Ana Redondo Villatoro1*, Álvaro Gutiérrez Domingo2, Inmaculada Rodríguez Jiménez1, María Nieves Cabezas Palacios1
1Obstetrics and Gynecology Clinical Management Unit of the Virgen Macarena University Hos-pital, Seville, Spain
2Pathological Anatomy Clinical Management Unit of the Virgen Macarena University Hospital, Seville, Spain
*Corresponding Author: Ana Redondo Villatoro, Obstetrics and Gynecology Clinical Management Unit of the Virgen Macarena University Hospital, Seville, Spain
Abstract
The objective of this article is to report a case of a synchronous tumor of the ovary, endometrium, and sigmoid in a 51-year-old woman that has taken place in our care practice. In addition, a systematic review of the existing literature on synchronous tumors has been performed. In conclusion, a correct diagnosis of synchronous versus metastatic tumors is essential, given that their prognosis differs.
Keywords: synchronous; endometrial cancer; ovarian cancer; sigma cancer
Introduction
Synchronous malignancies of 3 or more tumors are uncommon, and synchronous tumors of the gynecological tract are abnormal, representing 50 %–70 % of synchronous cancer of the ovaries and endometrium. [1] However, synchronous tumors affect fewer than 2 % of patients diagnosed with endometrial or ovarian cancer. [1]
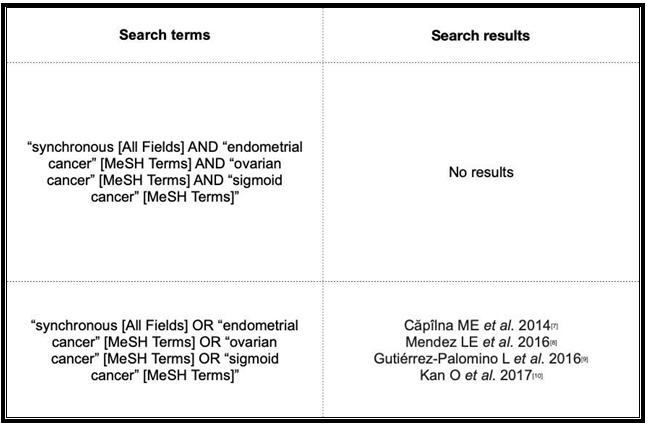
We conducted a systematic literature search in the PubMed and Cochrane databases using the terms “synchronous,” “ovarian cancer,” “endometrial cancer,” and “sigmoid cancer” but found no case similar to ours. As far as we know, ours is the first case of synchronous ovarian, endometrial, and endometrioid adenocarcinoma tumor on endometriosis in the sigmoid wall described in the international medical literature. We, therefore, consider this case of interest, given the difficulties that its rarity can present in this type of case. We reported the diagnostic-therapeutic management performed in our unit and conducted a detailed literature review.
Case Description
A 51-year-old woman with no family or medical history of interest underwent a transvaginal ultrasound as part of a routine gynecological check-up. The ultra-sound revealed a left ovarian cyst measuring 90x80 mm with solid vascularised papillae measuring 60 mm in its interior. The cancer antigen CA 19-9 reading was 277 U/mL (2.0– 37.0), and the CA 125 reading was 45 U/mL (0–35.0). Based on suspicion of an adnexal tumor with a high risk of malignancy, the patient was scheduled for an exploratory laparotomy, during which she underwent total hysterectomy with double adnexectomy, pelvic and paraaortic lymphadenectomy, omentectomy, appendectomy, and biopsy of the sigmoid wall. At the microscopic level, we observed a poorly differentiated mixed cystadenoma- cinema in the left ovary, with mucinous and endometrioid areas and solid areas of undifferentiated cells with large areas of necrosis. This tumor caused capsular disruption (stage IC of the Federation of Gynecology and Obstetrics [FIGO] staging system). The results of the immunohistochemical study were as follows: cytokeratin 7 positive (CK7+), epithelial membrane antigen-positive (EMA+), carcinoembryonic antigen-positive (CEA+), estrogen receptor-positive (ER+), and progesterone receptor-positive (PR+). We observed no neuroendo- crime differentiation of the undifferentiated component (chromogranin and synaptophysin negative). In the hysterectomy specimen, we observed a well-differentiated endometrioid adenocarcinoma (G1) with squamous differentiation, FIGO grade IB, with infiltration of the lower third of the myometrium but without affecting the parametria. The momentum and caecal appendix were free of neoplasia. The submitted lymph nodes were free of metastasis, and the peritoneal lavage was negative for neoplastic cells. In the sigmoid wall, we observed an endometrioid adenocarcinoma associated with foci of endometriosis.
