Theodote K. Pontikes, MD, MBA-HCM, Zachary Bloomberg, MD Candidate, Angelos Halaris, MD, PhD*
Loyola University Chicago Stritch School of Medicine, 2160 South First Avenue (Fahey Center), Maywood, Illinois 60153, USA.
*Corresponding Author: Angelos Halaris, MD, PhD, Loyola University Chicago Stritch School of Medicine, 2160 South First Avenue (Fahey Center), Maywood, Illinois 60153, USA.
Abstract
Background: The CDC’s National Center for Health Statistics estimates that nearly half of children treated for attention-deficit/hyperactivity disorder (ADHD) are initially diagnosed by their pediatricians. This ever-growing population will continue to call upon pediatricians, as well as pediatric psychiatrists to manage this neurodevelopmental disorder. One tool to guide physicians in the management of psychiatric illness and thereby reduce trial-and-error approaches is pharmacogenomic testing. The validity of pharmacogenomic testing regarding psychotropic medication management and clinical outcomes has mostly been studied in adults, especially for the treatment of depression. For children, dosing based on pharmacogenetic profiles has been common in the fields of oncology and gastroenterology.
Case Presentation: The following three cases illustrate how healthcare providers can utilize pharmacogenomic testing to understand certain reasons why some patients may not respond, as expected, to a particular medication, or may experience adverse effects at lower therapeutic doses, or may require more innovative, individually tailored treatment strategies that may not be suggested in the evidence-based literature as first- or second- line choices.
Conclusion: While being mindful of the guidelines of the Clinical Pharmacogenetics Implementation Consortium and limitations in pharmacogenetic testing in pediatric patients, there is potential for personalized metabolic insight to suggest or explain why certain psychotropic medications may offer greater therapeutic responses, and why others may have lower thresholds for adverse events or may not be effective. This knowledge may contribute to decreased morbidity and healthcare costs. The tool of pharmacogenomic testing can assist prescribers to identify functionally relevant genetic polymorphism(s) that may be related to medication response. For example, these include hepatic P450 enzymes, serotonin transporter gene alleles, and catecholamine pathway genes.
Keywords: ADHD, Stimulants, Pharmacogenomics
Background
Attention-deficit/hyperactivity disorder (ADHD) is characterized by non-physiological levels of hyperactivity, impulsivity, and inattentiveness. ADHD is the most common neurodevelopmental disorder of childhood, with an 8-10% prevalence rate in school-age children worldwide. The pathophysiology of ADHD is incompletely understood. However, converging evidence supports an underlying dysregulation of catecholaminergic pathways mediating higher cortical functions, in particular attention and executive functioning. The major determinants of the pathophysiology of ADHD are centered on abnormalities in dopamine and norepinephrine metabolism, along with changes in the expression and function of associated transporters and receptors. With respect to pharmacotherapies, multimodal treatment approaches have been advocated. Indeed, the majority of individuals with ADHD, independent of age, are treated with medication(s). Such pharmacotherapies include the psychostimulants, methylphenidate and amphetamine-dextroamphetamine, which are considered as first-line medications. Although psychostimulants have a significant track record of safety and efficacy, up to 30 % of patients treated with one of these agents may experience an inadequate clinical response or treatment-limiting side effects including behavioral changes, insomnia and appetite suppression. Non-stimulant medications are also prescribed, notably atomoxetine, clonidine, guanfacine and bupropion. Over the past decade, relationships have been established between cytochrome P450 genetic variants and plasma concentrations, pharmacokinetics affecting the central nervous system (CNS), and associated clinical implications for pharmacotherapy [1]. Selection of a “congruent” pharmacologic agent and/or dose adjustments based on the pharmacokinetic and pharmacodynamic profile of the patient provides additional information to the prescriber to inform selection of a pharmacologic agent that may more likely be both effective and well-tolerated; thereby minimizing trial and error [2] [3]. We have utilized GeneSight, a FDA-approved, marketed test kit, that provides guided treatment decisions and is covered by government and most private insurance plans. The test kit is marketed by Myriad Genetics, Inc., which recognizes that although a particular medication on its panels may “not have clinically proven genetic markers that allow it to be categorized, it may be an effective choice based on other clinical factors” [https://genesight.com]. This acknowledgment reinforces the significance of a comprehensive medical evaluation and sound clinical judgment that takes into account multiple factors when determining patient-centered, personalized treatment recommendations that can meaningfully improve quality of life.
