Iman AlDiri1*, Ibrahim Eskarose2, David Webb3, Ehab Atta2
1Pain and palliative care consultant, Hussain Makki Juma Center for Specialized Surgeries, Kuwait Cancer Control Center, Kuwait.
2Anaesthesiologist, Hussain Makki Juma Center for Specialized Surgeries, Kuwait Cancer Control Center, Kuwait.
3Houghton House Addiction & Mental Health Treatment Centers, 432 York Ave, Randburg, South Africa
*Corresponding Author: Iman AlDiri, Hussain Makki Juma Center for Specialized Surgeries, Kuwait Cancer Control Center, Kuwait
Abstract
Context: Constipation is a common side effect of opioid therapy that impairs quality of life, reduces adherence to therapy, and interferes with effective pain control. A prolonged-release (PR) fixed combination of oxycodone and the opioid antagonist naloxone (OXN PR) has been shown to effectively control pain while reducing the incidence of opioid-induced constipation.
Objectives: The purpose of this retrospective observational, real-world study was to evaluate the analgesic efficacy of OXN PR and incidence of constipation in outpatients with moderate-severe cancer-related pain who were switched from other opioids as part of their routine care.
Methods: Data were collected from a chart review of 270 patients with a confirmed diagnosis of cancer and cancer pain who had received a fixed dose of opioid for at least 4-6 weeks before being switched to OXN PR for at least 4-6 weeks. The primary endpoint was changed in subjective pain intensity score (visual analogue scale) from baseline to 4-6 weeks after the switch, and data were also collected relating to constipation and laxative use.
Results: In comparison with pain scores at the switching date, subjective pain was reduced by 53% (mean difference -30.8 mm on visual analogue scale, 95 % confidence interval -33.2 mm to -28.3 mm; P<0.001) after switch to OXN PR. Switching to OXN PR was not associated with an increase in constipation episodes or laxative use.
Conclusion: In everyday clinical practice, switching patients with poorly controlled moderate to severe cancer pain to OXN PR can significantly improve pain control without increasing opioid-related constipation.
Keywords: Cancer pain; opioid; analgesia; prolonged release; oxycodone/naloxone; constipation
Introduction
More than half of all patients with cancer suffer from pain, which may be associated with both the disease and treatment. [1,2] Pain is generally at least moderate in intensity, may be chronic, acute, and episodic, and it impacts significantly on emotional wellbeing, disability, and quality of life. The prevalence of cancer pain increases as the disease progresses, such that around 80 % of those with advanced cancer experience moderate or severe chronic pain. [2-5] Cancer pain treatment guidelines, including those from the European Society for Medical Oncology (ESMO) [6], European Association for Palliative Care (EAPC)[7], World Health Organisation (WHO)[8], and National Comprehensive Cancer Network (NCCN)[9] recommend strong opioids as a first-line option and the mainstay of analgesic therapy for the management of moderate to severe cancer- related pain.
Nevertheless, despite it being so common and the availability of clear treatment guidelines, cancer pain is frequently poorly managed and poorly controlled. Reasons include patients’ reluctance to report pain, poor treatment adherence, and fear of side effects. Physicians often feel insufficiently knowledgeable about pain management, concerned about opioid side effects, respiratory depression, and addiction, and afraid or unsure how to prescribe the higher-dose, high-potency opioid analgesics and/or combination therapies that may be necessary. [2,5,10,11]
The side effects associated with opioids are well described and include nausea and vomiting, bloating, constipation, drowsiness, cognitive impairment, hallucinations, pruritus, myoclonus, urinary retention, postural hypotension and rarely respiratory depression and opioid-induced hyperalgesia. Many of these adverse effects are transient and may be addressed by the temporary reduction of opioid dose followed by slower titration, or by switching to another opioid or alternative route of administration (e.g., transdermal).[6,12] However, opioid-induced constipation (OIC), characterized by straining at stool; the passage of lumpy or hard stools; sensation of incomplete evacuation or anorectal obstruction; the need to use manual maneuvers to facilitate defecation, and passing fewer than three stools per week, maybe persistent. It is, therefore, one of the most frequently reported and bothersome adverse events in patients receiving opioids. It is estimated that between 40 % and 95 % of patients on opioid therapy experience OIC, which not only impairs the quality of life, but also reduces adherence to therapy and interferes with effective pain control. [6,13-15]
Although laxatives are often recommended for the management of OIC, they do not address the underlying cause (opioid binding to μ receptors in the gastrointestinal tract) and are ineffective in at least half of patients to whom they are prescribed. [6,12,13]
An alternative approach to managing severe pain and reducing the incidence of OIC is to use a prolonged-release (PR) fixed combination of oxycodone and the opioid antagonist naloxone (OXN PR).[6] Oxycodone has comparable efficacy to morphine for the management of cancer pain, and oral naloxone prevents it from binding to the m- receptors in the gastrointestinal tract, which reduces the incidence of OIC.[16,17] Because the systemic availability of orally administered naloxone is negligible, it does not affect the analgesic efficacy of oxycodone. A number of randomized controlled trials and observational studies have shown that oxycodone combined with naloxone in a 2:1 ratio fixed-dose tablet significantly improves bowel function and quality of life with no loss of analgesic efficacy in comparison to PR oral oxycodone.[17] Because OXN PR is administered twice daily, it may offer a more convenient dosing schedule for managing pain in comparison to analgesics that require more frequent administration.