Based on these findings, we concluded that this case involved 3 independent neoplasms because the ovarian and endometrial tumors showed different histological results. In the endometrial tumor, we observed no vascular invasion or any other evidence of dissemination.
There were also foci of atypical hyperplasia adjacent to cancer. The ovarian tumor was unilateral and located in the ovarian parenchyma, with no imaging evidence of vascular invasion, superficial implants, or predominantly hilar location. There was no other evidence of dissemination. In the sigmoid wall, foci of en- endometriosis implied a developed adenocarcinoma on this endometriosis and ruled out the possibility of metastasis.
The patient subsequently underwent 6 cycles of adjuvant chemotherapy with carboplatin and taxol every 3 weeks. During the first year of follow-up by the medical oncology department, serum CA 125 and CA 19-9 measurements were requested with a periodicity of 3 months. After completing 1 year of follow-up with normal levels, the periodicity was changed to every 6 months. After 8 years of follow-up by the oncology department, the patient was asymptomatic, with no findings in the imaging tests and negative tumor markers. Therefore, the patient was discharged with the recommendation to undergo annual CA 125 and CA 19-9 readings by her primary care physician.
Discussion
Synchronous primary tumors are simultaneously or successively present in the 6 months following the diagnosis of the initial neoplasm. The tumours constitute a well-established clinical entity based on the criteria described by Warren and Gates in 1932. The incidence rate for these tumours ranges from 3.6 % to 11.9 %, according to the series reviewed. The criteria to be met are as follows:
1) Each tumour should have a defined malignancy pattern; 2) the possibility that the tumour constitutes a metastasis of another tumour needs to be ruled out; 3) each tumour should present different histology, and if they are similar within the same organ, they should have no demonstrated connection between them; 4) each tumour should follow its natural history and can present an independent progression; 5) each tumour can present with their symptoms or be a finding in the course of the study required for the diagnosis, staging or follow-up of the first tumour or found only during the post-mortem, during the autopsy; and 6) the diagnosis of the tumors can be successive or simultaneous. [1]
Histologically and according to the histopathological criteria of the US Armed Forces Institute of Pathology, the neoplasms are independent (Figure 1). Suppose we were talking about the metastasis of an ovarian tumour to the endometrium. In that case, we should have similar histology, a direct extension of the tumour to the external wall of the uterus and parametrial involvement, vascular invasion, absence of atypical hyperplasia in the endometrium, dissemination in another part with the typical pattern of ovarian carcinoma, etc. Suppose we were talking about the metastasis of an endometrial tumour to the ovaries. In that case, we should have similar histology, direct invasion of the tumour to the adnexal, and vascular invasion in the myometrium, bilateral and multinodular ovarian tumors, hilar ovarian location, with vascular invasion, superficial implants, etc. All of this is consistent with synchronous and nonmetastatic neoplasms. The foci of endometriosis in the third tumour (sigmoid wall) indicated that it had developed on this endometriosis, ruling out the possibility of metastasis. [2]
