presence of abundant extracellular amastigotes of Leishmania spp. (Figure 2-5). Finally, a diagnosis of visceral Leishmaniasis was made. The patient was assessed by an expert in infectious diseases and treated with liposomal Amphotericin B at a dose of 4 mg/kg/day for 5 days, after which 4 mg/kg/day on days 10, 17, 24, and 31 were initiated, considering we were upfront an immunocompromised patient.
Figure 2: 10X: Bone marrow aspirate: Hypercellular none marrow.
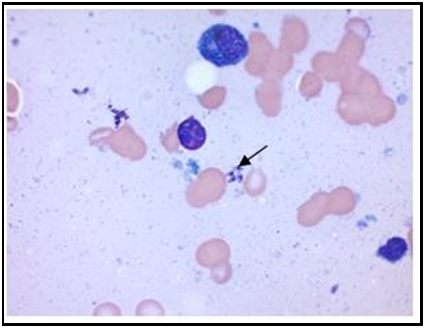
Figure 3: 100X: Bone marrow aspirate: Rouleaux phenomenon. Presence of extracellular amastigotes of Leishmania spp.
Figure 4: 100X: Bone marrow aspirate: Presence of amastigotes of extracellular Leishmania spp. Visualization of Kinetoplast.
Figure 5: 100X: Bone marrow aspirate: Histiocytes with phagocytosis of amastigotes.
Discussion
Visceral Leishmaniasis in 2015 had a higher incidence in Brazil, Ethiopia, India, Kenya, and Somalia1. Regarding the national epidemiology, as reported by the National Institute of Health, in Colombia, in 2018, 7 cases were reported, in order of presentation: Bolivar, Sucre, Córdoba, and Huila3. The most favorable ecological niche for Phlebotomus is humid sites in regions with a height less than 1000 meters above sea level. The domestic dog is one of the main reservoirs of L. infantum [2].
Transmission is not exclusively through the vector, and there are other routes such as transfusions, organ transplants, biological accidents, or intravenous illicit drug users in patients diagnosed with HIV [1,3] being all much less frequent than vectorial transmission. Patients with multiple myeloma are considered severely immunocompromised and have a high risk of opportunistic infections [4], Leishmaniasis being considered one of them. The approximate incubation time varies from 2 weeks to 6 months. Recurrence of latent infection is possible. The disease is caused by the bite of a vector (phlebotomine) and can be caused by more than 20 species of Leishmania spp. In a smaller proportion, transmission through transfusion of contaminated blood components is possible. Leishmaniasis is an endemic disease; in 90 % of cases, it occurs in tropical and subtropical areas5. Most cases have been reported in immunocompromised patients, including patients diagnosed with HIV, patients undergoing bone marrow transplantation, and patients with lymphoproliferative disorders managed with anti-CD52 and anti-CD20 monoclonal antibodies. However, recent epidemiological data report that anti-myeloma agents and their combinations significantly correlate with an increased risk of bacterial, viral, and parasitic infections [5].
Chronologically, the infection occurs in stages. Firstly, the sandfly injects the promastigotes into the skin through saliva, thus entering the bloodstream. After this, the promastigotes are phagocytosed by macrophages and other types of mononuclear phagocytic cells. Once the promastigotes are phagocytosed, they transform into amastigotes. Finally, amastigotes multiply in various tissues, gaining the ability to infect other cells [1]. In this patient's case, he was exposed to Leishmaniasis during his adolescence, being in an endemic area in the early stages of his life. Immunocompetent patients usually do not develop the disease after Leishmaniasis exposure and infection. They have an "inactive" condition throughout their lives, which manifests itself once the host's immunocompetent state gets compromised. This means immunosuppression is a prerequisite for the activation of the disease[4].
Regarding pathophysiology, numerous defects have been described in multiple myeloma, including hypogammaglobulinemia, altered lymphocyte function as mentioned throughout the article, and immunosuppression associated with corticosteroids and other treatments. All the above can predispose to severe opportunistic infections and promote tumor growth and resistance to chemotherapy6, enhancing immune system compromise. Other risk factors are those patients with a history of bone marrow transplantation, which, despite not being the case in our patient, represents one of the essential methods of therapeutic consolidation in patients with multiple myeloma.
Among the most used drugs for treating multiple myeloma we find proteasome inhibitors, immunomodulators, and steroids. Each confers an immunosuppressive effect and increases the risk of opportunistic infections through various mechanisms.
Firstly, steroids decrease the clonal expansion of B cells and the synthesis of antibodies, as well as impede the differentiation and proliferation of T lymphocytes, thus reducing the ability to initiate an adequate immune response. In addition, the inhibition of NF-kB suppresses the secretion of cytokines, significantly reducing the production of IL-2, which is an essential mediator in initiating the immune response. The effect of steroids is cumulative and depends on the exposure time and the doses used [4].