The purpose of this retrospective observational, real-world study was to evaluate the analgesic efficacy and tolerability of OXN PR, with particular attention to effects on gastrointestinal function, in outpatients with moderate-severe cancer-related pain who were switched from other opioids as part of their routine care.
Methods
This was a single-center, non-interventional, retrospective, non-inferiority, real-world observational cohort study conducted at the Hussain Makki Juma Center for Specialized Surgeries at the Kuwait Cancer Control Center. Data were collected from a chart review of patients with confirmed diagnosis of cancer and cancer pain who had received a fixed dose of opioids for at least 4-6 weeks before being switched to OXN PR for at least 4-6 weeks as part of their routine medical care.
Data were evaluated and compared at the time of switch (switch date) and after 4-6 weeks of treatment with OXN PR (endpoint). Data collected at the time of switch and at endpoint included bodyweight, vital signs, and pain intensity score measured using a visual analogue scale (VAS) ranging from 0 (no pain) to 100 mm (worst pain imaginable). Additional data included concomitant medications (including pain medications at baseline), other distressing symptoms, and constipation episodes and treatments during the previous 4-6 weeks. OXN PR dose was recorded at the endpoint.
The primary endpoint was a change in subjective pain intensity score (VAS) from baseline to 4-6 weeks after the switch. Secondary endpoints included a number of reported constipation episodes, laxative use assessed by number, dose, frequency, and name of laxative prescription; and number of recorded distressing symptoms other than pain (whether or not considered related to a medicinal product) during the 4-6 weeks before and after the switch. An adverse event (AE) was defined as any unfavorable and unintended sign (including an abnormal laboratory finding), symptom, or disease temporally associated with the use of a medicinal product, whether or not considered related to the medicinal product. AEs identified by the attending physician was rated as mild (AE was easily tolerated), moderate (AE resulted from insufficient discomfort to interfere with daily activities) or severe (AE was associated with significant impairment of usual activities).
Patients were not eligible for this study if they had any contraindications to OXN PR as per the locally approved summary of product characteristics. These included allergy to oxycodone hydrochloride, naloxone hydrochloride or excipients; severe respiratory depression; severe chronic obstructive pulmonary disease; cor pulmonale; severe bronchial asthma; non-opioid paralytic ileus; and moderate to severe hepatic impairment. Data were excluded for patients if their records showed any contraindications to OXN PR, if they failed to meet the inclusion criteria, or if there were missing data on pain control (using the VAS) or constipation and laxative use at either baseline or endpoint.
Ethics approval and consent
Before starting the study, the protocol was submitted for ethical review and approved by the internal review board (IRB) of the Hussain Makki Juma Center for Specialized Surgeries, Kuwait Cancer Control Center, Kuwait. Patient confidentiality was maintained at all times. All laboratory specimens, evaluation forms, reports, and other records were anonymous and were identified using a coded number. Due to the retrospective nature of the research and considering that patients were not exposed to any associated risks, a waiver of the informed consent was obtained from the IRB and independent ethics committee. This study was conducted in compliance with the clinical study protocol, Good Pharmacoepidemiological Practice Guidelines (GPP), and applicable regulatory requirements.
