Iraida Caballero Aguirrechu1*, Ernesto Arteaga2, Sonia Collazo Caballero3, Nino López Caballero4, Tania Crombet Ramos5
1Hermanos Ameijeiras Hospital, Cuba. https://orcid.org/0000-0002-1044-6052
2Hermanos Ameijeiras Hospital, Cuba. https://orcid.org/0000-0002-8213-6379
3Hermanos Ameijeiras Hospital, Cuba. https://orcid.org/0000-0002-7354-4928
4Hermanos Ameijeiras Hospital, Cuba. https://orcid.org/0000-0003-4651-2602
5Hermanos Ameijeiras Hospital, Cuba. https://orcid.org/0000-0002-2550-7292
*Corresponding Author: Iraida Caballero Aguirrechu, Hermanos Ameijeiras Hospital, Cuba. https://orcid.org/0000-0002-1044-6052.
Abstract
Introduction: Cutaneous T lymphomas (CTCL) are within the heterogeneous group of non-Hodgkin lymphomas (NHL) that do not originate in lymph nodes. The recommended treatment combines chemotherapy, radiotherapy and immunotherapy. Presentation of the case: a patient with mycosis fungoides in the tumour phase is presented, who receives treatment with anti-CD6 monoclonal antibody, itoluzumab, with good clinical response and low toxicity. Conclusions: There is evidence that relates the autoreactivity of T lymphocytes with increased expression of CD6 on their cell membrane. The murine monoclonal antibody ior®-t1 (IgG2a anti-CD6) recognizes the human CD6 molecule. This mAb recognizes human cell lines in cultures of T and B origin. The administration of the anti-CD6 mAb itolizumab is safe and reports a benefit in clinical response.
Keywords: cutaneous T-lymphoma, mycosis fungoide, CD-6, itolizumab
Introductions
Cutaneous T-lymphomas (CTCLs) are a heterogeneous group of non- Hodgkin lymphomas (NHL) that do not originate in the lymph nodes and are characterized by lymphoproliferative epidermotrophic disorder resulting from infiltration into the skin and bone marrow of neoplastic T lymphocytes and other organs, where the phenotype of mature CD4+ peripheral T cells predominates.
Within NHL, cutaneous lymphomas (CL) rank second in frequency and their annual incidence is 0.5-1 x 100 000 population. These lymphomas have also been classified as low-grade which implies a low potential for aggressiveness, with long evolution and prolonged survival, Annex 1. [1,2]
The prognosis of patients with CTCL is based on the clinical stage at diagnosis. Patients with early-stage disease have a median survival of 20 years or more. The most common clinical presentations are Mycosis Fungoides (MF) and its leukemic variant, Sézary Syndrome (SS). Both are considered distinct manifestations of a single neoplastic entity that appears between 40 and 60 years and is 2,2 times more frequent in males than in females.
In patients with CTCL, an increase in cells with characteristic markers of lymphocytes with natural killer (NK) activity (CD16+, CD56+) and an increase in activated T cells have been detected. Current therapies for CTCLs include topical and systemic treatments for skin manifestations. However, these therapies are not curative and patients frequently progress with extracutaneous lesions involving viscera. [2,3]
CTCLs with systemic spread are usually resistant to radio and chemotherapy. Multiple therapeutic schemes are used in the treatment, ranging from ionizing radiation in isolated fields or total cutaneous irradiation combined or not with chemotherapy, and systemic retinoids, adenosine deaminase inhibitors, monoclonal antibodies and interferons. [4,5]
Therapeutic effects are observed with the use of murine T101 and anti-Leu 1 monoclonal antibodies (MAbs), both directed against the CD5 molecule, but the clinical response is limited by the production of antibodies against the murine MAbs. CD6 is a membrane glycoprotein that is considered a leukocyte differentiation antigen. [5,6]
It is expressed primarily on peripheral blood T lymphocytes, where it constitutes the majority of the CD3+ cell population, and on a small subpopulation of B cells (B1a). The expression of CD6 on the surface of mature human thymocytes has been associated with resistance to apoptosis by these cells in the process of maturation of lymphocytes in this lymphoid organ. [1,6]
Itolizumab is a humanized MAb that recognizes CD6, like its murine parental antibody (ior-t1®), without inducing cell proliferation or complement-dependent cytotoxicity, and it is a new molecule that acts by immunomodulating the CD6 molecule which is a co-stimulatory molecule required for optimal T-cell stimulation by the antigen- presenting cells. [5,6]
The objective of this study is to describe a case of a female patient with a Cutaneous T lymphoma, mycosis fungoides type, that was treated with chemotherapy and with the humanized anti-CD6 antibody itolizumab, as maintenance.
