Duan Rubing*, Liang Xiaodong, Ji Kaili, Li Ping, Guo Jianghua
Reproductive Center of Jiangmen Central Hospital (Jiangmen 529030, Guangdong)
*Corresponding Author: Duan Rubing, Reproductive Center of Jiangmen Central Hospital (Jiangmen 529030, Guangdong).
Abstract
Objective: To systematically evaluate the effectiveness of the novel coronavirus pneumonia vaccine in preventing the infection of variant strains of the Omicron virus.
Methods: The databases of Web of Science, PubMed, Embase, The Cochrane Library, CNKI, WanFang Data, and CBM were searched by computer, and the case-control studies were collected to evaluate the effectiveness of the novel coronavirus pneumonia vaccine in preventing the infection of Omikron and its variants. The search time was from establishing the database to March 8, 2023. After two researchers independently screened the literature, extracted data, and evaluated the risk of bias included in the study, a meta-analysis was conducted using RevMan 5.3 software.
Results: A total of 9 studies were included, including 2865389 subjects. The meta-analysis results showed that the infection rate (OR=0.56, 95% CI (0.48, 0.65), P<0.001), hospitalization rate (OR=0.44, 95% CI (0.39, 0.51), P<0.001), and severe illness rate (OR=0.53, 95% CI (0.35, 0.81), P<0.001) of the vaccination group were significantly lower than those of the non-vaccination group.
Conclusion: Vaccination can effectively prevent the infection of the novel coronavirus Omikron strain and reduce the incidence of hospitalization and critical illness caused by the virus. This provides an essential reference for the formulation and implementation of epidemic prevention policies such as vaccine promotion.
Keywords: novel coronavirus pneumonia vaccine; Omicron variants Effectiveness; Meta-analysis; Case Control Study.
Introduction
Novel coronavirus pneumonia is an acute respiratory infectious disease caused by severe acute respiratory syndrome coronavirus 2. Since the first report of unknown coronavirus pneumonia in China in 2019, the pandemic has caused great harm at home and abroad, seriously affecting public health, economic development, and people's health in China and the world [10-11].
Epidemiological data shows that 20% of the confirmed patients with COVID-19 infection need to be hospitalized, of which the demand ratio of ICU is 1 ∶ 16000, the case fatality rate of patients under 65 years old is 0.6% ~ 2.8%, and that of patients over 70 years old is 5.4% ~ 16.6% [12-14]. Because of the Lack of effective antiviral drugs and the fact that the epidemic has never been fundamentally controlled, vaccines are considered one of the most effective means to prevent COVID-19 infection [15].
Since the first case of the Omicron variant was reported in South Africa in November 2021, the Omicron variant has developed into a dominant strain following the Alpha variant, Beta variant, Gamma variant, and Delta variant. However, most vaccines currently in the clinical evaluation stage are designed for the wild type of COVID-19 virus, and its effectiveness in preventing the infection of the Omicron variant remains to be discussed [16-17].
This study systematically evaluated the effectiveness of the COVID-19 vaccine in preventing the infection of the Omicron variant to provide medical evidence for optimizing the prevention and treatment strategy of COVID-19 and formulating the grading diagnosis and treatment system of COVID-19.
1. Materials and Methods
1.1 Inclusion and Exclusion Criteria
1.1.1 Study Type Case-Control Trials.
1.1.2 Subjects Patients with suspected symptoms of COVID-19 (cough, fever, shortness of breath, vomiting, diarrhoea) [18] who went to medical institutions for treatment.
1.1.3 Intervention test group: intramuscular injection of COVID-19 vaccine and primary immunization or reinforcer injection after basic immunization; Control group: did not receive any SARS-CoV-2 vaccine.
1.1.4 Outcome indicators: SARS-CoV-2 infection rate, hospitalization rate, critical illness rate, and mortality rate.
1.1.5 Exclusion criteria: ① Non-Chinese and English literature; ② Publish multiple articles on the same vaccinated population and select the most recent or informative one; ③ Non-Phase I/II, Phase II, and Phase II/III clinical trials: ④ Full text cannot be obtained; ⑤ Lack of outcome indicators or inability to extract raw data.