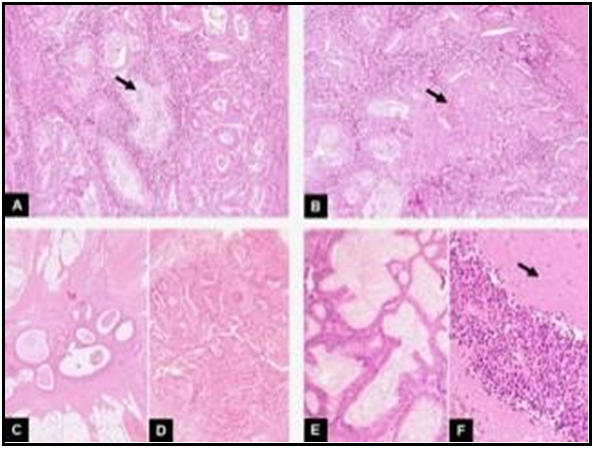
Figure 1. Synchronous tumor of ovary, endometrium and sigmoid colon. A) In this first image the endometrial tumour is seen. It is a well-differentiated endometrioid ade-nocarcinoma: a gland-forming tumor is seen (arrow) (HE,10x). B) Focal squamous dif-ferentiation was observed in other areas of the same tumour. We see solid nests of squamous cells interspersed with tumour glands (arrow) (HE,10x). C) In this third image, we see a focus of endometriosis within the sigmoid wall. They are benign glands with an accompanying endometrial like-stroma (HE,4x). D) Associated with this endo-metriosis, foci of endometrioid carcinoma were observed (HE,4x). E) Finally, at the le- vel of the left ovary, a mixed cystadenocarcinoma with endometrioid areas and muci- nous areas was observed. In this image, we can see the mutinous differentiation (HE,10x). F) In addition, there was a third component in this ovarian tumour. As we seein this image, it consisted of solid areas of undifferentiated cells with large areas of ne-crosis (arrow) (HE,10x).
For its part, tumor synchrony is a marker of hereditary disease in Lynch syndrome II and hereditary colorectal cancer not associated with polyposis. This autosomal dominant inheritance syndrome, triggered by various germline mutations, is responsible for 1 %–5 % of cases of colorectal cancer. [3] In addition to the 70 %–90 % chance of preventing colorectal cancer, these patients have a high risk of cancer in various extra colonic locations, including the endometrium, ovaries, breasts, stomach, small intestine, pancreas, and kidneys. [3] Given the absence of relevant family history in our case, there was no suspected relationship with this syndrome; we, therefore, did not conduct any genetic study for this.
In terms of the survival results, it has been reported that patients with synchronous malignancies have a better prognosis than patients with metastatic disease in the same organs. Furthermore, the higher survival rates for patients with synchronous endometrial and ovarian tumours versus patients with primary malignant ovarian tumours with dissemination to the endometrium are due to the de- section of ovarian cancer in an earlier stage. [4]
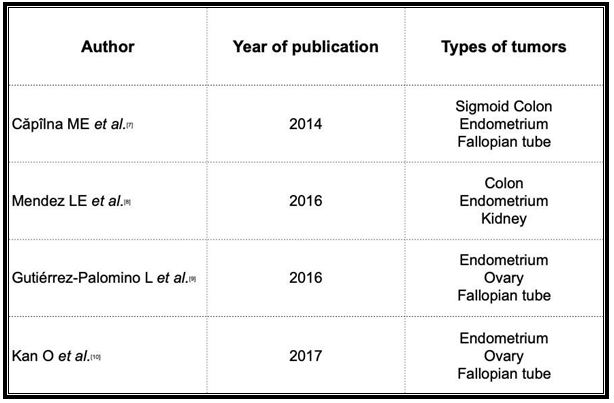
Regarding the planning of post-treatment follow-up, no specific strategy has demonstrated better results for synchronous tumours involving the genital tract. [7] However, the consensus regimens of the United States National Comprehensive Cancer Network for synchronous ovarian and endometrial cancer propose follow-up visits every 3 months up to 5 years after the surgery. Despite the lack of a clear impact of serial CA 125 and CA 19.9 measurements on overall survival, the recommendation is to measure these tumour markers every 3 months. [5,6] In our case, a close follow-up was conducted every 3 months for the first year. The patient’s good progression, the lack of pathological findings in the imaging tests, and the normality of the laboratory test results allowed us to set the periodicity of the visits to every 6 months after completing the first year after surgery. After conducting a comprehensive literature search in the PubMed and Cochrane databases (search date 2021-03-07) (Table 1), we found only 4 articles on synchronous tumours (Table 2), none of which described the involvement of the endometrium, ovaries, and sigmoid, thereby revealing the consider- able uniqueness of our case. We, therefore, consider its contribution to the scientific literature as highly interesting.
