Lattaphon Malaithong MD, Chatchai Choosaeng, MD, Worapot Srimanan MD*
Ophthalmology Division, Phramongkutklao Hospital, Bangkok, Thailand
*Corresponding Author: Worapot Srimanan, MD, Ophthalmology Division, Phramongkutklao Hospital, Bangkok, Thailand
Abstract
Introduction
Blepharoptosis is a common unintentional complication in Botulinum toxin injection for aesthetic practice. Even with spontaneous resolution, patients report a lot of aesthetic and visual discomfort due to decreased eyelid opening.
Methods
We reported two patients who had experienced unilateral blepharoptosis secondary to a cosmetic botulinum toxin injection for upper face wrinkles. Both cases developed eyelid ptosis about 7-10 days after the procedure. The severity of eyelid drooping was marked by only 1-2 mm of the palpebral fissure. The treatment with 5 % phenylephrine plus 0.075 % brimonidine solution was theoretically offered to stimulate the corresponding Muller muscle. The solution was given 1-2 drops twice daily at 8:00 am and 4:00 pm.
Result
Immediate improvement of eyelid ptosis was observed. The palpebral fissure was widened from 1-2 mm to 7-8 mm within 2-3 minutes and lasted for about 90 minutes after the treatment.
Conclusion
5 % phenylephrine plus 0.075 % brimonidine solution administration can mitigate unilateral blepharoptosis secondary to a cosmetic botulinum toxin injection for upper face wrinkles.
Keywords: ptosis, botulinum toxin, temporary, phenylephrine, brimonidine
Introduction
Background
Botulinum toxin is a drug made from a toxin produced by the bacterium Clostridium botulinum and related species. Its mechanism inhibits acetylcholine release from peripheral nerves at the neuromuscular junction [1]. Eight serotypes (from type A–H) of C. botulinum have been identified, of whichserotypes A and B are approved for clinical use. The United States Food Drug and Administration (FDA) first approved the use of botulinum neurotoxin for the treatment of blepharospasm, hemifacial spasm, and strabismus in 1989. [2] In 2002, the U.S. FDA approved the use of botulinum neurotoxin for the treatment of glabellar lines that was primarily for aesthetic conditions. [3] Since its discovery, botulinum toxin has become a versatile tool in managing various clinical and cosmetic conditions. Especially botulinum toxin type A (BoNTA) is the most common usage.
Nowadays, botulinum toxin injection to reduce facial wrinkles is one of the most practiced aesthetic procedures. The results are most promising and satisfactory. The common areas in the upper face typically include the forehead and glabella. It has possible side effects due to injection, including bleeding, bruising, swelling, erythema, and pain at injecting site. One of the common complications is blepharoptosis due to the accidental diffusion of the toxin into the culprit's muscle, levator palpebrae, a tiny muscle located in the upper eyelid. [4] The muscle voluntarily functions to elevate the upper lid, thus resulting in a droopy eyelid when affected.
The incidence of ptosis from BoNTA injection was estimated at 5.4 % among inexperienced injectors and less than 1 % in experienced injectors in a multicenter study conducted by Allergan. [5] The overall incidence of one review literature was about 2.5 %, and the trend of incidence declined when the injectors had more experience.
[6] The untoward effect usually occurs 2-10 days after the procedure, when the esthetic outcome begins to appear. The severity is determined by the amount of toxin injected in the session. Even if the effect of Botulinum toxin persists for 2- 4 weeks, affected cases may be uncomfortable from inadvertent ptosis for a waiting time. [7]
Our study report 2 cases that were successful in the application of a new combination of topical solution for temporary relief of complicated ptosis, which was a 5 % phenylephrine combination with 0.075 % brimonidine eye drops.
Case Series
Here we would like to report a series of 2 cases who suffered from blepharoptosis due to botulinum toxin injection of the upper face for aesthetic purposes.
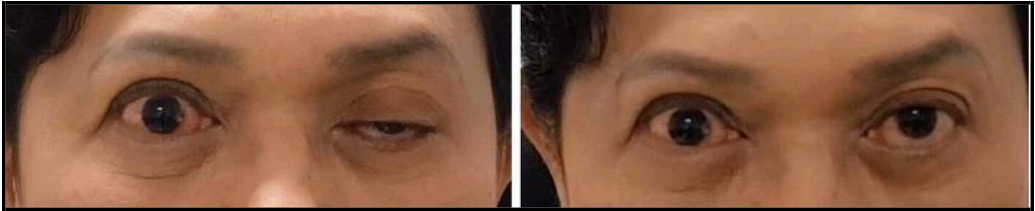
A 55-year-old Thai female received 50 units of botulinum toxin type A injection to correct upper facial wrinkles without immediate complications. She has no underlying medical or health conditions.
