Raymond Lamore, PharmD, BCCCP1*, Arshad Wani, MD2, Andrew Jarrah, MD, MBA3, Lauren Lacovara, MCMSc, PA-C3, Geoffrey D. Bass, MD, MBA2
1Department of Pharmacy, Penn Presbyterian Medical Center, Penn Medicine, Philadelphia, USA
2Division of Pulmonary and Critical Care Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, USA
3Department of Medicine, Penn Presbyterian Medical Center, Penn Medicine, Philadelphia, USA
*Corresponding Author: Raymond Lamore, PharmD, BCCCP, Department of Pharmacy, Penn Presbyterian Medical Center, 51 N. 39th St, Philadelphia, PA 19104, USA.
Abstract
A 54-year-old man presented with shock secondary to an amlodipine overdose. The patient admitted to ingesting 150 mg of amlodipine prior to presentation in a suicide attempt. He received intravenous fluid resuscitation and promptly required initiation of vasoactive therapy. Initial point- of-care ultrasonography (POCUS) demonstrated a markedly reduced ejection fraction (EF) of < 30% that subsequently improved with ongoing vasoactive support (hyper-dynamic left ventricle with an estimated EF of 70%). However, the patient’s shock persisted despite high dose infusion of multiple vasopressors (including vasopressin), high-dose insulin infusion, glucagon, and lipid therapy. Accounting for the aforementioned POCUS results, demonstrating recovery of heart function, a physiology-informed treatment approach led to the administration of intravenous methylene blue to enhance systemic vascular resistance in the setting of refractory distributive shock. A significant reduction in vasopressor requirements ensued with the eventual resolution of the patient’s shock state. This case reinforces that methylene blue should be deployed as a treatment modality for the treatment of distributive shock secondary to dihydropyridine calcium channel blocker overdose.
Introduction
Medication overdose, particularly driven by the opioid epidemic, continues to increase nationally [1]. These events, generally unintentional, may overshadow a similar increase in opioid and non- opioid suicidal overdoses [1,2]. As rates of suicide increase, the reduced availability of opioids may lead to the consideration of other classes of medications [2,3]. Since cardiovascular medications remain the most commonly prescribed pharmaceutical therapy, it supports the need for medical teams to be knowledgeable about not only their clinical application, but also their toxicological properties and rescue therapies [4-7].
We describe a case of a 54-year-old man who developed distributive shock secondary to an amlodipine overdose. Although the patient’s shock was refractory to multiple vasopressors (including vasopressin), hyperinsulinemia, glucagon, and lipid therapy, there was a clinically significant response to methylene blue [1-4].
Case Presentation
A 54-year-old man was brought to the emergency department (ED) with light headedness, nausea, and vomiting. He denied fever, chest pain, and dyspnea. He had no notable past medical history, including no known recent infection, coronary artery disease, venous thromboembolism, or anaphylaxis. Emergency medical services reported intentional ingestion of unspecified medications. He became agitated and combative, requiring 4-point restraints with periods of intermittent somnolence. Initial point-of-care ultrasonography (POCUS) demonstrated markedly reduced ejection fraction (EF) of < 30% by visual estimate. He received intravenous fluid resuscitation and promptly required initiation of vasoactive therapy.
He had some facial and upper chest flushing but was afebrile with a normal heart rate of 80 beats/min, normal respiratory rate of 14 breaths/min, and normal oxygen saturation on room air. His physical examination was otherwise unremarkable. He had no signs of volume depletion, oliguria, gastrointestinal hemorrhage, or trauma. He was intubated for airway protection, placed on mechanical ventilation, and ultimately required rapid escalation of epinephrine, norepinephrine, phenylephrine, and vasopressin in the setting of profound shock.
Initial laboratory studies were within normal limits, including a complete blood count, basic metabolic panel, and liver function tests; with exception of serum creatinine of 1.58 mg/dL, serum bicarbonate of 18 mmol/L, and arterial lactic acid of 3.4mmol/L. Cardiac enzymes were normal, and radiographic imaging did not reveal any source of infection or etiology for shock. POCUS in the ED demonstrated severely diminished EF estimated to be < 30% by visual estimate. EKG was notable for an ectopic atrial rhythm with new non-specific intra-ventricular conduction delay with a QRS and QTc duration of 120ms and 488ms, respectively. Six hours from the time of presentation, the patient’s shock continued to worsen with corresponding acidemia to pH 7.20, lactic acid of 6.0 mmol/L, and serum creatinine of 1.99 mg/dL. Subsequent serial POCUS in the ICU with visual estimates demonstrated improved EF, followed by a hyper-dynamic left ventricle with an estimated EF of 70%.