Presentation of the case
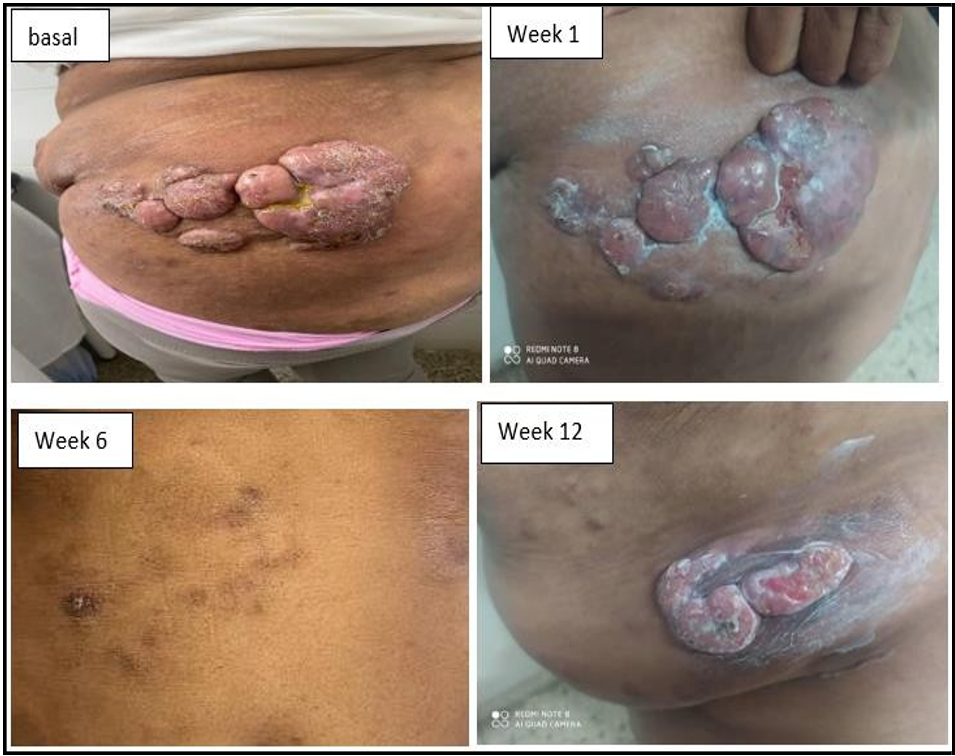
Afro-American 54-year-old female patient with a history of allergy, that 12 months ago began with reddened skin and intense pruritus that progresses in several weeks to nodular and systemic ulcerated lesions with biopsy reporting a cutaneous T cell lymphoma, mycosis fungoides type, Figure 1.
Figure 1: Skin lesions before and after treatment in a patient with Mycosis Fungoides (MF).
Physical examination showed elevated and irregular nodular skin lesions, with ulceration, in different anatomical regions (thorax, abdomen, buttocks, limbs) that respect the head, neck, hands and feet, Figure 1. All laboratory analysis including were normal (hemogram, and chemical test). Imaging: Abdominal ultrasound and chest X-ray revealed no bone, soft tissue or visceral alterations.