Table 1: Terms used in the bibliographic search and results obtained.
Table 2: Publications of synchronous tumours, obtained with the search criteria described above.
Conclusions
A correct diagnosis of synchronous tumours versus metastatic tumours is essential, given that their handling and prognosis differ. To this end, despite their rarity, it is vital to determine the presence of synchronous tumours at the gynaecological level. The participation of the pathology department is, of course, essential.
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose.
The authors have no conflicts of interest to declare that they are relevant to the content of this article.
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial or non-financial interest in the sub-subject matter or materials discussed in this manuscript.
The authors have no financial or proprietary interests in any material discussed in this article.
References
- Matsuo K, Machida H, Blake EA, Holman LL, Rimel BJ, et al. (2018) Trends and outcomes of women with synchronous endometrial and ovarian cancer. Oncotarget. 9(47): 28757-28771.
- Scully RE, Young RH, Philip B (1999) Tumors of the ovary, maldeveloped gonads, Fallo- pian tube and broad ligament: Atlas of tumor pahology. 51(5): 671.
- Mas-Moya J, Dudley B, Brand RE, Thull D, Bahary N, et al. (2015) Clinico- pathological comparison of colorectal and endometrial carcinomas in patients with Lynch-like syndrome versus patients with Lynch syndrome. Hum Pathol. 46(11): 1616- 1625.
- Wang Y, Yu M, Yang JX, Cao DY, Zhang Y, et al. (2018) [Clinicopathologic and sur- vival analysis of synchronous primary endometrial and ovarian cancer]. Zhonghua Fu Chan Ke Za Zhi. 53(12): 816-822.
- Morgan RJ Jr, Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Behbakht K, et al. (2016) Ovarian Cancer, Version 1.2016, NCCN Clinical Practice Guidelines in On- cology. J Natl Compr Canc Netw. 14(9): 1134-63.
- Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, et al. (2021) Ovarian Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in On- cology. J Natl Compr Canc Netw. 19(2): 191-226.
- Căpîlna ME, Rusu SC, Szabo B, Marian C (2014) Three synchronous primary pelvic can- cers--a case report. Rev Med Chir Soc Med Nat Iasi. 118(1): 107-10.
- Mendez LE, Atlass J (2016) Triple synchronous primary malignancies of the colon, endo- metrium and kidney in a patient with Lynch syndrome treated via minimally invasive techniques. Gynecol Oncol Rep. 17: 29-32.
- Gutiérrez-Palomino L, Romo-de Los Reyes JM, Pareja-Megía MJ, García-Mejido JA (2016) Tumores triple sincrónicos ginecológicos. Reporte de un caso [Triple synchronous primary gynaecological tumours. A case report]. Cir Cir. 84(1): 69-72.
- Kan O, Alkilic A, Turgay B, Gemici A, Atabekoglu CS (2017) Triple Synchronous Malignan- cies in Genital Tract; Primary Endometrial, Ovarian and Fallopian Tube Carcinoma: A Case Report. J Clin Diagn Res. 11(1): QD01-QD02.