Statistics
Descriptive statistics were used to describe the baseline demographic data and clinical characteristics at time point 1 (switch date) and time point 2 (endpoint) of the study. Categorical variables were presented by counts and percentages and continuous variables by mean and standard deviation when data were normally distributed. Where neither of these was applicable, the median and interquartile range (IQR) were used. Numerical data were explored for normality using the Kolmogorov-Smirnov test and the Shapiro-Wilk test. The VAS score was compared before and after the switch to OXN PR using paired t-test if the scores were normally distributed or Wilcoxon signed-rank test if scores were skewed. The percentage change was presented as means and standard deviations or medians and/or ranges and interquartile ranges together with 95 % confidence intervals and the non-inferiority margin was prespecified at 5 %. In total, it would be necessary to include 270 patients to estimate the change in VAS score to a margin of error of at most 0.25 standard deviations using a 95 % confidence interval. This also allowed the detection of a mean difference between the VAS score before and after the switch using the paired t-test with 90 % power and 5% confidence level. Categorical variables underwent a test of association using the McNemar test for paired data. The significance level was two-sided, with a type 1 error of 5%. The analysis was done using Statistical Package for Social Sciences version 24 (SPSS-24).
Results
In total, 270 patients with cancer and experiencing cancer pain met the inclusion criteria. Of the entire cohort, complete data relating to previously prescribed opioid medication at least 4-6 weeks prior to switch to OXN PR were available for 198 (73.33 %), and complete data relating to OXN PR therapy was available for 229 (84.81 %).
In the total group, the majority were female (57.04 %), Arab (80.74), and the mean age at the time of diagnosis was 54.71 years (Table 1).
There was a wide spread of cancer diagnoses representing more than 50 different tumour types and including patients with locally advanced and metastatic disease. The most common tumors were breast (28.51 %), rectal (8.15 %), pancreas (5.92 %), prostate (6.66 %), and cervical (4.44 %) cancers, whereas the majority of other tumour types occurred in fewer than 2 % of patients each.
|
Table 1. Baseline characteristics (N=270) |
|
|
Sex, n (%) Male Female |
116 (42.96) 154 (57.04) |
|
Age at time of diagnosis (years) (mean, SD) |
54.71 (12.31) |
|
Race, n (%) White Black Asian Other |
12 (4.44) 9 (3.33) 43 (15.93) 206 (76.30) |
|
Ethnicity, n (%) Arab Others |
218 (80.74) 52 (19.26) |
The most commonly reported pain medication prior to switch was tramadol, either alone or combined with paracetamol (Table 2).
|
Table 2. Pain medications taken during 4-6 weeks before switch |
|
|
Medication |
Number of patients, n (% of total enrolled patients) |
|
Tramadol |
128 (47.41) |
|
Tramadol/paracetamol |
15 (5.56) |
|
Oxycodone |
14 (5.19) |
|
Morphine sulfate |
9 (3.33) |
|
Oxycodone hydrochloride |
9 (3.33) |
|
Fentanyl |
7 (2.59) |
|
Buprenorphine |
6 (2.22) |
|
Codeine |
6 (2.22) |
|
Morphine |
2 (0.74) |
|
Oxycodone hydrochloride/naloxone hydrochloride |
2 (0.74) |
|
Not recorded |
72 (26.6) |
Mean body weight and vital signs
Mean body weight and vital signs before and after the switch are listed in Table 3. After switching to OXN PR there was a reduction in blood pressure, with significantly lower systolic and diastolic blood pressures at the endpoint in comparison with the switch date (122.6 vs. 119.46 mmHg; P < 0.001 and 77.37 vs. 75.04 mmHg; P < 0.001, respectively). There were no significant differences in body weight, heart rate, respiratory rate, or temperature.
|
Table 3. Mean body weight and vital signs at baseline (switch date) and endpoint |
|||||
|
Parameter |
Switch date |
Endpoint |
P value |
||
|
n |
Mean (SD) |
n |
Mean (SD) |
||
|
Body weight (kg) |
178 |
76.44 (15.09) |
185 |
75.86 (14.01) |
0.24 |
|
Systolic BP (mmHg) |
223 |
122.60 (13.79) |
226 |
119.46 (12.61) |
0.000 |
|
Diastolic BP (mmHg) |
223 |
77.37 (9.69) |
226 |
75.04 (9.27) |
0.000 |
|
Heart rate (beats/min) |
223 |
83.47 (5.62) |
225 |
83.52 (5.05) |
0.96 |
|
Respiratory rate (breaths/min) |
216 |
18.64 (3.90) |
220 |
18.80 (3.84) |
0.20 |
|
Temperature (oC) |
212 |
36.96 (0.18) |
217 |
36.96 (0.20) |
0.61 |
Pain intensity scores
VAS pain scores were significantly lower after switch to OXN PR. Mean VAS score decreased from 66 mm at switch date to 31 mm at endpoint, equating to a 53 % decrease (mean difference -30.8 mm, 95 % confidence interval -33.2 mm to -28.3 mm; P < 0.001). Median scores were 60 mm (range 80 mm) and 30 mm (range 80 mm), respectively (P<0.001). At switch date, 77.7% of patients reported a pain score ≥ 60 mm, whereas after switching to OXN PR, 72.3 % of patients reported a VAS to score ≤ 40 mm (Figure 1).
