Swarup K. Chakrabarti1*, Dhrubajyoti Chattopadhyay1,2
1H. P. Ghosh Research Center, New Town, Kolkata, West Bengal 700161, India.
2Sister Nivedita University, New Town, West Bengal 700156, India.
*Corresponding Author: Swarup K. Chakrabarti, H. P. Ghosh Research Center, HIDCO (II), EK Tower, New Town, Kolkata, West Bengal 700161, India.
Abstract
Climate change represents a monumental threat to both environmental stability and human health, exacerbated by activities such as deforestation and the burning of fossil fuels. The World Meteorological Organization's report, 'Global Climate 2011-2020: A Decade of Acceleration,' underscores the urgent need for immediate action to curb the rapid increase in global temperatures. Sustainable Development Goal 13 (SDG13) highlights the critical importance of integrating climate considerations into policy frameworks. While the direct impacts of climate change on ecosystems and weather patterns are well known, ongoing research is uncovering its complex links to the gut microbiome and human health. The delicate balance of the gut microbiota, which is crucial for digestion, metabolism, and immune function, is intricately tied to environmental conditions. Climate-induced changes in temperature can disrupt microbial ecosystems in the gut, potentially leading to dysbiosis and related health issues.
Moreover, shifts in food availability, as well as exposure to pollutants and pesticides, worsen these disruptions, highlighting the gut microbiota's vulnerability to environmental changes. These alterations upset the delicate equilibrium of the gut microbiome, contributing to health problems like obesity, diabetes, and inflammatory diseases. Moreover, climate change profoundly intersects with environmental epigenetics, altering gene expression patterns through microbial byproducts such as short-chain fatty acids. These epigenetic modifications, in turn, influence immune cell function and reshape the gut microbiome's composition. Grasping these complex interactions is vital for developing robust strategies to counteract the health impacts of climate change and champion sustainable practices. Tackling climate change requires acknowledging the intricate interplay between climate conditions, microbiota health, and epigenetics, thus providing crucial insights into disease mechanisms and steering resilient public health initiatives toward a more sustainable future.
Introduction
Deforestation and the combustion of fossil fuels represent major human activities accelerating climate change, posing severe global challenges affecting both the environment and human health. Additionally, rapid urbanization and environmental degradation compound these issues, significantly amplifying disruptions in global climate patterns and biodiversity loss [1–5]. The recent "Global Climate 2011-2020: A Decade of Acceleration" report from the World Meteorological Organization (WMO) underscores the imperative for decisive measures to cap global temperature rise at 1.5°C above pre-industrial levels [6]. Alarmingly, greenhouse gas levels soared to unprecedented heights in 2022, as reported by the WMO. Sustainable Development Goal 13 (SDG13) places utmost importance on climate action, advocating for initiatives to curb emissions, bolster resilience against climate risks, and embed climate considerations into policy and planning frameworks [7].
While the immediate impacts of climate change on ecosystems, sea levels, and weather patterns are widely recognized, emerging research is shedding light on its intricate connection to the gut microbiome and its implications for human health [8, 9]. The gut microbiota, an intricate ecosystem of microorganisms thriving in the gastrointestinal (GI) tract, is indispensable for human well-being [10–12]. Consisting of trillions of bacteria, viruses, fungi, and other microorganisms, it performs crucial functions in metabolism, immune response, nutrient absorption, and digestion. A multitude of factors, including diet, lifestyle choices, medication usage, and environmental factors, contribute to the diversity and composition of this microbial community [13–15].
The impact of climate change on the gut microbiome can be profound and multi-faceted. Increasing temperatures have the potential to reshape the distribution and abundance of microbial species, thus altering the overall composition of the gut microbiome [13–15, 16, 17]. Moreover, climate-induced shifts in food production and quality can indirectly influence gut health by changing the availability of essential nutrients [18, 19]. These alterations to the gut microbiome can have significant health ramifications, as disruptions in microbial equilibrium have been associated with a range of conditions, including obesity, diabetes, and mental health disorders [20–22]. Fully grasping the effects of climate change on individuals necessitates examining the dynamic interplay between environmental stressors and the gut microbiota.
Moreover, recent scientific discourse has increasingly acknowledged the intricate relationship among climate change, microbiota, and environmental epigenetics [23–25]. This underscores the pivotal role of epigenetics as a dynamic modulator of the genome, influencing the onset and risk of various diseases. Unlike genetic alterations, epigenetic changes are reversible, emphasizing their significance in disease etiology.
They can be reset by internal or external factors, offering intervention opportunities across critical developmental stages and an individual's lifespan [26]. To effectively tackle the health impacts of climate change and foster well-being in a shifting landscape, understanding and addressing these connections are paramount. In essence, comprehending these interrelationships is essential for devising strategies to mitigate climate change's adverse effects on human health and promoting sustainable practices that uphold both environmental resilience and gut health.
Given the pivotal insights into the key actors shaping climate change, microbiome health, and their potential impacts on human health, the subsequent section of this article endeavors to delve deeper into the intricate connection between them. Through various subsections, we aim to illuminate the nuances of this critical link, providing a comprehensive understanding and actionable insights for addressing these complex dynamics.
Impact of Climate Change on the Gut Microbiome
Climate change can exert both direct and indirect influences on the gut microbiota through multiple pathways [27–30]. Fluctuations in temperature and humidity directly create environments favorable for certain pathogenic or opportunistic microbes, potentially disrupting the delicate equilibrium of the gut microbiota.
Beyond simply affecting water and food accessibility, climate change-induced extreme weather events and altered precipitation patterns significantly disrupt agriculture and food production. Put differently, fluctuations in temperature and precipitation can dictate the types of crops viable in different regions, thereby impacting agricultural output. These changes in plant-based diets, a primary source of fiber and essential nutrients crucial for a healthy gut microbiota, may consequently alter the availability and diversity of these microbiota [31–33].
Furthermore, climate change may affect the utilization of antibiotics and other antimicrobial medications, directly influencing the composition and function of the gut microbiota and potentially leading to dysbiosis. For instance, escalating temperatures could facilitate the proliferation of infectious diseases, prompting increased antibiotic usage in humans and animals alike, consequently perturbing the gut microbiota [34, 35].
Against this backdrop, the subsequent sections aim to provide a comprehensive exploration of the key downstream mediators of climate change, building upon the foundational understanding established in the preceding discussion.
Effects of temperature changes on microbial diversity and composition
Temperature stands as a crucial environmental factor that profoundly impacts microbial diversity and composition. Across Earth's diverse habitats, varying ambient temperatures are a universal challenge that organisms must endure to ensure their survival and reproduction [36, 37]. Looking ahead, it is projected that, over the next century, both the frequency and intensity of temperature fluctuations will increase worldwide. This trend is largely driven by human-induced climate change, posing challenges for microorganisms to adapt swiftly to rapid temperature shifts essential for their survival.