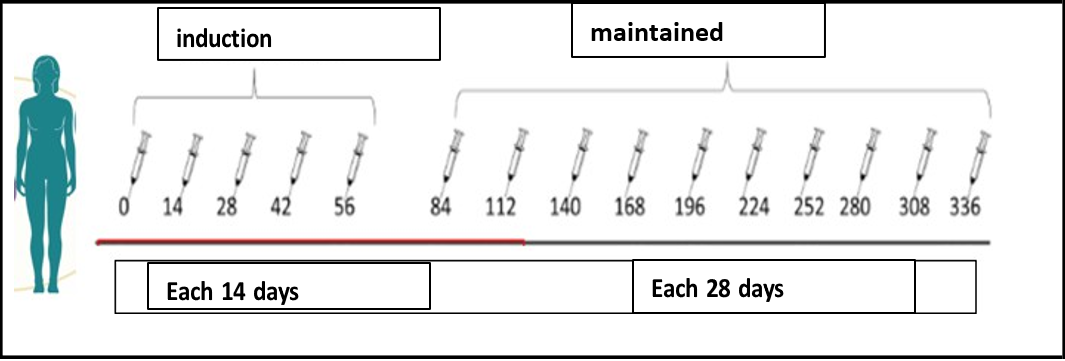
The patient was treated by the dermatology with topical steroids, subcutaneous interferon α2 recombined and daily oral cyclophosphamide. Upon treatment, the disease progressed and become to systemic treatment with intravenous chemotherapy, (COP scheme; cyclophosphamide, vincristine and prednisone). At the third cycle of chemotherapy, with partial response, the anti-CD6 MAb itolizumab was added in dosage of 1,5 mg/kg body weight, once every 14 days for 10 weeks (inductions time), and once in 28 days in maintenance regimen. MAb should be administered as IV infusion made by mixing 250 mL of sterile normal saline with slow infusion in one hour. The patient achieved complete response at week 15 (with the combinations, at 5th chemotherapy cycle) and remains in full response at month 32. In total, the patient has received 35 doses of itolizumab, including 30 maintenance infusions. Figures 1 and 2.
Figure 2: Treatment scheme with the humanized anti-CD6 antibody itolizumab.
Itolizumab was well tolerated. Adverse events consisted of fever and tremors in the third administration, both grade 2, and hypertension in the 5th administration due to discontinuation of previous medication. This event was classified as unrelated. No significant changes in routine laboratory parameters were observed.
Discussion
The response to treatment with itolizumab observed in patients with Cutaneous T Cell lymphoma (CTCL) evidenced the benefit of using an anti-CD6 antibody in lymphoproliferative syndromes (SL). [7,8] The antibody was also very well tolerated.
Although the exact mechanism is unknown, CD6 is reported to be associated with cellular activation mechanisms with co-stimulatory function in peripheral blood T lymphocytes. The interaction of CD6 with its natural ligands is related to the development of important cellular events such as adhesion, maturation, regulation of activation and survival, so this lymphocyte marker is currently identified as a potential target for therapeutic intervention. Itolizumab, a humanized monoclonal antibody recognizes the human CD6 molecule with high affinity. [9]
This MAb recognizes human lines of T cell origin in culture, as well as malignant peripheral mononuclear cells from patients with some types of chronic myeloid leukemia (B-CLL) (88-94%). [10,11] According to some evidence, the mechanism of action of T1h in CD6+ SL remains to be clarified. In a preliminary study, malignant B cells from a patient with B-CLL showed that itolizumab MAb induces apoptosis of these cells.
In this sense, it is reported that an anti-CD5 MAb, a molecule that is co-expressed with the CD6 molecule, induces apoptosis in tumour cells of patients with B-CLL. In addition, in in vitro studies with cells of the Jut 478 line, from B-CLL, it is observed that treatment with itolizumab induces cell death by antibody-mediated cell cytotoxicity (ADCC), similar to MAb cetuximab (anti-EGFR), with the same isotype as T1h (IgG1), which is a powerful ADCC inducer. [5,12,13] In a study of moderate to severe psoriasis, 70 % of the patients treated with itolizumab were classified as responders, 21 patients (26.25%) exhibited whitening of their lesions and only 3 were non-responders (3.75%). [2] Previous studies done in India in the same scenario, showed inferior results. In the phase II trial, only 45 % of the psoriasis patients showed complete response, while in the Phase III trial, complete response ranged from 27 to 36 %. [11,13] Polychemotherapy often induces immunosuppression, resulting in an increased risk of serious infection and poor tolerance. Therefore, overtreatment for this type of lymphoma should be avoided. Interferons have been shown to increase time to progression with low cure rates. The pathogenesis and prognosis of cutaneous T-cell lymphoma (CTCL) differ markedly between subtypes.