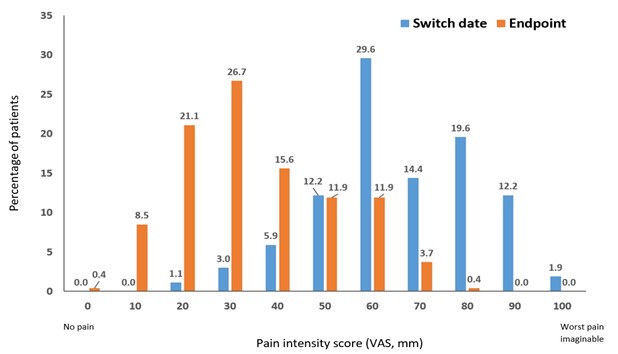
Figure 1: Pain intensity scores at time of switch (switch date) and 4-6 weeks after switch (endpoint) to OXN PR (N=270)
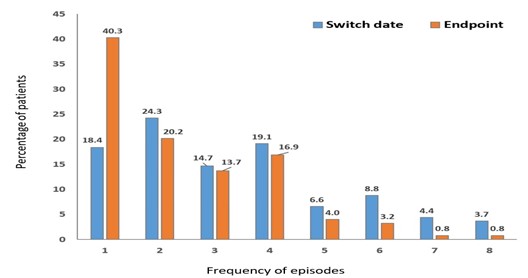
Figure 2: Frequency of constipation episodes during 4-6 weeks before (switch date, n=136) and after (endpoint, n=124) switch to OXN PR
Constipation episodes
Constipation episodes were reported by 136 patients at switch date and 124 patients at the endpoint. The median number of constipation episodes reported during the previous 4-6 weeks was higher at switch than after switch to OXN PR (3, interquartile range [IQR] 2 vs. 2, IQR 3), although this difference was not statistically significant (P=0.112).
Figure 2 shows the frequencies of constipation episodes before and after the switch. Laxative use was reported for 161 and 150 patients before and after the switch, respectively, with no significant differences between the two (Table 4).
|
Table 4. Laxative use before and after switch to OXN PR |
|||
|
Laxative type |
Switch date |
Endpoint |
P value |
|
Bisacodyl, n (%) |
46 (28.57) |
43 (28.67) |
0.176 |
|
Lactulose, n (%) |
48 (29.81) |
40 (26.67) |
|
|
Macrogol 3350/potassium chloride/ sodium bicarbonate/sodium chloride, n (%) |
61 (37.89) |
63 (42.00) |
|
|
Sodium biphosphate/Sodium phosphate, n (%) |
3 (1.86) |
1 (0.67) |
|
|
Sterculia, n (%) |
3 (1.86) |
3 (2.00) |
|
Distressing symptoms other than pain
Distressing symptoms other than pain were reported by 43.3 % (117/270) patients during the 4-6 weeks before the switch and by 40 % (108/270) during the 4-6 weeks after the switch. The most frequent were sleep disturbance (24.07 %), constipation (12.96 %), depression (8.52 %) and anorexia (4.81 %). Other symptoms were uncommon.
OXN PR dose and continuation
OXN PR was taken at the determined dosage twice daily according to a fixed schedule. While symmetric administration (the same dose mornings and evenings) subject to a fixed schedule (every 12 hours) was appropriate for the majority of patients, some patients, depending on the individual pain situation, benefited from asymmetric dosing tailored to their pain pattern. In general, the lowest effective analgesic dose was selected.
At the endpoint, all of the 229 patients who had been switched to OXN PR were still taking it. Change in dose had been made in 30 % (69/229) and change in dosing frequency in 23 % (53/229).
Discussion
This retrospective chart review showed that, in real life clinical practice, patients with cancer-related pain who were switched from another opioid to OXN PR for at least 4 weeks reported significantly improved pain intensity scores without an increase in OIC.