This is further compounded by the fact that microorganisms, including bacteria, archaea, fungi, and protists, exhibit varying responses to changes in temperature, which can have profound effects on microbial communities and ecosystem functioning [38, 39]. Any impact these alterations have on the microbial communities that inhabit the human gut may modify those communities' functions and have an impact on host phenotypes and fitness.
According to the World Health Organization (WHO), climate change-induced temperature increases could lead to more than 33,000 deaths of children under 15 from diarrheal diseases by 2050 [40, 41]. Additionally, a recent study suggests that body temperature is inversely related to the abundance of Firmicutes in the intestines, while intestinal Proteobacteria tend to increase with higher body temperatures across different hosts and conditions [42].
Furthermore, the rising temperatures attributed to human-induced climate change significantly increase the chances of harmful bacteria such as Salmonella and Campylobacter thriving [43]. These pathogens, identified by the Centers for Disease Control and Prevention (CDC) as major culprits of global foodborne illnesses, thrive in the temperature range of 40°F (4°C) to 140°F (60°C), commonly referred to as the "temperature danger zone" for food safety [44]. As global temperatures continue to climb, this danger zone expands, providing more favorable conditions for bacterial growth. As a result, the mounting impact of climate change amplifies the susceptibility of our food supply to contamination, emphasizing the urgent necessity for proactive measures to safeguard public health and ensure food safety.
Moreover, it's increasingly evident that a multitude of organisms rely on their microbiomes to endure elevated temperatures. A separate notable study highlights that heat tolerance can be transmitted to recipient flies by transplanting microbiomes from heat-resistant Drosophila melanogaster [45]. Conversely, transient heat waves have been observed to correlate with changes in the abundance of specific bacterial groups in corals, impacting the well- being of their hosts [46].
Understanding how microbiomes confer heat resilience, as seen in flies, holds promise for advancing therapies for heat-related ailments in humans. The potential manipulation of microbiomes to bolster heat tolerance presents novel avenues for treatment and prevention. This interplay between specific bacterial populations and host fitness amid heat stress, as observed in coral ecosystems, underscores the delicate equilibrium between microbiome composition and environmental pressures.
Similar dynamics may occur within the human microbiome, where disruptions triggered by factors such as temperature variations could influence health outcomes. Research suggests that changes in temperature can impact the composition and function of the human microbiome. For example, studies indicate that heat stress alters the gut microbiome composition in humans, leading to elevated levels of bacteria associated with inflammation and metabolic disorders [47, 48].
Additionally, recent research underscores the role of temperature in shaping microbial communities across diverse human body sites, including the skin, gut, and oral cavity [49, 50]. These findings imply that fluctuations in temperature, whether acute or chronic, have the potential to disrupt the delicate balance of microbial ecosystems within the body, potentially impacting immune function, metabolism, and overall health.
As climate change drives an escalation in both the frequency and severity of heat waves, insights derived from these studies could guide adaptive strategies for human populations. Understanding how organisms respond to heat stress through their microbiomes could inspire initiatives to enhance resilience in communities facing similar environmental challenges.
Influence of climate-induced changes in diet and food availability on the gut microbiome
As climate change continues to alter global food availability and quality, dietary shifts emerge, posing implications for the gut microbiome. While the gut microbiome demonstrates resilience and can adjust to dietary changes gradually, the rapid or extreme shifts induced by climate change may strain its adaptive capacity, potentially resulting in adverse health effects.
Climate change impacts dietary habits through shifts in food production and agricultural practices. Key factors like temperature fluctuations, changes in precipitation patterns, and the increased frequency of extreme weather events can significantly influence crop yields and food availability. For example, droughts often result in crop failures, reducing the supply of fruits, vegetables, and grains [31–33]. As a result, dietary patterns might shift, leading individuals to potentially increase their consumption of processed or high-fat foods while decreasing their intake of fiber-rich options.
Changes in diet can impact the composition of the gut microbiome, as specific dietary fibers and lipids can selectively promote the growth of particular bacterial species. For example, certain gut bacteria ferment soluble fibers such as inulin, pectin, and beta- glucans, notably Bifidobacteria and Lactobacilli [51,52]. These bacteria produce short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate through fermentation. Butyrate, in particular, is recognized for its role in providing energy for colon cells and its anti-inflammatory properties [53–55].
On the other hand, while insoluble fibers like cellulose and lignin are not fermented to the same extent as soluble fibers, they still contribute to gut health by increasing stool bulk and promoting regular bowel movements [56]. They may also indirectly influence the gut microbiota composition by providing substrates for other bacterial species. Additionally, dietary fats can influence the gut microbiota composition, with certain bacterial species preferring specific fatty acids [57,58].
For example, saturated fats are implicated in promoting the growth of Bilophila wadsworthia, a bacterium associated with inflammation and compromised gut barrier function [59,60]. In contrast, polyunsaturated fatty acids (PUFAs) such as omega-3s from fish oil are believed to bolster beneficial bacteria like Lactobacillus and Bifidobacterium [61]. Consequently, shifts in dietary habits driven by climate change could profoundly impact the diversity and health of the gut microbiome.
An imbalance in dietary intake can disrupt the delicate harmony of the gut microbiome, favoring the proliferation of harmful bacteria while diminishing beneficial ones. This disruption may lead to the production of toxic metabolites and the overgrowth of pathogenic microbes, compromising the integrity of the intestinal barrier. Consequently, these harmful agents may breach the barrier, entering the bloodstream and potentially impacting various human organs, resulting in mild illnesses initially. However, with the continued accumulation of toxins, these illnesses may escalate into more severe diseases over time.
Climate change also impacts the quality and safety of food. Increasing temperatures, for example, can result in higher levels of mycotoxins and other foodborne pathogens, contaminating food and heightening the threat of foodborne illnesses. These infections and toxins directly disrupt the balance and diversity of the gut microbiome [62, 63].
Furthermore, alterations in food storage and preservation techniques, driven by the effects of climate change, can exert significant effects on the abundance and diversity of beneficial bacteria present in our food. As climate patterns shift, so do agricultural practices and food storage methods, which can inadvertently alter the microbial composition of our food [31–33].
For example, variations in temperature and humidity levels can influence the growth and survival of certain bacterial strains, potentially reducing the abundance of beneficial microbes in stored or preserved foods. These changes in microbial balance not only impact the nutritional quality of food but also have significant implications for gut health and overall well-being.