1.2 Literature Retrieval Strategy
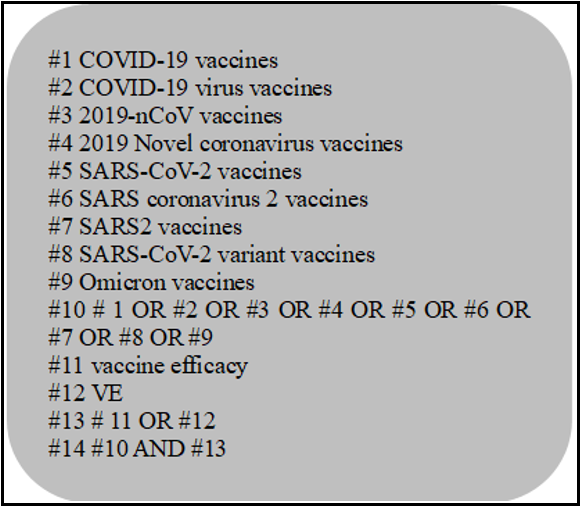
Computer searches Web of Science, PubMed, Embase, The Cochrane Library, CNKI, WanFang Data, and CBM databases to collect RCTs of different types of COVID-19 vaccines applied in the population. The search time limit is from establishing the database to March 8, 2023. The retrieval is carried out by combining theme words and free words. The keywords include COVID-19, novel coronavirus, novel coronavirus Pneumonia, New Coronavirus Variant, Omicron, novel coronavirus Pneumonia Vaccine, novel coronavirus Pneumonia Intensive Needle, effectiveness, vaccine efficacy, negative detection case-control study, etc. Taking PubMed as an example, the specific search strategy is shown in Box 1.
Box 1 PubMed Retrieval Strategy
1.3 Literature Screening and Data Extraction
Two researchers independently screened the literature, extracted data, and cross-checked it. If there are differences, they can be resolved through discussion or consultation with third parties. When selecting literature, first read the title, and after excluding significantly unrelated literature, further read the abstract and full text to determine whether to include it. If necessary, contact the original research author via email or phone to obtain information that still needs to be confirmed but is crucial to this study. The content of data extraction includes ① basic information contained in the study: research topic, first author, publication country, publication time, etc.; ② Baseline characteristics of the study subjects; ③ Specific details of exposure factors; ④ Key elements of bias risk assessment; ⑤ Outcome indicators and outcome measurement data of concern.
1.4 Risk assessment of bias included in the study
Two researchers independently evaluated the bias risk of inclusion in the cohort study using the Newcastle Ottawa Scale and cross-checked the results. Each item is assessed as' yes,' 'no,' or 'unclear'.
1.5 Statistical Analysis
Meta-analysis was conducted using RevMan 5.3 software. The binary variable uses relative risk ratio (RR) as the effect indicator, and their point estimates and 95% CI are given. Heterogeneity adoption among included studies χ 2 tests for analysis (inspection level: α= 0.1) while Combining I2 to determine the magnitude of heterogeneity quantitatively. If there is no statistical heterogeneity among the research results, a fixed effects model is used for meta-analysis. If there is statistical heterogeneity between the outcomes of each study, subgroup analysis and sensitivity analysis are used to explore the sources of heterogeneity further. After excluding the impact of significant clinical heterogeneity, a random effects model is used for meta-analysis. The testing level for meta-analysis is set to bilateral α= 0.05.