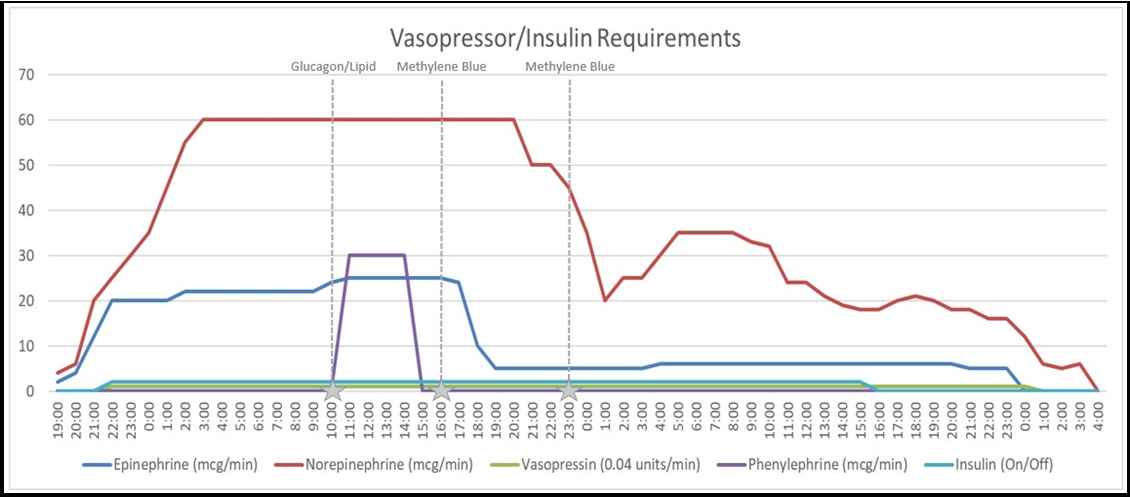
After obtaining a thorough history from the patient’s partner, it was verified that the patient took thirty tablets each of Amlodipine 5mg and Hydroxychloroquine 200mg as a suicide attempt. Poison control was contacted and a euglycemic hyperinsulinemia protocol was initiated with a goal of 2U/kg/hour of insulin. A 20% dextrose solution was initiated to facilitate the rapid up-titration of insulin. POCUS demonstrated restoration of cardiac contractility by a visual estimate of EF with an insulin infusion rate of 2.5U/kg/hour. Glucagon (10mg) and lipid emulsion (1.5mL/kg) were administered approximately 15 hours after presentation without an observed effect in vasopressor requirement for his ongoing distributive shock. Methylene blue 150mg (2mg/kg of total body weight) IV was then administered, with a subsequent dose given 7 hours thereafter, which was followed by a progressive reduction of insulin and vasopressors (Figure 1). Approximately 60 hours after ingestion, he was successfully weaned from all vasoactive medications and insulin. He was subsequently extubated and transferred to a medical floor then inpatient psychiatric hospital.
As noted in the case, serial bedside POCUS and subsequent formal echocardiography demonstrated recovery of normal ventricular function with the administration of inotropic support. Thus, a physiology-informed determination that the patient’s refractory distributive shock was a result of vasoplegia due to dihydropyridine calcium channel blocker (DCCB) overdose guided the administration of intravenous methylene blue to enhance systemic vascular resistance. A significant reduction in vasopressor requirements and ultimately resolution of the shock state correlated with methylene blue administration (Figure 1).
Discussion
The American College of Cardiology and the American Heart Association recommend dihydropyridine calcium channel blockers (DCCB) as a preferred initial antihypertensive therapy [4-5]. This subclass of oral calcium channel blockers (CCBs) includes amlodipine, nifedipine, and felodipine. These agents when taken as prescribed are generally well tolerated, with common side effects of hypotension, edema, flushing, and headache.
While DCCBs are a highly effective and widely prescribed therapy, they are the most common cardiovascular medication reported with fatal overdose [6,7]. Common manifestations of CCB toxicity include hypotension and/or bradycardia and hyperglycemia due to antagonism of calcium channels in pancreatic islet cells, progressing into a cardiogenic and distributive shock state. The management of symptomatic CCB toxicity is challenged by low-quality evidence, with usual recommendations including fluid resuscitation, calcium supplementation, atropine, vasopressors, inotropic support, high-dose insulin euglycemia therapy (HIET), glucagon, lipid therapy, and veno-arterial extracorporeal membrane oxygenation [7].