Consequently, as dietary sources of beneficial bacteria decline, the fragile equilibrium of the gut microbiome could be further disturbed, potentially increasing the risk of various health issues. Therefore, comprehending and mitigating the effects of climate-induced changes on food microbiology is imperative for protecting both food security and human health amidst environmental challenges.
Additionally, climate change poses a threat to food security and access to nutrient-rich foods, particularly for marginalized populations. Disruptions in food supply networks due to severe weather or environmental degradation can lead to malnutrition and food shortages. In such circumstances, individuals may lack access to a diverse range of meals, potentially harming their overall health and gut microbiota [64, 65].
It's important to emphasize that the impacts of dietary shifts caused by climate change and variations in food availability on the gut microbiota are intricate and can vary among individuals based on various factors such as age, genetics, and health status.
For instance, age plays a significant role in shaping the composition and functionality of the gut microbiome. Infants and young children undergo rapid changes in their gut microbiota as they transition from breastfeeding to solid foods, while the microbiota of adults tends to stabilize over time [66]. Thus, dietary alterations driven by climate change may have distinct effects on the gut microbiota of different age groups, with potential implications for their health and development.
On the other hand, genetic predispositions play a significant role in how individuals' gut microbiota respond to changes in diet [67]. Research highlights the impact of genetic variations on these responses. These variations influence how our bodies metabolize different nutrients and interact with specific types of gut microbes, shaping our unique microbial makeup. For instance, studies have revealed associations between specific genetic markers and the response of individuals' gut microbiota to dietary alterations, influencing the diversity and abundance of microbial populations [68,69]. This emphasizes how genetics can influence the dynamics of the gut microbiota in response to dietary shifts. Consequently, the interaction between genetics and dietary habits contributes to varying susceptibilities to disruptions in gut microbiota balance among individuals [70].
Moreover, the health condition of individuals significantly modulates the effects of dietary alterations on the gut microbiota. Chronic ailments such as inflammatory bowel disease (IBS) or obesity can disturb the microbial balance, increasing susceptibility to dietary changes [71]. Conversely, a healthy gut microbiota can enhance resilience, aiding in maintaining equilibrium in the face of environmental fluctuations [72]. To preserve the resilience of food systems and promote human health, efforts to mitigate and adapt to climate change must consider these interconnected aspects.
Hence, addressing climate change and its environmental ramifications is crucial not only for global health but also for the well-being of future generations, considering the transgenerational epigenetic inheritance of climate effects on molecular imprints within hosts. For instance, studies have shown that exposure to certain environmental toxins or dietary changes in one generation can lead to altered DNA methylation patterns that persist across multiple generations [73,74]. These epigenetic changes can influence susceptibility to diseases, behavior, and physiological responses to environmental stimuli in offspring, even in the absence of continued exposure to the original environmental stressor.
The triad of climate change, microbiota, and epigenetics
Every organism adapts to its surroundings by changing its epigenetic processes, altering gene expression and promoting cellular and organismal response. This principal molecular process through which the environment impacts biology is known as environmental epigenetics. Emerging research suggests that early epigenetic markings, influenced by experiences specific to various life stages, continuously shape health outcomes and disease susceptibility through ongoing editing involving adding or erasing epigenetic marks over a lifetime [75].
In this context, while Genome-Wide Association Studies (GWAS) have been pivotal in identifying genetic variations linked to diseases, they often struggle to accurately predict individual disease risk or pinpoint causative loci for complex diseases influenced by environmental factors [76]. On the other hand, Epigenome-Wide Association Studies (EWAS) excel in uncovering epigenetic changes associated with disease risk, revealing how environmental shifts, like climate changes, induce molecular alterations that contribute to disease onset [77]. By integrating insights from GWAS and EWAS, we can uncover genetic variations that shape epigenetic modifications, thereby amplifying their role in influencing disease susceptibility [78]. This combined approach not only enhances our understanding of disease mechanisms but also holds promise in identifying new therapeutic targets for diseases with a significant epigenetic component, offering fresh avenues for treatment and prevention.
Importantly, microbial metabolites, particularly SCFAs, serve as pivotal mediators in the intricate crosstalk between gut microbiota and host cells, profoundly influencing gene expression patterns through epigenetic mechanisms. Research findings underscore the significant impact of SCFAs, such as acetate, propionate, and butyrate, on the epigenetic landscape of host cells [79].
A notable example is butyrate, a significant SCFA produced when gut bacteria ferment dietary fibers. Butyrate is well-studied for its role as a histone deacetylase inhibitor (HDACi) [80]. By blocking HDAC enzymes, butyrate encourages histone hyperacetylation, which relaxes chromatin and improves the accessibility of transcription factors to gene promoters. This epigenetic modification promotes increased transcription of genes involved in essential cellular processes such as metabolism, inflammation, and immune response [81].
Studies have elucidated the impact of SCFAs on DNA methylation, another vital epigenetic mechanism regulating gene expression. For instance, butyrate has been shown to modulate DNA methylation patterns by inhibiting DNA methyltransferase enzymes [82, 83]. This inhibition results in alterations in DNA methylation profiles across the genome, influencing the expression of genes associated with various physiological functions.
Recent research has provided compelling evidence supporting the role of microbial metabolites in shaping host epigenetics and subsequent physiological outcomes. For instance, research has demonstrated that SCFAs produced by the gut microbiota play a crucial role in regulating immune cell differentiation and function via epigenetic modifications. This underscores the complex interaction between microbial metabolites and host immunity [84, 85].
Moreover, epidemiological studies have linked dysbiosis of the gut microbiota and alterations in SCFA production to various health conditions, including IBD, metabolic disorders, and cancer [86, 87]. For instance, a meta-analysis reported associations between decreased SCFA levels and IBD progression, underscoring the clinical relevance of microbial metabolites in disease pathogenesis [88].
Furthermore, heightened exposure to pollutants and pesticides, worsened by the impacts of climate change, represents a substantial hazard to human health as it directly impacts the regulation of epigenetics within host cells. Studies have consistently demonstrated that a multitude of environmental pollutants, including heavy metals, airborne toxins, and pesticides used in agriculture, can trigger epigenetic alterations, leading to detrimental health consequences [73–75].
For instance, studies have revealed that exposure to air pollutants such as particulate matter (PM), nitrogen dioxide (NO2), and ozone (O3) leads to modifications in DNA methylation patterns within blood leukocytes [89,90]. These alterations in DNA methylation profiles are linked to increased vulnerability to respiratory diseases, cardiovascular diseases (CVDs), and cancer.
Similarly, agricultural pesticides, such as organophosphates and glyphosate-based herbicides, have been implicated in disrupting epigenetic regulation [91,92]. Research demonstrated that exposure to glyphosate was associated with changes in histone modifications and DNA methylation patterns in human cell lines [92]. These epigenetic alterations have been linked to adverse health effects, including neurodevelopmental disorders and reproductive abnormalities [93, 94].