2. Results
2.1 Literature screening process and results
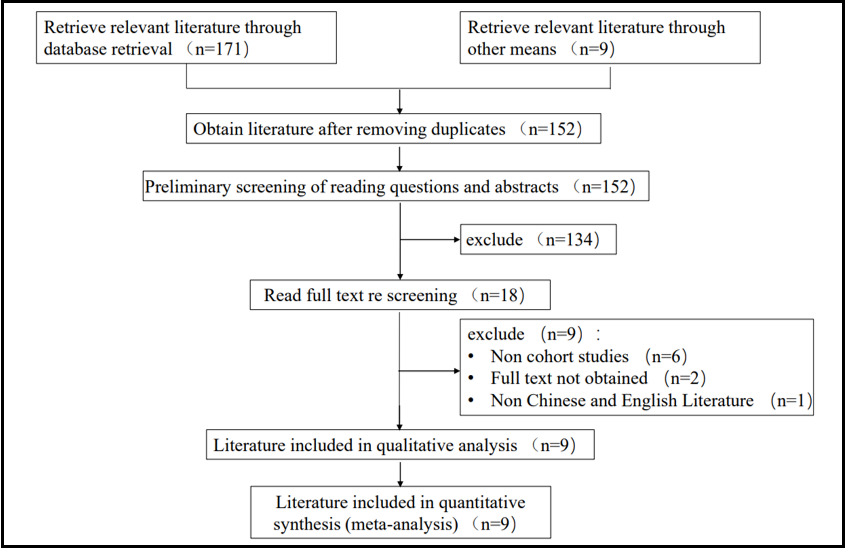
180 relevant literatures were initially identified, and after layer-by-layer screening, they were ultimately included in 9 cohort studies, with a total of 2865389 people. The literature screening process and results are shown in Figure 1.
Figure 1: Literature screening process and results
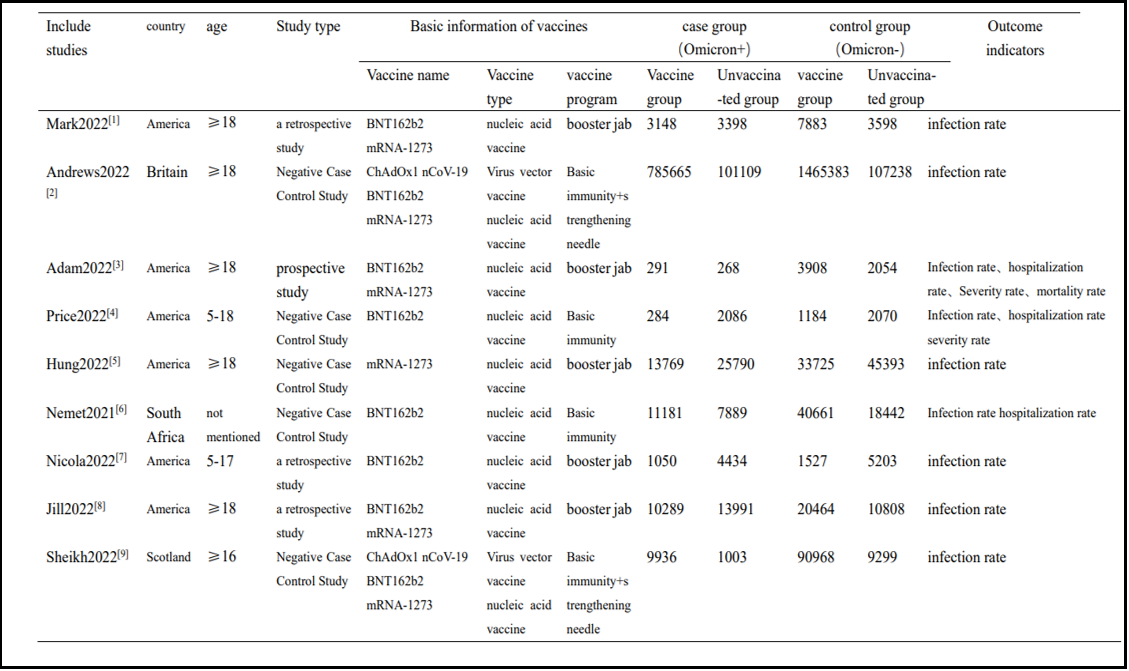
2.2 Basic characteristics and bias risk assessment results included in the study.
The basic characteristics included in the study are shown in Table 1, and the results of bias risk assessment are shown in Table 2.