Case #1
A 10-year-old male patient was referred to our Child and Adolescent Psychiatry Clinic by an outside psychiatrist. The patient initially presented to a pediatrician with difficult-to-treat symptoms of ADHD. His most notable symptoms of hyperactivity, inattention, and impulsivity had not yet remitted at initial presentation. Previous medication trials had included methylphenidate, dexmethylphenidate, lisdexamfetamine and guanfacine.
Prior to referral, his pediatrician ordered pharmacogenomic testing, which suggested a reduced response to methylphenidate, dexmethylphenidate, guanfacine, and clonidine (ADRA2A genotype) (Figure 1). The results also suggested slower rates of metabolism for lisdexamfetamine, amphetamine salts, and clonidine (CYP2D6 genotype), thus alerting to the possibility of inducing toxic blood levels and thereby increased risk of undesirable side effects.
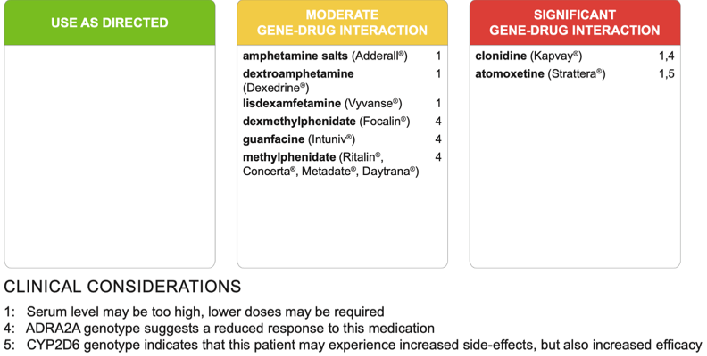
Figure 1: A panel of medications used in the management of ADHD indicating moderate or significant gene-drug interactions for Case 1.
Since a trial of amphetamine salts had never been prescribed, it was reasonable to recommend a slow titration. Over the course of two years with close symptom monitoring and due to normative physical growth, several micro-adjustments were made to the patient’s pharmacologic regimen. His ADHD symptoms significantly improved on Adderall extended-release (XR) in the morning, an Adderall immediate-release (IR) afternoon booster dose, and clonidine immediate-release three times daily (morning, afternoon and bedtime). Recently, oxcarbazepine was added to help address the pre-existing irritability related to impulse dyscontrol, difficulty coping with intense negative emotions (preceding or subsequent to impulsive behaviors) and residual symptoms not fully managed by Adderall XR, Adderall IR and clonidine due to dose limitations. Of note, his teachers’ feedback included a significant reduction in classroom interruptions due to excessive talking and an improved ability to be redirected when distracted. His parents commented that his ability to tolerate frustration and distress had improved, that he could follow directions without multiple repetitions and overall improvement in his social skills with peers and adults.
Case # 2
A 10-year-old female with a past psychiatric history significant for ADHD with severe hyperactivity and impulsivity, an unspecified mood disorder, generalized anxiety disorder, and intellectual impairment (evident on neuropsychological testing) was transferred to our care. Past medication history for ADHD included methylphenidate, dexmethylphenidate, and a brief trial of Adderall XR. She was also treated with lamotrigine and gabapentin for mood and anxiety symptoms, respectively.