Moreover, research has underscored the transgenerational impacts of environmental exposures on epigenetic inheritance. For instance, studies have demonstrated that prenatal exposure to environmental pollutants like polycyclic aromatic hydrocarbons (PAHs) induces epigenetic changes in the germline cells of offspring, resulting in altered patterns of gene expression across successive generations [95, 96].
Pollutants and pesticides have a direct impact on microbiota by altering their growth, diversity, and metabolic activities. For instance, pesticides like glyphosate, neonicotinoids, and organophosphates disrupt gut microbiota in animals and soil microbial communities, affecting ecosystem processes [97,98]. These changes subsequently influence the microbiome of plants and organisms in the soil, creating a ripple effect. Altered plant microbiomes, in turn, may affect the microbiomes of animals consuming them, potentially impacting their health and physiology. Disruptions in soil microbiota decrease soil resilience and increase susceptibility to pathogens, further destabilizing ecosystems [99, 100]. Thus, understanding and mitigating the impacts of pollutants and pesticides on microbial communities are crucial for maintaining healthy ecosystems and biodiversity.
Air pollutants, such as particulate matter and polycyclic aromatic hydrocarbons, have been linked to changes in respiratory and soil microbiota, respectively [101,102]. In aquatic environments, heavy metals and pesticide runoff can lead to shifts in microbial communities, impacting nutrient cycling and ecosystem stability. These pollutants disturb the delicate balance of microbial ecosystems, highlighting the interconnectedness between soil microbiomes, host microbiomes, and overall ecosystem health. Efforts to address these impacts are essential for preserving environmental integrity and ensuring the well-being of both terrestrial and aquatic ecosystems [103, 104].
Impact of climate-induced immune dysfunction on the gut microbiome and mucosal integrity
Climate-induced alterations in epigenetic patterns can profoundly influence the development, differentiation, and function of immune cells in the intestinal milieu [90,105]. Notably, studies have elucidated how shifts in temperature and other environmental variables can reshape the epigenetic landscapes of key immune cell types such as T cells, B cells, and dendritic cells, consequently altering their reactivity and responsiveness [106].
Temperature fluctuations, among other environmental cues, have emerged as significant regulators of immune cell dynamics within the intestinal environment [107,108]. The investigative results emphasize the susceptibility of T cells, pivotal in adaptive immunity, to temperature fluctuations. Such variations can magnify T-cell receptor signaling and the synthesis of cytokines.
Moreover, the intricate interplay of epigenetic mechanisms, including DNA methylation and histone modifications, exerts precise control over T cell differentiation processes, dictating gene expression patterns crucial for immune function [109]. Hence, environmental factors, notably temperature, wield influence over these epigenetic processes, thereby shaping pivotal aspects of T cell fate determination and functional outcomes [12-14].
Dendritic cells, essential antigen-presenting cells that bridge innate and adaptive immunity, are similarly affected by temperature fluctuations. Studies have revealed that variations in temperature can impact the maturation and activation of dendritic cells, influencing their ability to prime T cell responses [113, 114]. Furthermore, epigenetic mechanisms play a role in regulating dendritic cell function, including antigen presentation and cytokine production. Environmental cues, including temperature changes, can modulate the epigenetic regulation of dendritic cells, ultimately shaping their immune-stimulatory capacity and ability to orchestrate adaptive immune responses within the intestine [115].
Importantly, climate-induced immune dysfunction can significantly affect the composition and function of the gut microbiome. For instance, immune dysfunction can profoundly impact the integrity of the mucosal barrier in the intestine, leading to significant alterations in the gut microbiome. The intestinal mucosal barrier acts as a vital defense mechanism, comprising epithelial cells, mucus layers, antimicrobial peptides, and immune cells that collectively prevent the entry of pathogens while maintaining tolerance to beneficial commensal microorganisms [116, 117].
Immune dysfunction leads to the disruption of the mucosal barrier, fostering an environment conducive to dysbiosis. This imbalance enables pathogenic bacteria to flourish while suppressing beneficial ones. Consequently, this dysbiotic state further aggravates mucosal barrier dysfunction, perpetuating a cycle of inflammation and microbial imbalance [118].
Collectively, the amassed body of evidence unequivocally emphasizes the profound impact of exposure to pollutants and pesticides, instigated by climate change, on epigenetic regulation and human health (Figure 1). Unraveling the intricate molecular mechanisms underpinning these epigenetic adaptations is imperative for formulating robust strategies to mitigate the adverse health consequences of environmental exposures and uphold public health amid the tumultuous effects of climate change. Further elucidation of the molecular intricacies governing these interplays is indispensable for the development of precision medicine approaches tailored to address the complex interactions within the microbiota- host epigenetic axis.
In conclusion, comprehending the intricate interplay among climate change, the gut microbiome, and epigenetics emerges as a pivotal cornerstone for bolstering human health in a dynamic and evolving global landscape. By illuminating the underlying mechanisms orchestrating these relationships, we stand to gain deeper insights into the disease processes across diverse conditions and innovate novel approaches to bolster health resilience amid shifting climatic patterns. By marshaling research, innovation, and collaborative efforts, we can forge pathways to transformative solutions that bolster resilience, equity, and sustainability for generations to come.