Second, immunomodulators such as lenalidomide, used in this patient's case, have both a stimulating and an inhibitory effect. As a stimulating effect, there is evidence of greater activation of NK cells, dendritic cells, and the production of cytokines, all of this causing an inhibition of the expansion of regulatory T cells. However, in coadministration with dexamethasone, which corresponds to the maintenance protocol of the exposed patient, there is an expansion of regulatory T cells, reduction of NK cell cytotoxicity, and suppression of the total number of CD4 T lymphocytes in patients with multiple mieloma [4,7].
On the other hand, bortezomib (a proteasome inhibitor) suppresses the activity of dendritic cells, significantly decreases the amount of CD4 and CD8 T lymphocytes, and reduces the production of interferon-gamma. This suggests the importance of monitoring CD4 T lymphocytes, as well as in patients diagnosed with HIV, especially after the administration of chemotherapy, consisting of any of the drugs that have been mentioned in this article [6].
Other drugs are directed explicitly against myeloma tumor cells, and they are increasingly used for their overall benefit in controlling this oncological disease. Among these, we find daratumumab, a monoclonal anti-CD38 antibody, which can increase regulatory T cells, decreasing the number of active CD4 T lymphocytes in the blood. This alteration in immunological homeostasis could halter the immune system against intracellular parasites, allowing, in turn, the reactivation of opportunistic infections such as leishmaniasis [4].
Clinically, visceral Leishmaniasis is mainly characterized by hepatosplenomegaly, pancytopenia, and weight loss. Incubation time is 2 weeks to 8 months. Without treatment, it is a pathology with high mortality. In patients with a certain degree of immunosuppression, it could present unusually, mainly involving the gastrointestinal tract, oral mucosa, or liver.
Diagnosis is made through the visualization of amastigotes in tissues, being this the gold standard. For this, a tissue biopsy must be done, with an expected sensitivity of approximately 90 % [2]. Complementary molecular studies include indirect fluorescent antibody test, detection of antibodies against specific antigens (protein k39, rapid test), direct agglutination test, and urinary antigen test. In general, diagnostic confirmation is performed by tissue PCR, with a sensitivity greater than 95 % and an approximate specificity of 63-76 %. On the other hand, in clinical practice, there is the Montenegro test, which is the direct inoculation of Leishmania in the skin, where, if the patient is positive for the disease, they will present a type IV hypersensitivity reaction, manifested by the appearance of an induration more significant than 5 mm (positive test) [2].
It is essential to consider the differences regarding diagnosis in immunocompromised patients. An unusual presentation is possible in this type of patient, involving the oral, duodenal, and hepatic mucosa. The bone marrow and the spleen, which correspond to the most compromised sites, could be damaging. The sensitivity of diagnostic tests is generally lower, which is why it is recommended to have at least two different methods to make the diagnosis [8,9].
Regarding treatment, in both immunocompetent and immunocompromised patients, it is based on the administration of amphotericin B. Management with antimonials such as sodium stibogluconate or puromycin is also possible, the main adverse effect of which is cardiotoxicity and cardiac arrhythmias [2].
Nevertheless, there are essential modifications that must be taken into account in the immunocompromised patient, in whom a higher dose of liposomal Amphotericin B is administered (4 mg IV/kg on days 1-5, 10, 17, 24, 31, and 38), the usual dose being 3 mg/kg on days 1-5, 14 and 21[2]. If the liposomal presentation is not available, it is possible to administer amphotericin deoxycholate 0.5-1 mg/kg per day for a total dose of 1.5 to 2 grams. Pentavalent antimonials (sodium stiboglucanate) are administered at a dose of 20 mg/kg IV for 28 days. Another therapeutic option is miltefosine for patients weighing 34-44 kg (50 mg orally every 12 hours for 28 days), or for patients weighing more than 45 kg, a dose of 50 mg orally every 8 hours for 28 days is administered—28 days [2]. In patients immunosuppressed secondary to chemotherapy or pathologies such as HIV, a maintenance dose is recommended as follows: liposomal amphotericin B (4 mg/kg every 2-4 weeks); as an alternative therapy, pentavalent antimonials (sodium stibogluconate 20 mg/kg IV or IM every 4 weeks) [2]. It is considered possible to discontinue maintenance therapy in patients diagnosed with HIV or after the administration of specific chemotherapy agents such as daratumumab or bortezomib if the CD4 lymphocyte count is between 200 and 350 cells/mm3. However, other authors recommend continuing the therapy indefinitely. The differences concerning the treatment in immunocompromised patients are that in this population, the risk of relapse and death is much higher [1,2]. Finally, it is essential always to remember that these types of patients benefit from secondary prophylaxis, as is done in the treatment protocols for Leishmaniasis in patients diagnosed with HIV, to reduce the relapse rate. However, there is not a clear recommendation for the administration of prophylaxis [4].
