Mostafa Badr1*, Tobias Baumgartner1, Davide Cucchi2, Rainer Surges1, Randi von Wrede1
1Department of Epileptology, University Hospital Bonn, Germany
2Department of orthopedic surgery, University Hospital Bonn, Germany
*Corresponding Author: Mostafa Badr, Department of Epileptology, University Hospital Bonn, Germany
Abstract
Objective: Vertebral compression fractures occur in a significant proportion of people with epilepsy in the context of tonic-clonic seizures due to the severe and uncontrolled motor activity. In contrast, the association between vertebral compression fractures and syncope without fall has not been reported as yet.
Case Report: A 56-year-old male patient with multiple vertebral compression fractures following an observed episode with transient loss of consciousness without fall is reported. The detailed description of the episode suggested causal vasovagal syncope, supported by the absence of abnormalities in brain imaging and prolonged electroencephalography recordings.
Discussion and Conclusion: This is a very unusual case of vertebral fractures most likely due to generalized body stiffening occurring during the hypoxic phase of a vasovagal syncope. A detailed history taking was key to the proper diagnosis, with important implications on psychological, socioeconomic and legal aspects of the patient's life.
Keywords: syncope; case report; convulsions; vertebral compression fractures.
1. Introduction
Background
Transient loss of consciousness (TLOC) is known to be a common cause of emergency department (ED) admissions [1]. The most common causes of non-traumatic transient loss of consciousness are syncope, seizures, and psychogenic pseudosyncope or non-epileptic seizures [2]. The differentiation between epileptic seizures and syncope is crucial due to different therapeutic and psychosocial implications [3]. Unfortunately, the correct diagnosis is hindered for several reasons: I: Epileptic seizures and syncope have similar semiological elements. II: The patient himself often has only an incomplete perception of the loss of consciousness. III: Observers' descriptions are also often unreliable [4,5]. ECG, EEG, CT /MRI, blood tests, and tilt tests can help to identify the underlying cause of the TLOC. Nevertheless, sometimes the cause of TLOC remains unclear. Syncope is defined as a TLOC due to global cerebral hypoperfusion with relatively rapid onset and rapid and spontaneous recovery [6]. The International League against Epilepsy (ILAE) defines an epileptic seizure as a transient onset of signs and/or symptoms due to abnormal excessive or synchronous neuronal activity in the brain. Vertebral compression fractures can occur as a result of violent muscle contractions during tonic-clonic seizures [7,8], but, to our knowledge, have not been reported in association with convulsive syncope until now. In this case report, we present a patient with compression fractures in the setting of convulsive syncope.
2. Case presentation
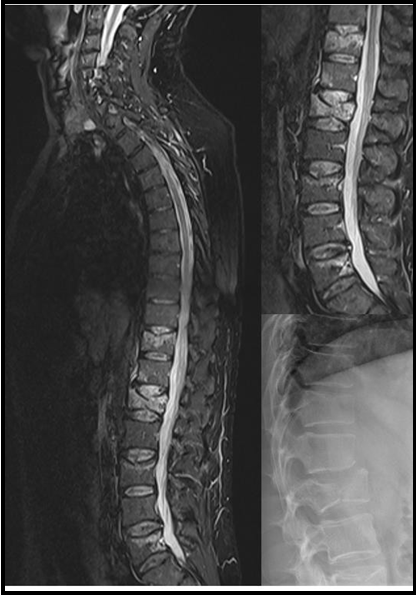
A 56-year-old male police officer with BMI of 26.6 kg/ m2 and a past medical history of hypertension, hyperlipidaemia and coronary artery disease was referred to our department for a second opinion after suffering an episode of TLOC with convulsions. The patient himself reported that he suddenly felt a strange sensation while driving, with severe nausea and marked epigastric pressure, that did not rise, and non-specific abdominal discomfort. He became sweaty, pale and his hearing was dull. The patient lost consciousness. His wife described that the patient's head was tilted back into the headrest, the whole body was stiff. The eyes were open, then he developed arrhythmic and irregular myoclonic jerks of the limbs with varying involvement, and enuresis. The reported duration of the whole episode from the loss of consciousness to the recovery ranged between 30-60 seconds. Afterwards, he was immediately reoriented to all qualities, and complained shortly after recovery about strong back pain. After the patient presented to the emergency department, X-ray and whole- body CT scans were performed, followed by MRI imaging of the spine on the following day. These investigate/ons showed multiple lower thoracic and lumbar fractures compromising the stability of the spine (AO A1 fracture of the 11th thoracic vertebra, AO A2 fracture of the 1st lumbar vertebra, AO A1 fracture of the 3rd lumbar vertebra, AO A1 fracture of the 5th lumbar vertebra). In addition, a CT brain with angiography, ECG and cardiac enzymes were performed, which showed no relevant findings.