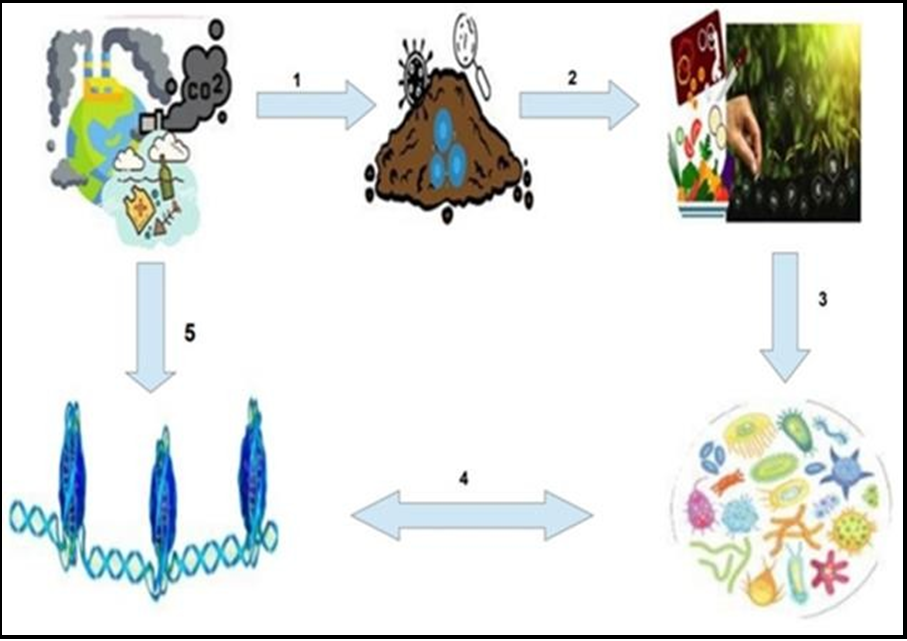
Figure 1: The Eco-Gut Axis and Human Health: The intricate intersectionality of climate changes, epigenomics, microbiota dynamics, and health endpoints presents a formidable and expansive terrain for exploration. (1) Anthropogenic activities emerge as the principal catalysts propelling climatic shifts, instigating cascading challenges across environmental domains such as atmospheric and aquatic milieu, thereby precipitating consequential alterations in soil microbiome composition. (2) Oscillations in soil microbial ecosystems wield substantive influence over the nutritive essence of our daily sustenance and the agricultural yield, thus sculpting the dietary landscape. (3) Deteriorating food quality exerts a profound impact on the ecological equilibrium of the gut microbiome, an indispensable cornerstone of physiological homeostasis. (4) Perturbations in the gut microbiome orchestrate epigenomic modifications via mediators like short-chain fatty acids (SCFAs), while reciprocal alterations in the host epigenome intricately shape the structural and functional profile of the gut microbiota, intimately involving immune cell populations juxtaposed to the gut microbiome and the mucosal barrier. (5) Lastly, the direct assault of climate exigencies on the host epigenome underscores the intricate interplay between environmental stimuli and epigenetics.The figure comprises individual images sourced from relevant web sources, including Google, which were edited and compiled to create a comprehensive visual representation.
Future Direction
In essence, the complex interplay between the gut microbiota, climate change, and human health underscores the need for comprehensive approaches to address their interconnectedness. To counteract the impact of environmental factors on gut microbiota alterations, proactive measures such as reducing greenhouse gas emissions and advocating for sustainable agricultural practices are crucial. Additionally, implementing strategies like incorporating probiotic supplements and adjusting dietary patterns can help maintain a healthy gut microbiota amidst environmental challenges. Prioritizing sustainable farming methods, reducing reliance on chemical inputs, and promoting diverse and nutritious diets emerge as essential steps in safeguarding both planetary and human health. To mitigate the effects of climate-induced changes in diet on the gut microbiome, it is essential to promote diverse and sustainable food systems, support local agriculture, and educate communities about the importance of a balanced diet for gut health. Protecting the delicate balance of the gut microbiome, crucial for immune function, requires implementing strategies that emphasize sustainable food practices, improved sanitation, and the prevention of infectious diseases. These efforts are vital for maintaining overall health and resilience in the face of environmental challenges.
Exploring the complexities of disease pathophysiology through the prism of microbiome-epigenetics-climate interactions opens a promising frontier for advancing therapeutic approaches and mitigating the adverse health effects of climate change. Future research efforts should prioritize unraveling the molecular mechanisms underlying these intricate interactions, paving the way for the identification of new therapeutic targets and the implementation of evidence-based interventions to alleviate the health challenges posed by climate change.
Funding: Financial support for the research project is provided by an intramural grant from the Bandhan Group, specifically designated to bolster the H. P. Ghosh Research Center based in Kolkata, India.
Conflict of Interest: The authors declare that they have no conflicts of interest to disclose regarding the publication of this research.
References
- Li Y, Brando PM, Morton DC, Lawrence DM, Yang H, et al. (2022) Deforestation-induced climate change reduces carbon storage in remaining tropical forests. Nat Commun. 13(1): 1964.
- Balch JK, Nagy RC, Archibald S, Bowman DM, Moritz MA, et al. (2016) Global combustion: the connection between fossil fuel and biomass burning emissions (1997-2010). Philos Trans R Soc Lond B Biol Sci. 71(1696): 20150177.
- Perera FP (2017) Multiple Threats to Child Health from Fossil Fuel Combustion: Impacts of Air Pollution and Climate Change. Environ Health Perspect. 125(2): 141-148.
- McMichael AJ, Friel S, Nyong A, Corvalan C (2008) Global environmental change and health: impacts, inequalities, and the health sector. BMJ. 336(7637): 191-4.
- Schmeller DS, Courchamp F, Killeen G (2020) Biodiversity loss, emerging pathogens and human health risks. Biodivers Conserv. 29(11): 3095-3102.
- Fletcher C, Ripple WJ, Newsome T, Barnard P, Beamer K, et al. (2024) Earth at risk: An urgent call to end the age of destruction and forge a just and sustainable future. PNAS Nexus. 3(4): 106.
- Filho WL, Wall T, Salvia AL, Dinis MAP, Mifsud M (2023) The central role of climate action in achieving the United Nations' Sustainable Development Goals. Sci Rep. 13(1): 20582.
- Microbes and Climate Change – Science, People & Impacts: Report on an American Academy of Microbiology Virtual Colloquium held on November 5, 2021. Washington (DC): American Society for Microbiology; 2022.
- Kim BJ, Lee SY, Kim HB, Lee E, Hong SJ (2014) Environmental changes, microbiota, and allergic diseases. Allergy Asthma Immunol Res. 6(5): 389-400.
- Colella M, Charitos IA, Ballini A, Cafiero C, Topi S, et al. (2023) Microbiota revolution: How gut microbes regulate our lives. World J Gastroenterol. 29(28): 4368-4383.
- Altveş S, Yildiz HK, Vural HC (2020) Interaction of the microbiota with the human body in health and diseases. Biosci Microbiota Food Health. 39(2): 23-32.
- Krishnamurthy HK, Pereira M, Bosco J, George J, Jayaraman V, et al. (2023) Gut commensals and their metabolites in health and disease. Front Microbiol. 14: 1244293.
- Ahn J, Hayes RB (2021) Environmental Influences on the Human Microbiome and Implications for Noncommunicable Disease. Annu Rev Public Health. 42: 277-292.
- Pedroza Matute S, Iyavoo S (2023) Exploring the gut microbiota: lifestyle choices, disease associations, and personal genomics. Front Nutr. 10: 1225120.
- Panthee B, Gyawali S, Panthee P, Techato K (2022) Environmental and Human Microbiome for Health. Life (Basel). 12(3): 456.
- Hylander BL, Repasky EA (2019) Temperature as a modulator of the gut microbiome: what are the implications and opportunities for thermal medicine? Int J Hyperthermia. 36(sup1): 83-89.
- Li J, Bates KA, Hoang KL, Hector TE, Knowles SCL, et al. (2023) Experimental temperatures shape host microbiome diversity and composition. Glob Chang Biol. 29(1): 41-56.