Table 1: Basic characteristics of inclusion in the study
Table 2: Bias Risk Assessment Results for Inclusion in the Study
|
Include studies |
Selection of research subjects |
Intergroup comparability |
outcome measure |
|||||
|
Representative exposure group |
Selection of non-exposed groups |
Determination of exposure |
Outcome events before the start of the study |
comparability |
Outcome event evaluation |
Is the follow-up sufficient |
Integrity of follow-up |
|
|
Mark2022[1] |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
Andrews2022[2] |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
Adam2022[3] |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
Yes |
Yes |
|
Price2022[4] |
Yes |
Yes |
Yes |
Yes |
unclear |
Yes |
Yes |
Yes |
|
Hung2022[5] |
No |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
Nemet2021[6] |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
Nicola2022[7] |
Yes |
Yes |
Yes |
Yes |
Yes |
unclear |
Yes |
Yes |
|
Jill2022[8] |
Yes |
Yes |
unclear |
Yes |
Yes |
Yes |
Yes |
Yes |
|
Sheikh2022[9] |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
2.3 Meta analysis results
2.3.1 Meta analysis of novel coronavirus vaccine for prevention of infection of Omicron mutant
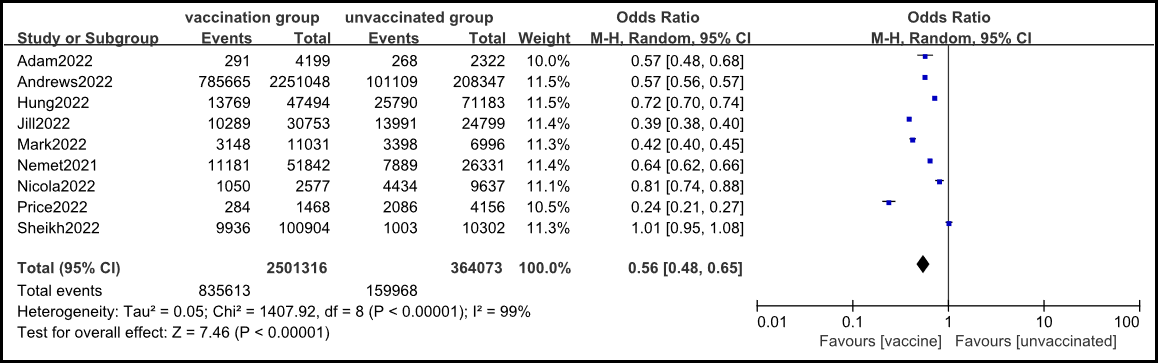
A total of 9 cohort studies were included [1-9], and the random effects model meta-analysis results showed that the infection rate of the Omicron virus strain in the vaccinated group was significantly lower than that in the non-vaccinated group [OR=0.56, 95% CI (0.48, 0.65), P<0.001] (Figure 2).
Figure 2: Meta analysis of the comparison of infection rates of Omicron virus strains between vaccinated and non-vaccinated groups
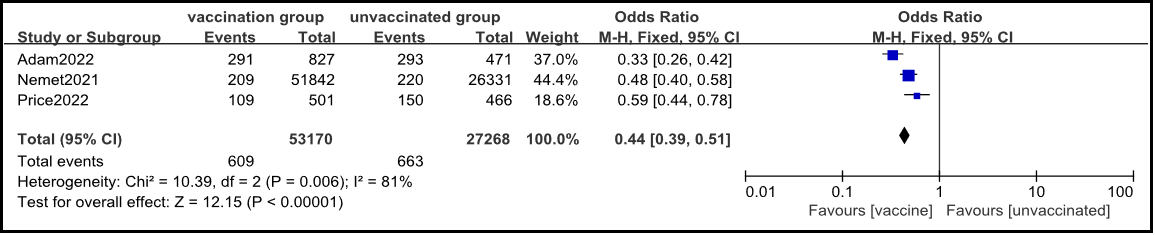
2.3.2 novel coronavirus vaccine prevents hospitalization caused by Omicron.
Three cohort studies were included [3,4,6], and the fixed effects model meta-analysis results showed that the hospitalization rate caused by COVID-19 in the vaccinated group was lower than that in the non-vaccinated group [OR=0.44, 95% CI (0.39, 0.51), P<0.001] (Figure 3).