An orthopaedic consultation occurred and, considering to the highly unstable fracture of the 1st lumbar vertebra, a surgical intervention was indicated and performed on the 3rd day after the episode (minimally invasive posterior instrumentation from the 10th thoracic vertebra to the 1st sacral vertebra). Detailed history taking revealed that he has suffered in early adulthood from episodes with prodromal symptoms which typically occur in the context of vasovagal syncope, such as severe nausea, dizziness, abdominal discomfort, sweating, paleness, diminution of hearing and blurred vision. Further, during the lumbar puncture, he had transient symptoms (namely nausea, abdominal discomfort, sweating, paleness, diminution of hearing and blurred vision) that typically occur in the prodromal phase of vasovagal syncope. Neither other seizure-like events nor deterioration of memory, concentration, attention, or mood were reported. There were no risk factors for epilepsy and the family history for epilepsy was negative. All external examinations including routine EEG, EEG after sleep deprivation, cranial MRI, and lumbar puncture were unremarkable. The treating neurologist considered this episode of TLOC to be a first epileptic seizure. Anticonvulsant therapy was even recommended because of the fractures. The following investigations have been carried out in our institution. The physical examination of the patient did not reveal any abnormalities except for a limited mobility of the spine related to the previous aforementioned surgery. The long-term-EEG showed normal findings. The tilt test showed a habitual syncope with predominant hypotension and moderate bradycardia (35 bpm) on ECG. The EEG during the tilt test initially showed a physiological background activity, followed by typical signs of hypoxia (generalized rhythmic delta waves, then transition to generalized EEG flattening, then rhythmic delta activity again, then physiological activity).
In summary, a habitual episode was documented. Electroclinical findings were consistent with a causal neurocardiogenic vasovagal, mixed vasodepressive and cardioinhibitory syncope. A review of the medical history revealed no evidence of unclear or spontaneous fractures. Dual-energy X-ray absorptiometry revealed normal bone density values for the age of the patient. The calcium levels and the results of the electrophoresis were within the normal range, ruling out the possibility of a spontaneous osteoporotic vertebral fracture. A telephone follow-up at 15 months after the episode of TLOC showed no further events or spontaneous osteoporotic vertebral fractures, thereby underscoring the necessity for monitoring the skeletal aspect (further lesions).
Figure 1: X-ray and MRI scans demonstrating the vertebral fractures
Discussion
This unusual case report illustrates the difficulty, but also the importance, of taking a comprehensive history taking in patients with TLOC. Vertebral compression fractures were reported to occur particularly during tonic and tonic-clonic seizures due to forceful uncontrolled muscle contractions [7-9]. The occurrence of vertebral fractures with syncope in the absence of a fall, however, is uncommon and likely to prompt the false diagnosis of epileptic seizures. Syncope is frequently accompanied by motor phenomena, including initial loss of muscle tone and irregular myoclonic jerks [10]. However, it is less well known that partial or generalized body stiffening occurs in up to two thirds of syncope [10]. Our patient suffered from a TLOC with tonic muscle activity (generalized body stiffening) with secondary vertebral body fractures. Although the patient had a habitual episode during the tilt-test, no myoclonic jerks were noted. One explanation may be that rapid correction of the head position and elevation of the legs probably resulted in a rapid recovery of cerebral blood flow. The occurrence of tonic spasms and myoclonic jerks associated with syncope is explained as follows: the resulting cerebral hypoperfusion triggers the medullary reticular formation [11], which may lead to myotonic activity. Some inputs to the medullary reticular formation are responsible for controlling or regulating posture, tone, balance, and various signals to the cerebellum [12]. In summary, based on the semiological criteria as well as the results of the additional examinations, it can be assumed that a neurocardiogenic vasovagal syncope occurred, followed by a tonic contraction of the paravertebral muscles leading to the vertebral compression fractures. Moreover, no clinical or anamnestic evidence of pathological fractures was identified in medical history or at the follow-up. However, further monitoring of the skeletal aspect is recommended.