- Farooq MS, Uzair M, Raza A, Habib M, Xu Y, et al. (2022) Uncovering the Research Gaps to Alleviate the Negative Impacts of Climate Change on Food Security: A Review. Front Plant Sci. 13: 927535.
- Elechi JOG, Sirianni R, Conforti FL, Cione E, Pellegrino M (2023) Food System Transformation and Gut Microbiota Transition: Evidence on Advancing Obesity, Cardiovascular Diseases, and Cancers-A Narrative Review. Foods. 12(12): 2286.
- Durack J, Lynch SV (2019) The gut microbiome: Relationships with disease and opportunities for therapy. J Exp Med. 216(1): 20-40.
- Panda SS, Nayak A, Shah S, Aich P (2023) A Systematic Review on the Association between Obesity and Mood Disorders and the Role of Gut Microbiota. Metabolites. 13(4): 488.
- Bruce-Keller AJ, Salbaum JM, Berthoud HR (2018) Harnessing Gut Microbes for Mental Health: Getting From Here to There. Biol Psychiatry. 83(3): 214-223.
- Pulliero A, Traversi D, Franchitti E, Barchitta M, Izzotti A, et al. (2021) The Interaction among Microbiota, Epigenetic Regulation, and Air Pollutants in Disease Prevention. J Pers Med. 12(1): 14.
- Ho SM, Johnson A, Tarapore P, Janakiram V, Zhang X, et al. (2012) Environmental epigenetics and its implication on disease risk and health outcomes. ILAR J. 53(3-4): 289-305.
- Skinner MK (2022) Environmental epigenetics and climate change. Environ Epigenet. 9(1): dvac028.
- Moosavi A, Motevalizadeh Ardekani A (2016) Role of Epigenetics in Biology and Human Diseases. Iran Biomed J. 20(5): 246-58.
- Liang J, Wang Y, Liu B, Dong X, Cai W, et al. (2024) Deciphering the intricate linkage between the gut microbiota and Alzheimer's disease: Elucidating mechanistic pathways promising therapeutic strategies. CNS Neurosci Ther. 30(4): e14704.
- Tu P, Chi L, Bodnar W, Zhang Z, Gao B, et al. (2020) Gut Microbiome Toxicity: Connecting the Environment and Gut Microbiome-Associated Diseases. Toxics. 8(1): 19.
- Williams CE, Williams CL, Logan ML (2023) Climate change is not just global warming: Multidimensional impacts on animal gut microbiota. Microb Biotechnol. 16(9): 1736-1744.
- Montenegro J, Armet AM, Willing BP, Deehan EC, Fassini PG, et al. (2023) Exploring the Influence of Gut Microbiome on Energy Metabolism in Humans. Adv Nutr. 14(4): 840-857.
- Seppelt R, Klotz S, Peiter E, Volk M (2022) Agriculture and food security under a changing climate: An underestimated challenge. iScience. 25(12): 105551.
- Godde CM, Mason-D'Croz D, Mayberry DE, Thornton PK, Herrero M (2021) Impacts of climate change on the livestock food supply chain; a review of the evidence. Glob Food Sec. 28: 100488.
- Lake IR, Hooper L, Abdelhamid A, Bentham G, Boxall AB, et al. (2012) Climate change and food security: health impacts in developed countries. Environ Health Perspect. 120(11): 1520-6.
- Kurane I (2010) The effect of global warming on infectious diseases. Osong Public Health Res Perspect. 1(1): 4-9.
- Luchen CC, Chibuye M, Spijker R, Simuyandi M, Chisenga C, et al. (2023) Impact of antibiotics on gut microbiome composition and resistome in the first years of life in low- to middle-income countries: A systematic review. PLoS Med. 20(6): e1004235.
- Sepulveda J, Moeller AH (2020) The Effects of Temperature on Animal Gut Microbiomes. Front Microbiol. 11: 384.
- Bernhardt JR, O'Connor MI, Sunday JM, Gonzalez A (2020) Life in fluctuating environments. Philos Trans R Soc Lond B Biol Sci. 375(1814): 20190454.
- Tiedje JM, Bruns MA, Casadevall A, Criddle CS, Eloe-Fadrosh E, et al. (2022) Microbes and Climate Change: a Research Prospectus for the Future. mBio. 13(3): e0080022.
- Rocca JD, Yammine A, Simonin M, Gibert JP (2022) Protist Predation Influences the Temperature Response of Bacterial Communities. Front Microbiol. 13: 847964.
- Dhimal M, Bhandari D, Karki KB, Shrestha SL, Khanal M, et al. (2022) Effects of Climatic Factors on Diarrheal Diseases among Children below 5 Years of Age at National and Subnational Levels in Nepal: An Ecological Study. Int J Environ Res Public Health. 19(10): 6138.
- Sheffield PE, Landrigan PJ (2011) Global climate change and children's health: threats and strategies for prevention. Environ Health Perspect. 119(3): 291-8.
- Huus KE, Ley RE (2021) Blowing Hot and Cold: Body Temperature and the Microbiome. mSystems. 6(5): e0070721.
- Dietrich J, Hammerl JA, Johne A, Kappenstein O, Loeffler C, et al. (2023) Impact of climate change on foodborne infections and intoxications. J Health Monit. 8(Suppl 3): 78-92.
- Coorey R, Ng DSH, Jayamanne VS, Buys EM, Munyard S, et al. (2018) The Impact of Cooling Rate on the Safety of Food Products as Affected by Food Containers. Compr Rev Food Sci Food Saf. 17(4): 827-840.
- Moghadam NN, Thorshauge PM, Kristensen TN, de Jonge N, Bahrndorff S, et al. (2018) Strong responses of Drosophila melanogaster microbiota to developmental temperature. Fly (Austin). 12(1): 1-12.
- Bergman JL, Ricci F, Leggat W, Ainsworth TD (2023) Characteristics of The Bleached Microbiome of The Generalist Coral Pocillopora damicornis from Two Distinct Reef Habitats. Integr Org Biol. 5(1): obad012.
- Liu S, Wen D, Feng C, Yu C, Gu Z, et al. (2023) Alteration of gut microbiota after heat acclimation may reduce organ damage by regulating immune factors during heat stress. Front Microbiol. 14: 1114233.
- He Y, Maltecca C, Tiezzi F (2021) Potential Use of Gut Microbiota Composition as a Biomarker of Heat Stress in Monogastric Species: A Review. Animals (Basel). 11(6): 1833.
- Aggarwal N, Kitano S, Puah GRY, Kittelmann S, Hwang IY, et al. (2023) Microbiome and Human Health: Current Understanding, Engineering, and Enabling Technologies. Chem Rev. 123(1): 31-72.