Figure 3: Meta analysis of comparison of COVID-19 admission rates between vaccinated and non-vaccinated groups
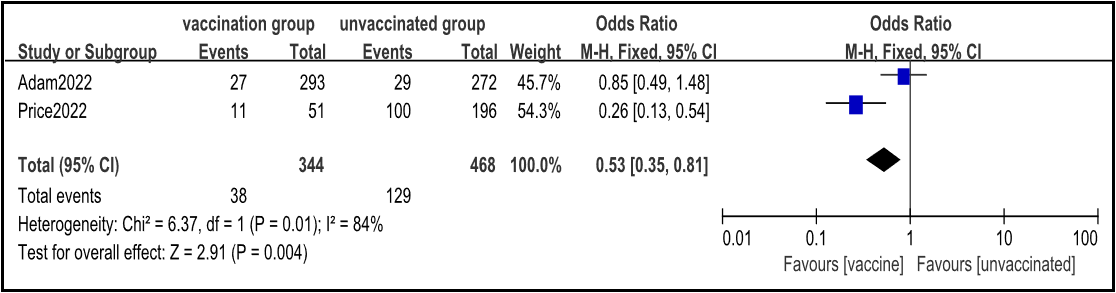
2.3.3 Novel coronavirus Vaccine Prevents Critical Illness Caused by Omicron
Two cohort studies were included [3,4], and the fixed effects model meta-analysis results showed that the rate of progression to critical illness in the vaccinated group was lower than that in the non-vaccinated group [OR=0.53, 95% CI (0.35, 0.81), P<0.001] (Figure 4).
Figure 4: Meta analysis of the rate of progression to critical illness between vaccinated and non-vaccinated groups
2.3.4 Sensitivity analysis
Sensitivity analysis was conducted using the method of removing each study one by one, and the merged results showed no significant changes, indicating that the results were relatively stable.
2.4 Publication bias
Using a funnel plot to test publication bias on outcome indicators, it can be seen that the distribution of each study is symmetrical, indicating a low possibility of publication bias (due to space limitations, the funnel plot can be obtained by contacting the corresponding author).
3. Discussion
Since the first report of a novel coronavirus in November 2019, the virus sequence has undergone multiple mutations. At present, the dominant strains at home and abroad are mainly the Omicron mutant strains, while the new coronavirus vaccines in use are specifically designed based on the wild-type virus sequence [19]. This study showed that the infection rate [OR=0.56, 95% CI (0.48, 0.65), P<0.001] of the group vaccinated with the novel coronavirus vaccine was significantly lower than that of the group not vaccinated. This may be because the design targets of existing vaccines mainly focus on relatively conserved spike protein domains, which requires more extensive epidemiological investigations to verify [20].
The pathogenesis of novel coronavirus is still unclear. Chen et al. [21] showed that the vaccine designed based on the SARA-CoV-2 RBD sequence can produce specific antibodies in mice, alleviate eosinophil infiltration, and prevent hospitalization and severe cases caused by inflammatory storms. This is consistent with the significantly lower hospitalization rate (OR=0.44, 95% CI (0.39, 0.51), P<0.001) and severe illness rate (OR=0.53, 95% CI (0.35, 0.81), P<0.001) in the vaccination group shown in this study compared to the non-vaccination group. This may be related to the mixed Th1/Th2 responses of helper T cell 1 and helper T cell 2 [22].
Pollet et al. [23] found in their study of humoral and cellular immunity induced by COVID-19 vaccine that the total IgG and neutralizing antibody content in the experimental group were higher than those in the control group after 35, 43, and 57 days of vaccination. Flow cytometry results showed that CD4 (+) T cells were highly expressed in IL-4 and IFN- γ、Downregulation of IL-2 suggests an increase in the number of functional T cell follicular helper cells, which promote the production of follicular plasma cells and long-term memory B cells. These studies support the conclusion that the infection rate [OR=0.56, 95% CI (0.48, 0.65), P<0.001], hospitalization rate [OR=0.44, 95% CI (0.39, 0.51), P<0.001], and severity rate [OR=0.53, 95% CI (0.35, 0.81), P<0.001] of the vaccination group are significantly lower than those of the non-vaccination group from the perspective of immune protection.