Over time and in the context of puberty, it became evident that the patient’s symptoms of impulsivity, hyperactivity, inattention and poor frustration tolerance were no longer adequately treated on her medication regimen, and her mood was therefore also negatively impacted. Pharmacogenomic testing was ordered at age 13-years and suggested a reduced response to methylphenidate, dexmethylphenidate, clonidine and guanfacine (ADRA2A genotype; Figure 2).
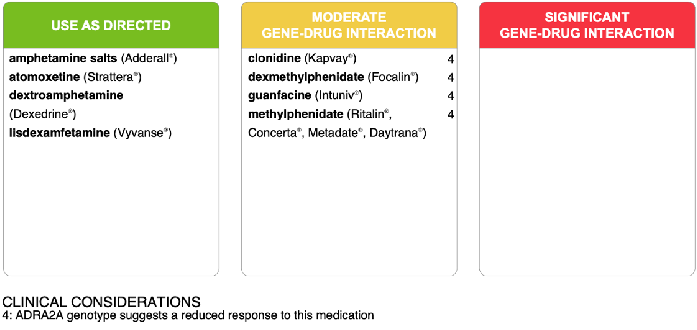
Figure 2: A panel of medications used in the management of ADHD indicating moderate or significant gene-drug interactions for Case 2.
Following a review of the test results, dexmethylphenidate was discontinued and a trial of Adderall XR was initiated, along with an afternoon booster dose of Adderall IR. Clonidine extended-release was also prescribed, as clonidine immediate-release was discontinued due to the side effect of sedation. While on the new ADHD medication regimen, the patient’s hyperactivity, impulsivity, and attention span improved significantly. She was less distracted, better able to follow directions, and less prone to defiance. Additionally, she was more responsive to redirection, and her ability to tolerate frustration and distress improved.
Case # 3
A 14-year-old transgender male (no hormonal treatment) presented to our clinic after previously being treated by a psychiatrist and pediatrician for major depressive disorder, generalized anxiety disorder, and insomnia with nightmares. On initial evaluation at our clinic, there was also evidence of sensory disintegration disorder, motor tics, and concern for possible ADHD. Family psychiatric history was positive for treatment of ADHD in one parent and an older sibling. The patient was a gifted student who performed well academically through sixth grade, but then experienced a significant decline in academic performance. Neuropsychological testing at age 14 showed evidence of difficulty with cognitive flexibility, and the diagnoses given were “Frontal Lobe and Executive Function Deficit” and “Specific Learning Disorder, with impairment in mathematics.” A few months later, it was also established that the patient met criteria for gender dysphoria. Several medications were tried to manage mood dysregulation, severe anxiety, insomnia and tics. When stabilized, an attempt was made to target his ADHD symptoms.
Successive trials of a low-dose methylphenidate extended-release (Concerta) and a low-dose trial of Adderall resulted in intolerable adverse effects. Pharmacogenomic testing had been ordered prior to the initiation of any stimulant medication. Results showed a COMT metabolic genotype associated with gene-drug interaction suggesting reduced therapeutic responses to all stimulant medications (Figure 3). The patient’s inattentive symptoms not only contributed to his impaired functioning, but also exacerbated his depressed mood, thus, prompting a decision to begin a trial of bupropion XL 150 mg every morning to help target both depression and inattention secondary to ADHD. Bupropion was an appropriate choice, as two previous trials with a selective serotonin reuptake inhibitor (SSRI) had failed to treat his anxiety and depressive symptoms, and his family history was significant for bupropion effectively treating a parent’s depression [4]. Within two months of initiation, the patient was tolerating bupropion XL (as well as guanfacine and clonazepam), attending a therapeutic school regularly, and demonstrating notable improvements in frustration tolerance and attention.