In the patient's medical history, there was evidence for presyncopes and once syncope, but no evidence of epileptic seizures or epilepsy was found in subsequent diagnostics. Thus, the initial consideration of antiseizure medications was due to a misdiagnosis, and such treatment was unnecessary. The proper diagnosis has important socioeconomic consequences and psychosocial implications, as legal regulations for syncope and seizures differ. The patient is now able to work as a police officer again and will not receive anti-seizure medications which could even worsen bradycardias and cardiac arrhythmia in some cases [13]. Such a misdiagnosis can have a negative impact on employment status. The economic consequences can include underemployment, driving restrictions and loss of autonomy, and even insurance problems. The risk of seizure-related injury is also high [14]. This report describes a case of TLOC-related vertebral fractures not related to epilepsy/seizures, highlighting the relevance of timely referral to a specialized centre/second-level tests to rule in/rule out epilepsy in order to prescribe adequate medication/avoid unnecessary treatment. A comprehensive history is an essential element in the accurate diagnosis of these cases, which can subsequently influence the course of treatment and have implications for the patient's overall well-being and quality of life.
Ethical Statement: Informed consent was obtained from the patient.
Declaration of Interests: none.
References
- Ungar A, Tesi F, Chisciotti VM, Pepe G, Vanni S, et al. (2016) Assessment of a structured management pathway for patients referred to the Emergency Department for syncope: results in a tertiary hospital. Europace. 18(3): 457–62.
- Petkar S, Cooper P, Fitzpatrick AP (2006) How to avoid a misdiagnosis in patients presenting with transient loss of consciousness. Postgrad Med J. 82(972): 630–41.
- Sheldon R (2015) How to Differentiate Syncope from Seizure. Cardiology Clinics. 33(3): 377–85.
- Baumgartner T, Surges R (2019) How to Distinguish Syncope from Epileptic and Psychogenic Non-Epileptic Seizures. Dtsch Med Wochenschr. 144(12): 835–41.
- Duplyakov D, Golovina G, Garkina S, Lyukshina N (2010) Is it possible to accurately differentiate neurocardiogenic syncope from epilepsy? Cardiol J. 17(4): 420–7.
- Brignole M, Moya A, de Lange FJ, Deharo JC, Elliott PM, et al. (2018) 2018 ESC Guidelines for the diagnosis and management of syncope. European Heart Journal. 39(21): 1883–948.
- Robles LA, Guerrero-Maldonado A (2022) Seizure-Induced Spinal Fractures: A Systematic Review. Int J Spine Surg. 16(3): 521–9.
- Frey K, Zöllner JP, Knake S, Oganian Y, Kay L, et al. (2020) Risk incidence of fractures and injuries: a multicenter video-EEG study of 626 generalized convulsive seizures. J Neurol. 267(12): 3632– 42.
- Cucchi D, Baumgartner T, Walter SG, Menon A, Ossendorff R, et al. (2022) Epidemiology and specific features of shoulder injuries in patients affected by epileptic seizures. Arch Orthop Trauma Surg. 143(4): 1999-2009.
- Shmuely S, Bauer PR, van Zwet EW, van Dijk JG, Thijs RD (2018) Differentiating motor phenomena in tilt-induced syncope and convulsive seizures. Neurology. 90(15): e1339–46.
- Patel DP, Cohen TJ (2022) Director of Electrophysiology D of the PAC. Cardiac Syncope Versus Seizure: The Value of the EP Consult. EP Lab Digest. 13(2).
- Task Force for the Diagnosis and Management of Syncope, European Society of Cardiology (ESC), European Heart Rhythm Association (EHRA), Heart Failure Association (HFA), Heart Rhythm Society (HRS), Moya A, et al. (2009) Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 30(21): 2631–71.
- Yadav R, Schrem E, Yadav V, Jayarangaiah A, Das S, et al. (2021) Lacosamide-Related Arrhythmias: A Systematic Analysis and Review of the Literature. Cureus. 13(12): e20736.
- Strine TW, Kobau R, Chapman DP, Thurman DJ, Price P, et al. (2005) Psychological distress, comorbidities, and health behaviors among U.S. adults with seizures: results from the 2002 National Health Interview Survey. Epilepsia. 46(7): 1133–9.