Limitations of the study: 1. Due to the lack of basic research on vaccine efficacy in China, all 9 articles included were from non-Asian countries. Due to different vaccination policies in other countries, the age distribution of the population included in this study is ≥ 5 years old. Children under 5 years old and infants and young children do not apply to the conclusions of this study. 3. COVID-19 vaccines abroad are primarily nucleic acid vaccines or viral vector vaccines, such as BNT162b2 (Pfizer Biotechnology, USA) and mRNA-1273 (Modner, USA), which are commonly used in the United States and ChAdOx1 nCoV-19 (AstraZeneca, UK), which are commonly used in the United Kingdom; In China, inactivated vaccines are mainly used, such as Sinovac V (Kexing, China).
In summary, this study is the first to explore the effectiveness of existing vaccines against SARS-CoV-2 Omicron and its variants in terms of infection rate, hospitalization rate, and risk/severity rate after COVID-19 vaccination, providing an essential reference for the formulation of epidemic prevention policies, especially for vaccine promotion.
References
- Thompson MG, Natarajan K, Irving SA, Rowley EA, Griggs EP, Et Al. (2022) Effectiveness Of A Third Dose Of Mrna Vaccines Against COVID-19-Associated Emergency Department And Urgent Care Encounters And Hospitalizations Among Adults During Periods Of Delta And Omicron Variant Predominance - VISION Network, 10 States, August 2021-January 2022. 71(4): 139-145.
- Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Et Al. (2022) Covid-19 Vaccine Effectiveness Against The Omicron (B.1.1.529) Variant[J]. The New England Journal Of Medicine. 386(16): 1532- 1546.
- Lauring AS, Tenforde MW, Chappell JD, Gaglani M, Ginde AA, Et Al. (2022) Clinical Severity Of, And Effectiveness Of Mrna Vaccines Against, Covid-19 From Omicron, Delta, And Alpha SARS-Cov-2 Variants In The United States: Prospective Observational Study. BMJ. 376: E069761.
- Price AM, Olson SM, Patel MM (2022) BNT162b2 Protection Against The Omicron Variant In Children And Adolescents Reply[J]. The New England Journal Of Medicine. 386(24): 2346.
- Tseng HF, Ackerson BK, Luo Y, Sy LS, Talarico CA, Et Al. (2022) Effectiveness Of Mrna-1273 Against SARS-Cov-2 Omicron And Delta Variants. Nature Medicine. 28(5): 1063-1071.
- Gray G, Collie S, Goga A, Garrett N, Champion J, Et Al. (2022) Effectiveness Of Ad26.COV2.S And BNT162b2 Vaccines Against Omicron Variant In South Africa[J]. The New England Journal Of Medicine. 386(23): 2243-2245.
- Klein NP, Stockwell MS, Demarco M, Gaglani M, Kharbanda AB, Et Al. (2022) Effectiveness Of COVID-19 Pfizer-Biontech BNT162b2 Mrna Vaccination In Preventing COVID-19- Associated Emergency Department And Urgent Care Encounters And Hospitalizations Among Nonimmunocompromised Children And Adolescents Aged 5-17 Years - VISION Network, 10 States, April 2021-January 2022. 71(9): 352-358.
- Ferdinands JM, Rao S, Dixon BE, Mitchell PK, Desilva MB, Et Al. (2022) Waning 2-Dose And 3-Dose Effectiveness Of Mrna Vaccines Against COVID-19-Associated Emergency Department And Urgent Care Encounters And Hospitalizations Among Adults During Periods Of Delta And Omicron Variant Predominance - VISION Network, 10 States, August 2021-January 2022. 71(7): 255-263.
- Sheikh A, Kerr S, Woolhouse M, Mcmenamin J, Robertson C (2022) Severity Of Omicron Variant Of Concern And Effectiveness Of Vaccine Boosters Against Symptomatic Disease In Scotland (EAVE II): A National Cohort Study With Nested Test-Negative Design. The Lancet Infectious Diseases. 22(7): 959-966.