- Prescott SL, Larcombe DL, Logan AC, West C, Burks W, et al. (2017) The skin microbiome: impact of modern environments on skin ecology, barrier integrity, and systemic immune programming. World Allergy Organ J. 10(1): 29.
- Holscher HD (2017) Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 8(2): 172-184.
- Ferenc K, Jarmakiewicz-Czaja S, Filip R (2022) Components of the Fiber Diet in the Prevention and Treatment of IBD-An Update. Nutrients. 15(1): 162.
- Deleu S, Machiels K, Raes J, Verbeke K, Vermeire S (2021) Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EBioMedicine. 66: 103293.
- Topping DL (1996) Short-chain fatty acids produced by intestinal bacteria. Asia Pac J Clin Nutr. 5(1): 15-9.
- Silva YP, Bernardi A, Frozza RL (2020) The Role of Short- Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne). 11: 25.
- Khalid W, Arshad MS, Jabeen A, Muhammad Anjum F, Qaisrani TB, et al. (2022) Fiber-enriched botanicals: A therapeutic tool against certain metabolic ailments. Food Sci Nutr. 10(10): 3203- 3218.
- Lemons JMS, Liu L (2022) Chewing the Fat with Microbes: Lipid Crosstalk in the Gut. Nutrients. 14(3): 573.
- Xu AA, Kennedy LK, Hoffman K, White DL, Kanwal F, et al. (2022) Dietary Fatty Acid Intake and the Colonic Gut Microbiota in Humans. Nutrients. 14(13): 2722.
- Natividad JM, Lamas B, Pham HP, Michel ML, Rainteau D, et al. (2018) Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat Commun. 9(1): 2802.
- Jiang S, Miao Z (2023) High-fat diet induces intestinal mucosal barrier dysfunction in ulcerative colitis: emerging mechanisms and dietary intervention perspective. Am J Transl Res. 15(2): 653-677.
- Fu Y, Wang Y, Gao H, Li D, Jiang R, et al. (2021) Associations among Dietary Omega-3 Polyunsaturated Fatty Acids, the Gut Microbiota, and Intestinal Immunity. Mediators Inflamm. 2021: 8879227.
- Smith BA, Fazil A (2019) How will climate change impact microbial foodborne disease in Canada? Can Commun Dis Rep. 45(4): 108-113.
- Kępińska-Pacelik J, Biel W (2021) Alimentary Risk of Mycotoxins for Humans and Animals. Toxins (Basel). 13(11): 822.
- Cheruiyot SJ, Kimanthi M, Shabani JS, Nyamu NF, Gathu C, et al. (2022) Climate change poses a threat to nutrition and food security in Kilifi County, Kenya. Afr J Prim Health Care Fam Med. 14(1): e1-e4.
- Schnitter R, Berry P (2019) The Climate Change, Food Security and Human Health Nexus in Canada: A Framework to Protect Population Health. Int J Environ Res Public Health. 16(14): 2531.
- Marrs T, Jo JH, Perkin MR, Rivett DW, Witney AA, et al. (2021) Gut microbiota development during infancy: Impact of introducing allergenic foods. J Allergy Clin Immunol. 147(2): 613-621.e9.
- Cahana I, Iraqi FA (2020) Impact of host genetics on gut microbiome: Take-home lessons from human and mouse studies. Animal Model Exp Med. 3(3): 229-236.
- Huda MN, Salvador AC, Barrington WT, Gacasan CA, D'Souza EM, et al. (2022) Gut microbiota and host genetics modulate the effect of diverse diet patterns on metabolic health. Front Nutr. 9: 896348.
- Nova E, Gómez-Martinez S, González-Soltero R (2022) The Influence of Dietary Factors on the Gut Microbiota. Microorganisms. 10(7): 1368.
- Cuevas-Sierra A, Ramos-Lopez O, Riezu-Boj JI, Milagro FI, Martinez JA (2019) Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv Nutr. 10(suppl_1): S17-S30.
- Godala M, Gaszyńska E, Zatorski H, Małecka-Wojciesko E (2022) Dietary Interventions in Inflammatory Bowel Disease. Nutrients. 14(20): 4261.
- Dogra SK, Doré J, Damak S (2020) Gut Microbiota Resilience: Definition, Link to Health and Strategies for Intervention. Front Microbiol. 11: 572921.
- Marsit CJ (2015) Influence of environmental exposure on human epigenetic regulation. J Exp Biol. 218(Pt 1): 71-9.
- Breton CV, Landon R, Kahn LG, Enlow MB, Peterson AK, et al. (2021) Exploring the evidence for epigenetic regulation of environmental influences on child health across generations. Commun Biol. 4(1): 769.
- Al Aboud NM, Tupper C, Jialal I. Genetics, Epigenetic Mechanism.. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024 Jan.
- Marigorta UM, Rodríguez JA, Gibson G, Navarro A (2018) Replicability and Prediction: Lessons and Challenges from GWAS. Trends Genet. 34(7): 504-517.
- Birney E, Smith GD, Greally JM (2016) Epigenome-wide Association Studies and the Interpretation of Disease -Omics. PLoS Genet. 12(6): e1006105.
- Verma M (2016) Genome-wide association studies and epigenome-wide association studies go together in cancer control. Future Oncol. 12(13): 1645-64.
- Stein RA, Riber L (2023) Epigenetic effects of short-chain fatty acids from the large intestine on host cells. Microlife. 4: uqad032.
- Steliou K, Boosalis MS, Perrine SP, Sangerman J, Faller DV (2012) Butyrate histone deacetylase inhibitors. Biores Open Access. 1(4): 192-8.
- Davie JR (2003) Inhibition of histone deacetylase activity by butyrate. J Nutr. 133(7 Suppl): 2485S-2493S.
- Wang L, Shannar AAF, Wu R, Chou P, Sarwar MS, et al. (2022) Butyrate Drives Metabolic Rewiring and Epigenetic Reprogramming in Human Colon Cancer Cells. Mol Nutr Food Res. 66(12): e2200028.
- Gutierrez-Angulo M, Ayala-Madrigal ML, Moreno-Ortiz JM, Peregrina-Sandoval J, Garcia-Ayala FD (2023) Microbiota composition and its impact on DNA methylation in colorectal cancer. Front Genet. 14: 1037406.
- Rooks MG, Garrett WS (2016) Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 16(6): 341-52.
- Liu XF, Shao JH, Liao YT, Wang LN, Jia Y, et al. (2023) Regulation of short-chain fatty acids in the immune system. Front Immunol. 14: 1186892.
- Alshehri D, Saadah O, Mosli M, Edris S, Alhindi R, et al. (2021) Dysbiosis of gut microbiota in inflammatory bowel disease: Current therapies and potential for microbiota-modulating therapeutic approaches. Bosn J Basic Med Sci. 21(3): 270-283.