In some aggressive subtypes of CTCL, such as primary cutaneous gamma/delta T-cell lymphoma and cytotoxic epidermotropic T-cell CD8+ primary cutaneous lymphoma, hematopoietic stem cell transplantation should be considered, while overtreatment with other favourable subtypes should be avoided. Therefore, a solid understanding of the pathogenesis and immunological history of cutaneous lymphoma is required to better treat patients suffering from this disease. [14]
Conclusion
In conclusion, the use of anti-CD6 MAb in the combined treatment of MF is a safe therapeutic option that might prolong the progression free survival. These encouraging results should be confirmed in a well-designed clinical trial.
References
- Willemze R, Cerroni L, Kempf W, Berti E, Facchetti F, et al. (2019) The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 133(16): 1703-1714.
- Batista-Romagosa M, Gray-Lovio O, Falcón-Lincheta L, Pérez- Alonso T, Mantecón-Fernández B, et al. (2020) Eficacia y seguridad clínica del itolizumab en fase de inducción, en pacientes con psoriasis grave. Revista Cubana de Medicina Militar. 49(2): 230-245.
- Hernández P, Montero E, Suárez E, Cuenca I, Lorente AF, et-al. (2016) Itolizumab, Anticuerpo monoclonal humanizado, un tratamiento novedoso para los pacientes con Psoriasis Severa. Anales de la Academia de Ciencias de Cuba. 5(3).
- Crombet-Ramos T, Ramos-Suzarte M, Saavedra-Hernández D, LeónMonzón K, Betancourt-Cervantes J, et al. (20201) Reposicionamiento del anticuerpo monoclonal humanizado anti- CD6 itolizumab en el tratamiento de pacientes con COVID-19. Anales de la Academia de Ciencias de Cuba.11(3).
- Menon R, David B (2015) Itolizumab - a humanized anti-CD6 monoclonal antibody with a better side effects profile for the treatment of psoriasis. Clin Cosmet Investig Dermatol. 8: 215-22.
- Brugnoni D, Airò P, Tosoni C, Taglietti M, Lodi-Rizzini F, et al. (1997) CD3-CD4+ cells with a Th2-like pattern of cytokine production in the peripheral blood of a patient with cutaneous T cell lymphoma. Leukemia. 11: 1983-1985.
- Izquierdo Cano LM, Espinosa Estrada EE, Hernández Padrón C, Ramón Rodríguez LG, Ávila Cabrera OM, et al. (2014) Anticuerpo monoclonal humanizado Itolizumab (anti-cd6) en síndromes linfoproliferativos cd 6+. Experiencia preliminar. Revista Cubana de Hematol, Inmunol y Hemoter. 30(3): 257-264.
- Bughani U, Saha A, Kuriakose A, Nair R, Sadashivarao RB, et al. (2017) T cell activation and differentiation are modulated by a CD6 domain 1 antibody Itolizumab. PLoS ONE. 12(7): e0180088.
- García CA, Faxas ME, Gavilondo J, Amador JF, Expósito G (1990) Topical treatment of cutaneous T-cell lymphoma skin lesion with the Mouse anti-CD6 antibody ior t1. Biotecnología Aplicada. 7: 176-181.
- Huhn D, von Schilling C, Wilhelm M, Ho AD, Hallek M, et al. (2001) Rituximab therapy of patients with B-cell chronic lymphocytic leukemia. Blood. 98(5): 1326-1331.
- Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, et al. (2007) Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 110(6): 1713-22.
- Fujii K (2018) New Therapies and Immunological Findings in Cutaneous T-Cell Lymphoma. Front Oncol. 8: 198.