She developed left upper lid ptosis 8 days after the aesthetic procedure. Initially, in the first 3 days, the upper lid gradually drooped, starting with 1-2 mm of the palpebral fissure, until the complete unilateral ptosis was diagnosed on the fourth day after the injection. No other visual functions were impaired. The pupillary light reaction was typical.
Figure 1: Before (left picture) and after (right picture) topical instillation of 5 % phenylephrine plus 0.075 % brimonidine solution for 3 minutes [case 1]
A 45-year-old Thai female received 50 units of botulinum toxin type A injection to correct upper facial wrinkles without immediate complications. She has no underlying medical or health conditions. She developed right upper lid ptosis 6 days after the aesthetic procedure. Initially, in the first 3 days, the upper lid gradually drooped, starting with 1-2 mm of palpebral fissure until the complete unilateral ptosis was diagnosed on the fourth day after the injection. No other visual functions were impaired. The pupillary light reaction was typical.
Figure 2: Before (left picture) and after (right picture) topical instillation of 5 % phenylephrine plus 0.075 % brimonidine solution for 3 minutes [case 2]
Treatment
Blepharoptosis is a common aesthetic complication after botulinum toxin injection, particularly when treating upper face wrinkles. The difficulty usually occurs 7-10 days after the performed procedure. The natural history of ptosis is characterized by gradual eyelid drooping in the first 3 to 5 days after onset. Clinical severity is generally determined by the amount of toxin administered into the glabellar area.
The topical eye drops were mixed for individual patients at room temperature (25 °C) using the aseptic technique currently used in hospitals. This included the use of a clean and sterilized, long laboratory coat, cap, hat, mask, and gloves; a sterile syringe to extract and transfer 5 ml of the 10 % phenylephrine solution from the bottle; and a new sterile needle to extract 5 ml of 0.15 % brimonidine and inject it back into the Phenylephrine solution bottle. The answer was mixed, and the bottle was tightly closed. Preparation was conducted in a clean room under a laminar flow.
The administration of 5 % phenylephrine plus 0.075 % brimonidine solution into the eye stimulates Muller muscle twice daily at 0800 am and 0400 pm until the botulinum effect on the neuromuscular function wears off, which is typically around 4-6 weeks. The immediate improvement of eyelid ptosis can be noticed within 2-3 minutes after administration of the solution and can last up to 90 minutes.
Participants had written Informed consent. The side effects of topical eyedrops were declared, including hypertension, palpitation, and arrhythmia. The side effect was continuous monitoring for 4 hours according to drug duration. Closing the eye for 5 minutes and digital compression at the punctum was done to decrease drug absorption.
Outcome And Follow-Up
5 % phenylephrine plus 0.075 % brimonidine solution can dramatically improve eyelid ptosis due to aesthetic botulinum toxin injection. We report 2 cases who presented with unilateral complete eyelid ptosis with only 1-2 mm of palpebral fissure remaining. After administration of 5 % phenylephrine plus 0.075 % brimonidine eye drop solution twice daily, immediate improvement can be appreciated within 2-3 minutes after treatment, and the effect can last up to 90 minutes.
Discussion
Our case series showed drastic improvement in unintentional ptosis after BoNTA injection using topical 5 % phenylephrine and 0.075 % brimonidine eye drop.
Physiologically there are 2 major retractor muscles of the upper eyelid, the levator palpebrae superioris and the Müller muscle, responsible for elevating the upper eyelid and maintaining it in an open position. The β1-adrenergic receptors are predominantly found in the levator muscle and cause voluntary contraction when stimulated, whereas α2-Adrenergic receptors are the predominant subtype in the Müller's muscle and result in involuntary contraction when enabled. [8] Since the levator muscle is paralyzed due to the botulinum toxin blocking effect on the neuromuscular junction. The Muller's muscle is aimed to be activated by using adrenergic pharmacologic agents.
Pathophysiology blepharoptosis occurs when the ipsilateral levator muscle has been inadvertently hit by the botulinum toxin, presumably during glabellar frown line aesthetic treatment. The botulinum toxin is postulated to travel down the orbital septum to reach the ipsilateral levator muscle.