- Laake I, Skodvin SN, Blix K, Caspersen IH, Gjessing HK, Et Al. (2022) Effectiveness Of Mrna Booster Vaccination Against Mild, Moderate, And Severe COVID-19 Caused By The Omicron Variant In A Large, Population-Based, Norwegian Cohort. The Journal Of Infectious Diseases. 226(11): 1924-1933.
- Fan YJ, Chan KH, Hung IF (2021) Safety And Efficacy Of COVID- 19 Vaccines: A Systematic Review And Meta-Analysis Of Different Vaccines At Phase 3. Vaccines (Basel). 9(9): 989.
- Chemaitelly H, Tang P, Hasan MR, Almukdad S, Yassine HM, Et Al. (2021) Waning Of BNT162b2 Vaccine Protection Against SARS-Cov-2 Infection In Qatar. New England Journal Of Medicine. 385(24): E83.
- Barda N, Dagan N, Cohen C, Hernán MA, Lipsitch M, Et Al. (2021) Effectiveness Of A Third Dose Of The BNT162b2 Mrna COVID-19 Vaccine For Preventing Severe Outcomes In Israel: An Observational Study. The Lancet. 398(10316): 2093-2100.
- Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Et Al. (2022) Final Analysis Of Efficacy And Safety Of Single-Dose Ad26.COV2.S. New England Journal Of Medicine. 386(9): 847- 860.
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Et Al. (2021) Safety And Efficacy Of The Chadox1 Ncov-19 Vaccine (AZD1222) Against SARS-Cov-2: An Interim Analysis Of Four Randomised Controlled Trials In Brazil, South Africa, And The UK. Lancet. 397(10269): 99-111.
- Liu Chang, Chen Ying'an, Zhao Shuangyu, Et Al. Meta Analysis Of The Safety Of Novel Coronavirus Pneumonia Vaccine [J]. Chinese Journal Of Evidence Based Medicine, 2021. 21(06): 676-682.
- Li Chunhe, Liu Nan, Wang Chaoyu, Et Al. Systematic Evaluation Of The Efficacy And Safety Of Novel Coronavirus Pneumonia Vaccine [J]. Chinese Journal Of Evidence Based Medicine, 2022,22 (09): 1027-1032
- Chen Tao, Chen Guang, Guo Wei, Et Al. Quick Guide To Diagnosis And Treatment Of Pneumonia Infected By Novel Coronavirus (Third Edition) [J]. Medical Herald, 2020, 39(03): 305-307.
- Zinatizadeh MR, Zarandi PK, Zinatizadeh M, Yousefi MH, Amani J, Et Al. (2022) Efficacy Of Mrna, Adenoviral Vector, And Perfusion Protein COVID-19 Vaccines. Biomedicine & Pharmacotherapy. 146: 112527.
- Oladipo EK, Ajayi AF, Ariyo OE, Onile SO, Jimah EM, Et Al. (2020) Exploration Of Surface Glycoprotein To Design Multi- Epitope Vaccine For The Prevention Of Covid-19. Informatics In Medicine Unlocked. 21: 100438.
- Chen WH, Tao X, Agrawal AS, Algaissi A, Peng BH, Et Al. (2020) Yeast Expressed SARS-Cov Recombinant Receptor-Binding Domain (RBD219-N1) Formulated With Alum Induces Protective Immunity And Reduces Immune Enhancement. Vaccine. 38(47): 7533-7541.
- Hotez PJ, Bottazzi ME, Corry DB (2020) The Potential Role Of Th17 Immune Responses In Coronavirus Immunopathology And Vaccine-Induced Immune Enhancement. Microbes Infect. 22(4-5): 165–167.
- Pollet J, Chen WH, Versteeg L, Keegan B, Zhan B, Et Al. (2020) SARS-Cov-2 RBD219-N1C1: A Yeast-Expressed SARS-Cov-2 Recombinant Receptor-Binding Domain Candidate Vaccine Stimulates Virus Neutralizing Antibodies And T-Cell Immunity In Mice. Hum Vaccin Immunother. 17(8): 2356-2366.