Conclusions
The loss of complex mechanisms of humoral and cellular immunity in patients diagnosed with multiple myeloma treated with chemotherapy agents is important [13]. It is essential to consider factors such as residence, recent trips to endemic areas, or transfusion history. The development of fever of unknown origin, hepatosplenomegaly, and pancytopenia are fundamental signs that will allow us to suspect visceral Leishmaniasis and establish treatment promptly, ideally with liposomal amphotericin B at the recommended doses for immunosuppressed patients to avoid complications and reduce the risk of relapse and death. On the other hand, it is essential to consider, depending on the patient's context, the establishment of secondary prophylaxis with mainly liposomal amphotericin B and regular follow-up with quantification of CD4 T lymphocytes.
Early recognition of clinical signs and symptoms will suggest visceral Leishmaniasis among the possible differential diagnoses, with biopsy and bone marrow aspiration being important in the presence of evidence of pancytopenia, as in the case of the exposed patient. The diagnostic gold standard is the visualization of amastigotes in the biopsy, being the kinetoplast recognition fundamental for a correct diagnosis. It is important to always perform PCR as a complementary and confirming study. After diagnosis in the context of patients undergoing chemotherapy, it is vital to suspend immunosuppressive treatment until the end of antiparasitic management. This is essential to carry out effective control of the disease.
Acknowledgments: None to declare.
Financial Disclosure: No funding was received. None of the authors have disclosures relevant to this manuscript.
Conflict of Interest: None to declare.
Informed Consent: The manuscript has been sufficiently de-identified to protect the patient. However, before death, informed written consent was obtained for the publication of the case.
Author Contributions: All authors contributed to the editing of the manuscript. CTV and MCFL wrote the manuscript. YDT, MBAB, and JF contributed to the manuscript corrections.
Data Availability: The authors declare that data supporting the findings of this study are available within the article.
References
- Burza S, Croft SL, Boelaert M (2018) Leishmaniasis. Lancet. 392(10151): 951-70.
- Van Griensven J, Diro E (2019) Visceral Leishmaniasis: Recent Advances in Diagnostics and Treatment Regimens. Infect Dis Clin North Am. 33(1): 79-99.
- Boletín Epidemiológico Semanal: Semana epidemiológica 41. Instituto Nacional de Salud: Julio 22 2018.
- Ziogas DC, Terpos E, Gavriatopoulou M, Migkou M, Fotiou D, et al. (2018) Coexistence of leishmaniasis and multiple myeloma in the era of monoclonal antibody (anti-CD38 or anti-SLAMF7) containing triplets: one shared story of two exceptional cases, Leukemia & Lymphoma. 59(4): 983-987.
- Nucci M, Anaissie E (2009) Infections in patients with multiple myeloma in the era of high-dose therapy and novel agents. Clin Infect Dis. 49(8): 1211-25.
- Kaye P, Scott P (2011) Leishmaniasis: complexity at the host- pathogen interface. Nat Rev Microbiol. 9(8): 604-15.
- Boelaert M, Verdonck K, Menten J, Sunyoto T, van Griensven J, et al. (2014) Rapid tests for the diagnosis of visceral leishmaniasis in patients with suspected disease. Cochrane Database Syst Rev. 2014(6): CD009135.
- Muthu Raja KR, Kovarova L, Hajek R (2012) Induction by lenalidomide and dexamethasone combination increases regulatory cells of patients with previously untreated multiple myeloma. Leuk Lymphoma. 53(7): 1406-8.
- Hurissa Z, Cuevas LE, Lalloo DG, Hailu A (2010) Challenges in HIV and visceral Leishmania co-infection: future research directions. Trop Med Int Health. 15(11): 1401.
- Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, et al. (2007) Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 5(11): 873-82.
- Murray HW, Berman JD, Davies CR, Saravia NG (2005) Advances in leishmaniasis. Lancet. 366(9496): 1561-77.
- Basset D, Faraut F, Marty P, Dereure J, Rosenthal E, et al. (2005) Visceral leishmaniasis in organ transplant recipients: 11 new cases and a review of the literature. Microbes Infect. 7(13): 1370-5.
- Hsu A, Ritchie DS, Neeson P (2012) Are the immuno- stimulatory properties of Lenalidomide extinguished by co- administration of Dexamethasone?. OncoImmunology. 1(3): 372-374.
- Piro E, Kropp M, Cantaffa R, Lamberti AG, Carillio G, et al. (2012) Visceral leishmaniasis infection in a refractory multiple myeloma patient treated with bortezomib. Ann Hematol. 91(11): 1827–1828.
- Radujkovic A, Hundemer M, Eisenbach C, Luft T, Penzel R, et al. (2014) Visceral leishmaniasis in a patient with relapsed multiple myeloma receiving high-dose melphalan and autologous stem cell transplant. Leukemia & Lymphoma. 55(12): 2967-2969.
- Torti L, Pulini S, Morelli AM, Bacci F, Di Bartolomeo P (2015) Visceral leishmaniasis in relapsed and overtreated multiple myeloma in the era of high dose and "novel agent" therapy. Int J Hematol. 102(4): 391-3.