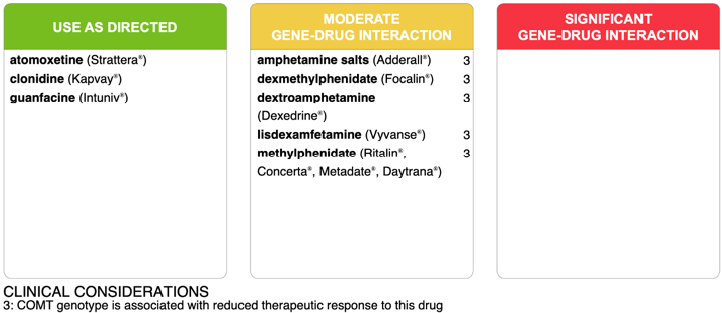
Figure 3: A panel of medications used in the management of ADHD indicating moderate or significant gene-drug interactions for Case 3.
Discussion
The positive outcomes described in these cases illustrate the promising clinical utility of pharmacogenomic testing in the pharmacologic management of children and adolescents with ADHD. While initial prescription of first-line medications for ADHD may eventually lead to effective treatment in some cases, there may also be a series of failed medication trials before a favorable response is achieved. Until then, patients may be at risk for experiencing numerous adverse reactions. For example, prescribing medications without factual knowledge of the patient’s pharmacogenomic profile, as it relates to a specific pharmacologic agent, may unnecessarily expose the patient to adverse side effects, such as insomnia, anxiety, or irritability. Moreover, successive medication trials inevitably prolong an individual’s negative experience with impairing ADHD symptoms and associated consequences adversely impacting their education and overall brain development [5]. The individual may also develop a comorbid psychiatric illness during the period of non-response or poor response, especially among the child and adolescent population [6]. Undertreating children with ADHD compromises cognitive, social, and academic functioning, resulting in a decline in school performance, interference with reaching age-appropriate developmental milestones, and experiences with peer bullying, all of which are independent risk factors for developing anxiety and mood disorders [6] [7]. Therefore, effectively treating ADHD as early as possible after it is identified is critical in improving quality of life and optimizing a child’s developmental trajectory. Another important consideration is when children have other systemic comorbidities, such as seizure disorders or diabetes mellitus. Inadequately treating ADHD can further heighten the stress these patients experience, which may then contribute to decompensation in their physical status and treatment adherence, possibly resulting in further impairment in executive functioning [8]. Multiple and successive failed medication trials may prolong suffering and intensify the severity of an untreated comorbid condition, while also adversely impacting the co-occurring ADHD symptoms.
References
- Wehry AM, Ramsey L, Dulemba SE, Mossman SA, Strawn JR (2018) Pharmacogenomic Testing in Child and Adolescent Psychiatry: An Evidence-Based Review. Current Problems in Pediatric and Adolescent Health Care. 48(2): 40-49.
- Kieling C, Genro JP, Hutz MH, Rohde LA (2010) A current update on ADHD pharmacogenomics. Pharmacogenomics. 11(3): 407-419.
- Myer NM, Boland JR, Faraone SV (2018) Pharmacogenetics predictors of methylphenidate efficacy in childhood ADHD. Molecular psychiatry. 23(9): 1929-1936.
- Ng QX (2017) A systematic review of the use of bupropion for attention-deficit/hyperactivity disorder in children and adolescents. Journal of child and adolescent psychopharmacology. 27(2): 112-116.
- Friedman LA, Rapoport JL (2015) Brain development in ADHD. Current Opinion in Neurobiology. 30: 106-111.
- Goksøyr PK, Nøttestad JA (2008) The burden of untreated ADHD among adults: The role of stimulant medication. Addictive Behaviors. 33(2): 342–346.
- Saluja G, Iachan R, Scheidt PC, Overpeck MD, Sun W, et al. (2004) Prevalence of and Risk Factors for Depressive Symptoms Among Young Adolescents. Archives of Pediatrics & Adolescent Medicine. 158(8): 760.
- Kendall PC, Brady EU, Verduin TL (2001) Comorbidity in Childhood Anxiety Disorders and Treatment Outcome. Journal of the American Academy of Child & Adolescent Psychiatry. 40(7): 787–794.