- Afzaal M, Saeed F, Shah YA, Hussain M, Rabail R, et al. (2022) Human gut microbiota in health and disease: Unveiling the relationship. Front Microbiol. 13: 999001.
- Hu Y, Chen Z, Xu C, Kan S, Chen D (2022) Disturbances of the Gut Microbiota and Microbiota-Derived Metabolites in Inflammatory Bowel Disease. Nutrients. 14(23): 5140.
- Rider CF, Carlsten C (2019) Air pollution and DNA methylation: effects of exposure in humans. Clin Epigenetics. 11(1): 131.
- Prunicki M, Cauwenberghs N, Lee J, Zhou X, Movassagh H, et al. (2021) Air pollution exposure is linked with methylation of immunoregulatory genes, altered immune cell profiles, and increased blood pressure in children. Sci Rep. 11(1): 4067.
- Nicolella HD, de Assis S (2022) Epigenetic Inheritance: Intergenerational Effects of Pesticides and Other Endocrine Disruptors on Cancer Development. Int J Mol Sci. 23(9): 4671.
- Rossetti MF, Canesini G, Lorenz V, Milesi MM, Varayoud J, et al. (2021) Epigenetic Changes Associated With Exposure to Glyphosate-Based Herbicides in Mammals. Front Endocrinol (Lausanne). 12: 671991.
- Grezenko H, Ekhator C, Nwabugwu NU, Ganga H, Affaf M, et al. (2023) Epigenetics in Neurological and Psychiatric Disorders: A Comprehensive Review of Current Understanding and Future Perspectives. Cureus. 15(8): e43960.
- Tran NQV, Miyake K (2017) Neurodevelopmental Disorders and Environmental Toxicants: Epigenetics as an Underlying Mechanism. Int J Genomics. 2017: 7526592.
- Perera F, Herbstman J (2011) Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol. 31(3): 363-73.
- Herbstman JB, Tang D, Zhu D, Qu L, Sjödin A, et al. (2012) Prenatal exposure to polycyclic aromatic hydrocarbons, benzo[a]pyrene-DNA adducts, and genomic DNA methylation in cord blood. Environ Health Perspect. 120(5): 733-8.
- Gama J, Neves B, Pereira A (2022) Chronic Effects of Dietary Pesticides on the Gut Microbiome and Neurodevelopment. Front Microbiol. 13: 931440.
- Ali A, AlHussaini KI (2024) Pesticides: Unintended Impact on the Hidden World of Gut Microbiota. Metabolites. 14(3): 155.
- Christian N, Whitaker BK, Clay K (2015) Microbiomes: unifying animal and plant systems through the lens of community ecology theory. Front Microbiol. 6: 869.
- Wang X, Chi Y, Song S (2024) Important soil microbiota's effects on plants and soils: a comprehensive 30-year systematic literature review. Front Microbiol. 15: 1347745.
- Srogi K (2007) Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: a review. Environ Chem Lett. 5(4): 169-195.
- Roslund MI, Grönroos M, Rantalainen AL, Jumpponen A, Romantschuk M, et al. (2018) Half-lives of PAHs and temporal microbiota changes in commonly used urban landscaping materials. Peer J. 6: e4508.
- Wallen-Russell C, Pearlman N, Wallen-Russell S, Cretoiu D, Thompson DC, et al. (2023) A Catastrophic Biodiversity Loss in the Environment Is Being Replicated on the Skin Microbiome: Is This a Major Contributor to the Chronic Disease Epidemic? Microorganisms. 11(11): 2784.
- Giambò F, Costa C, Teodoro M, Fenga C (2022) Role- Playing Between Environmental Pollutants and Human Gut Microbiota: A Complex Bidirectional Interaction. Front Med (Lausanne). 9: 810397.
- Lussier AA, Bodnar TS, Weinberg J (2021) Intersection of Epigenetic and Immune Alterations: Implications for Fetal Alcohol Spectrum Disorder and Mental Health. Front Neurosci. 15: 788630.
- Kondilis-Mangum HD, Wade PA (2013) Epigenetics and the adaptive immune response. Mol Aspects Med. 34(4): 813-25.
- Weisberg SP, Ural BB, Farber DL (2021) Tissue-specific immunity for a changing world. Cell. 184(6): 1517-1529.
- Ghulam Mohyuddin S, Khan I, Zada A, Qamar A, Arbab AAI, et al. (2022) Influence of Heat Stress on Intestinal Epithelial Barrier Function, Tight Junction Protein, and Immune and Reproductive Physiology. Biomed Res Int. 2022: 8547379.
- Calle-Fabregat C, Morante-Palacios O, Ballestar E (2020) Understanding the Relevance of DNA Methylation Changes in Immune Differentiation and Disease. Genes (Basel). 11(1): 110.
- Bajnok A, Serény-Litvai T, Temesfői V, Nörenberg J, Herczeg R, et al. (2023) An Optimized Flow Cytometric Method to Demonstrate the Differentiation Stage-Dependent Ca2+ Flux Responses of Peripheral Human B Cells. Int J Mol Sci. 24(10): 9107.
- Zan H, Casali P (2015) Epigenetics of Peripheral B-Cell Differentiation and the Antibody Response. Front Immunol. 6: 631.
- MacGillivray DM, Kollmann TR (2014) The role of environmental factors in modulating immune responses in early life. Front Immunol. 5: 434.
- Ostberg JR, Repasky EA (2006) Emerging evidence indicates that physiologically relevant thermal stress regulates dendritic cell function. Cancer Immunol Immunother. 55(3): 292-8.
- Hatzfeld-Charbonnier AS, Lasek A, Castera L, Gosset P, Velu T, et al. (2007) Influence of heat stress on human monocyte- derived dendritic cell functions with immunotherapeutic potential for antitumor vaccines. J Leukoc Biol. 81(5): 1179- 87.
- Tian Y, Meng L, Zhang Y (2017) Epigenetic Regulation of Dendritic Cell Development and Function. Cancer J. 23(5): 302-307.
- Takiishi T, Fenero CIM, Câmara NOS (2017) Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers. 5(4): e1373208.
- Sun Z, Song ZG, Liu C, Tan S, Lin S, et al. (2022) Gut microbiome alterations and gut barrier dysfunction are associated with host immune homeostasis in COVID-19 patients. BMC Med. 20(1): 24.
- Toor D, Wsson MK, Kumar P, Karthikeyan G, Kaushik NK, et al. (2019) Dysbiosis Disrupts Gut Immune Homeostasis and Promotes Gastric Diseases. Int J Mol Sci. 20(10): 2432.