Importantly, understanding the unique pharmacology of the DCCB subclass may further inform therapy. DCCBs have a higher affinity for the vascular smooth muscle, compared to the cardiac selective non-dihydropyridines (e.g., diltiazem and verapamil), and thus are expected to have a more prominent blood pressure effect due to vasodilation. The existing evidence-base suggests that overdose of CCB leads to a loss of specificity in receptor binding [7]. Additionally, oral DCCBs vary in half-life, impacting the predicted time of recovery. In the case of amlodipine overdose (Half-life of 30- 52 hours), it would be reasonable to see refractory shock persist for multiple days [7-9]. Therefore, a more nuanced understanding of the specific CCB subclass complemented by dynamic re-assessment of physiology with serial echocardiography may substantially inform treatment.
Methylene blue, more commonly known for its use in the treatment of methemoglobinemia and post-operative vasoplegia, is a potent inhibitor of nitric oxide synthase and guanylate cyclase [8]. This leads to a decrease in vascular smooth muscle relaxation and an increase in systemic vascular resistance. It is generally used as a single dose (1- 2mg/kg) that can be repeated or as a continuous infusion [9,10]. As a monoamine oxidase inhibitor, use is cautioned in the presence of other serotonergic agents. Additionally, its ability to absorb light emissions can lead to spurious pulse oximetry measurements [8-10]. Similar to other antidotes, supportive evidence of methylene blue is limited to a small sampling of case reports [8-10]. Outside of these cautionary points, methylene blue is generally well tolerated and is far safer than the majority of other “first line” therapies.
Methylene blue may be a potent and underutilized agent in rescue from life-threatening CCB. Specifically, for patients with restored cardiac output but persistent distributive shock after DCCB overdose, we assert that methylene blue therapy is a generally potent, safe, and well-tolerated therapy with a physiologic basis for efficacy.
Conclusion
We conclude that a high index of clinical suspicion should be maintained for calcium channel blockers in the differential for shock and bradycardia, as they are the most common cardiovascular medications reported with intentional overdose. Recognizing the pharmacologic differences between the subclasses of calcium channel blockers (dihydropyridine versus non-dihydropyridine) is key to identifying preferential rescue therapies, such as methylene blue. Methylene blue can augment systemic vascular resistance and restore blood pressure in patients with distributive shock from CCB overdose.
Declarations
Funding source: None
Conflicts of Interest: The authors have no conflicts of interest to declare.
Data Availability: The data used to support this case report is located within a repository that can be provided upon request.
Author Roles:
Conceptualization: Raymond Lamore
Supervision: Raymond Lamore, Geoffrey Bass, Arshad Wani
Validation: Raymond Lamore, Geoffrey Bass , Arshad Wani
Visualization: Raymond Lamore
Writing – original draft: Raymond Lamore, Geoffrey D. Bass; Andrew Jarrah, Lauren Lacovara, Arshad Wani
Writing – review & editing: Raymond Lamore, Geoffrey D. Bass, Arshad Wani
References
- Hedegaard H, Miniño AM, Spencer MR, Warner M (2021) Drug overdose deaths in the United States, 1999–2020. NCHS Data Brief, no 428. Hyattsville, MD: National Center for Health Statistics.
- Miller TR, Swedler DI, Lawrence BA, Ali B, Rockett IRH, et al. (2020) Incidence and Lethality of Suicidal Overdoses by Drug Class. JAMA Netw Open. 3(3): e200607.
- Stone D, Mack K, Qualters J (2023) Recent Changes in Suicide Rates, by Race and Ethnicity and Age Group — United States, 2021. MMWR. 72(6): 160-162.
- Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, et al. (2018) 2017. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 71(6): e13.
- Nathan S, Pepine C, Bakris G (2005) Calcium antagonists. Hypertension. 46: 637-642.
- Gummin DD, Mowry JB, Beuhler MC, Spyker DA, Bronstein AC, et al. (2021) 2020 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 38th Annual Report. Clinical Toxicology. 59(12): 1282–1501.
- Alshaya OA, Alhamed A, Althewaibi S, Fetyani L, Alshehri S, et al. (2022) Calcium Channel Blocker Toxicity: A Practical Approach. J Multidiscip Healthc. 15: 1851–1862.
- Ginimuge P, Jyothi SD (2010) Methylene Blue: Revisited. J Anaesthesiol Clin Pharmacol. 26(4): 517–520.
- Jang DH, Donovan S, Nelson LS, Bania TC, Hoffman RS, et al. (2015) Efficacy of methylene blue in an experimental model of calcium channel blocker-induced shock. Ann Emerg Med. 65(4): 410-5.
- Pellegrini JR Jr, Munshi R, Tiwana MS, Abraham T, Tahir H, et al. (October 29, 2021) “Feeling the Blues”: A Case of Calcium Channel Blocker Overdose Managed with Methylene Blue. Cureus. 13(10): e19114.