There are several case reports of ptosis due to BoNTA injections in the literature. The symptom onset is about 3–14 days post- injection, typically resolving within 3–4 weeks. Ptosis may be mild and unnoticeable, but the patient will likely report a heavy feeling in their eyebrow or eyelid and may not be able to open the affected eye fully. The total reversal of ptosis has been reported to take up to 3 months. Nowadays, several treatment options have been proposed to relieve this problem. The topical eyedrop, oral anticholinesterase, and Botulinum toxin injection were options for correcting this problem. The topical eyedrop, which affects α1 and α2 adrenergic receptors, could be the correct ptosis problem. We used 0.5 % apraclonidine, an antiglaucoma drug, to stimulate α2 adrenergic receptors. At the sametime, it can also mildly stimulate α1 receptors, thus resulting inslightly improved eyelid ptosis when topically instilled twice to thricedaily. 5 Several kinds of literature support this medication foralleviating ptosis symptoms. Nowadays, 0.5 % apraclonidine is irritating and has annoying side effects such as conjunctivitis anduncomfortable sensation. [9,10] A few cases were reported in literature improving ptosis by this medication. [11] Further, this medication is currently unavailable worldwide, and its role is limited. The newer developed 0.15 % brimonidine, a selective α2 adrenergic agonist, has replaced its applications. The latter is more effective with less frequent daily instillations, thus becoming more widely used among ophthalmologists. Additionally, since brimonidine is a selective α2 adrenergic agonist with no α1 agonistic effect, it can only aid in mild eyelid elevation in ptotic cases. Previous reports showed insignificant differences in upper eyelid position between the eye of healthy participants after using topical 0.15 % brimonidine ophthalmic solution. [12,13] Contrastly, Alotaibi GF et al. report a case with complicated ptosis improvement after BoNTA injection by 0.33 % brimonidine topical gel. [14]
The 0.1 % oxymetazoline hydrochloride eyedrops are currently the only FDA-approved pharmacologic treatment for acquired blepharoptosis in adults. It is a potent α1 and α2 adrenergic receptor agonist classically developed for nasal congestion. Two randomized clinical trials confirm its efficacy for ptosis treatment, including increased marginal-reflex distance 1 and visual field. [15] By the way, these eyedrops are unavailable in Thailand. It could be found in nasal sprays and nasal drops form. The most widely used pharmacologic agent is 0.1 % naphazoline, primarily for allergic conjunctivitis. It is a weak α1 adrenergic agonist and has been prepared in a low concentration. Therefore, it can only exert a mild elevation effect on the drooping eyelids.
Another agent that can be used to treat ptotic patients is 10% phenylephrine, a potent α1 adrenergic agonist commercially available in a highly concentrated preparation. It is a conventionally preoperative topical test indicator for successful Müller's muscle resection ptosis repair. [16,17] Currently, there was no report of phenylephrine eyedrops in the treatment of ptosis due to BoNTA injection.
Oral anticholinesterase drugs were proposed to treat ptosis induced by BoNTA injection. Pyridostigmine was reported to alleviate this complication. It was the second line of treatment because of systemic side effects in gastrointestinal and gastro-urinary systems.
We use the combination of topical 5 % phenylephrine and 0.075 % brimonidine in our report for reliving ptosis temporarily. The α1 and α2 adrenergic receptor agonists are the keys to our treatment. The solution has about 90 minutes of duration of action. Since Muller's muscle is prone to fatigability, it is advisable to administer the answer no more than twice daily.
Our case series reveals a new modality to subside ptosis complications after BoNTA injection. Whether there was no adverse event in our series, the use of these combinations should be supervised by an ophthalmologist. Side effects should be advised, and continuous monitoring should be done. Generally, it is recommended to observe intraocular pressure, systemic blood pressure, and other potential side effects, including photophobia and blurred vision, in the patients at least once weekly.
Conclusion
In summary, a few commercial eyedrop preparations are commonly available in our clinical practice to treat eyelid ptosis due to botulinum toxin injection for upper facial wrinkle correction. 0.15 % brimonidine and 0.1 % naphazoline are the two most common drugs to treat such conditions. In this article, we propose using 5 % phenylephrine plus 0.075 % brimonidine solution as another best option to treat droopy eyelids secondary to aesthetic botulinum toxin injection. We recommend topical instillation of 5 % phenylephrine plus 0.075 % brimonidine solution twice daily at 8:00 am and 4:00 pm until botulinum toxin effects and eyelid ptosis clinically disappear. An ophthalmologist should periodically evaluate intraocular pressure, systemic blood pressure, photophobia, and blurred vision at least once a week.
Acknowledgments: General support by a departmental chair and chief director, Ornwasee Jatuthong, MD
Statement of Ethics
Study approval statement: Institutional Review Board Royal Thai Army Medical Department reviewed and approved this study protocol, approval number S030h/65_Exp.
Consent to publish information: Written informed consent was obtained from the patient to publish this case report and any accompanying images.
Conflict of Interest Statement: The authors of this manuscript do not have any conflict of interest to declare
Funding Sources: This manuscript did not receive any funding
Author Contributions
Worapot Srimanan, MD, treated the subject and collected the clinical data. Worapot Srimanan, MD, wrote the manuscript, Chatchai Choosaeng, MD and Lattaphon Malaithong, MD, revised the manuscript. All authors approved the final version of the manuscript. The authors agree to be responsible for all aspects of this work.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary material files. Further inquiries can be directed to the corresponding author.
References
- Dhaked RK, Singh MK, Singh P, Gupta P (2010) Botulinum toxin: bioweapon & magic drug. Indian J Med Res. 132(5): 489- 503.
- Frevert J (2015) Pharmaceutical, biological, and clinical properties of botulinum neurotoxin type A products. Drugs R D. 15(1): 1-9.
- Small R (2014) Botulinum toxin injection for facial wrinkles. Am Fam Physician. 90(3): 168-75.
- Satriyasa BK (2019) Botulinum toxin (Botox) A for reducing the appearance of facial wrinkles: a literature review of clinical use and pharmacological aspect. Clin Cosmet Investig Dermatol. 12: 223–228.
- King M ( 2016) Management of Ptosis. J Clin Aesthet Dermatol. 9(12): E1-E4.
- Cavallini M, Cirillo P, Fundarò SP, Quartucci S, Sciuto C, et al. (2014) Safety of Botulinum Toxin A in Aesthetic Treatments: A Systematic Review of Clinical Studies. Dermatologic Surgery. 40(5): 525-36.
- Klein AW (2004) Contraindications and complications with the use of botulinum toxin. Clinics in Dermatology. 22(1): 66-75.
- Esmaeli-Gutstein B, Hewlett BR, Pashby RC, Oestreicher J, Harvey JT (1999) Distribution of Adrenergic Receptor Subtypes in the Retractor Muscles of the Upper Eyelid. Reconstructive Surgery. 15(2): 92-9.
- Silvestre JF, Carnero L, Ramón R, Albares MP, Botella R (2001) Allergic contact dermatitis from apraclonidine in eyedrops. Contact Dermatitis. 45(4): 251.
- Armisen M, Vidal C, Quintans R, Suarez A (1998) Allergic contact dermatitis from apraclonidine. Contact Dermatitis. 39(4): 193.
- Omoigui S, Irene S (2005) Treatment of Ptosis as a Complication of Botulinum Toxin Injection. Pain Med. 6(2): 149-151.
- Mendonça TB, Lummertz AP, Bocaccio FJ, Procianoy F (2017) Effect of Low-Concentration, Nonmydriatic Selective Alpha- Adrenergic Agonist Eyedrops on Upper Eyelid Position. DS. 43(2): 270-274.
- Tuncer I, Bilgin S, Zengin MÖ, Mangan MS, Karaca A, et al. (2021) Effect of brimonidine tartrate 0.15% on scotopic pupil size and upper eyelid position: controlled trial. Eye. 35(2): 672-675.
- Alotaibi GF, Alsukait SF, Alsalman HH, Turkmani MG (2022) Eyelid ptosis following botulinum toxin injection treated with brimonidine 0.33% topical gel. JAAD Case Reports. 22: 96-98.
- Slonim CB, Foster S, Jaros M, Kannarr SR, Korenfeld MS, et al. (2020) Association of Oxymetazoline Hydrochloride, 0.1%, Solution Administration with Visual Field in Acquired Ptosis. JAMA Ophthalmol. 138 (11): 1168-1175.
- Grace Lee N, Lin LW, Mehta S, Freitag SK (2016) Response to phenylephrine testing in upper eyelids with ptosis. Digit J Ophthalmol. 21(3): 1–12.
- Glatt HJ, Fett DR, Putterman AM (1990) Comparison of 2.5% and 10% phenylephrine in the elevation of upper eyelids with ptosis. Ophthalmic Surg. 21(3): 173-6.