Optimal cut-off points for the VAS in patients with cancer-related pain have previously been defined as 0 (no pain), 1-35 (mild), 36-70 (moderate) and 71-100 (severe). [18] It is notable that, accordingly, at the time of switch almost two-thirds of the patients reported moderate-intensity pain and one-third reported severe pain. After switch to OXN PR, the pain was reduced to mild in 56 % and, with the exception of one patient, the remainder reported moderate pain. The study design was to test for non-inferiority and that should be taken into consideration when interpreting changes in pain score. Nevertheless, the mean reduction in VAS pain intensity of approximately 30 mm and 53 % after the switch to OXN PR is clinically meaningful.[19]
It is notable that before the switch, despite all of our patients reporting cancer pain, approximately one-quarter did not have adequate data relating to previous opioid therapy. Furthermore, before the switch slightly more than half of the patients were receiving tramadol for pain control, either alone or combined with paracetamol. Although tramadol is widely used in palliative care, it is a weak opioid and its analgesic effect in patients with cancer pain may be limited.[6] Adverse effects can be severe and it requires multiple daily doses, which might affect adherence to therapy. [6,7] Accordingly, guidelines suggest that early use of low-dose morphine (step III opioid) may be preferable to using a weak step II opioid in patients with cancer pain. [6,7]
The type of pain was not recorded in our patients. However, pain may be exacerbated near the end of the analgesic dosing interval when blood levels fall below the minimum effective analgesic level (end of dose pain). [20] Furthermore, up to approximately 60 % of cancer patients experience breakthrough pain, which is moderate to severe pain against a background of seemingly well-controlled pain. [10,21] There are a number of reasons why switching to OXN PR may have improved pain control in our patients and the results in this study are consistent with those of previous randomized controlled and observational studies. In addition to being a strong opioid, OXN PR is dosed twice daily, which may help to reduce the frequency of end of dose pain, facilitate adherence and provide consistent pain control. It is generally well tolerated.[17] Various dosing options allow individualized dosing according to pain intensity. The sustained analgesic efficacy of OXN PR in patients with moderate to severe cancer pain has previously been demonstrated in an open-label 24- week extension study.[22] In this population, mean rescue analgesia per day was 1.1 (range 0-3.9) capsules of immediate-release oxycodone 5 mg. In a second 24-week extension study in patients with malignant and non-malignant pain requiring opioids and suffering from opioid-induced constipation, average pain over the last 24 hours in patients treated with OXN PR remained stable with a median pain score of 4.0 (mild pain) and mean pain score of 3.8 to 4.0 throughout the study.[23,24] In a 60-day observational study in which patients with uncontrolled moderate-severe chronic pain or intolerant to other analgesics were switched to OXN PR, OXN PR resulted in a significant reduction in pain overall as well as fewer episodes of breakthrough cancer pain. The impact of pain on quality of life was also reduced and was accompanied by an improvement in bowel function.[25]
Opioid-induced bowel dysfunction, and especially OIC, is the most frequent adverse event reported by patients receiving opioid therapy and it can significantly impair both pain management and quality of life.[13] A number of studies have demonstrated that, in comparison with equivalent doses of oxycodone PR in patients with cancer pain, OXN PR improves measures of bowel function and constipation symptoms while still providing comparable efficacy.[22,23,25-30] In randomized clinical trials and real-world observational studies, improvement in bowel function in patients using OXN PR was associated with significantly improved quality of life.[15,28] In our study, switching to OXN PR was associated with improved pain control and reduced frequency of constipation, although the difference was not statistically significant. Laxative use did not change after the switch, indicating that regardless of the frequency, constipation remained a significant problem to our patients, and the majority still required additional treatment. However, it is notable that despite the frequency of laxative use, and the number of patients in whom constipation episodes were recorded (approximately 60 % and 50 %, respectively), constipation was only reported by approximately 13 %, suggesting that either it remains under-reported, or, perhaps with laxative use, may not have been perceived as significantly distressing by this patient group. Immediate-release opioids used for breakthrough pain may also be associated with OIC and could help to account for a similar frequency of constipation before and after the switch to OXN-PR.
The prevalence of reported distressing symptoms other than the pain was similar before and after the switch. After 4 to 6 weeks of treatment, none of the patients discontinued treatment with OXN PR. Furthermore, the dose of OXN PR remained the same for the majority of patients throughout follow-up. Treatment with OXN PR was associated with a small (approximately 2 mmHg) reduction in mean blood pressure, which is unlikely to be of clinical significance.
We recognize that there are some significant limitations to our study. Because we only included patients who had received at least 4 weeks of OXN PR, patients who might have discontinued OXN PR before that time due to lack of efficacy or poor tolerability were excluded. Consequently, there may have been a biased selection of patients with a good response and fewer significant adverse effects. Because of the retrospective nature of this study, although we have listed the most common distressing symptoms other than pain and constipation, we were unable to comment further on the incidence of treatment-related adverse events or their possible relationship to a specific medication. Our patient population was very ill and most of the distressing symptoms reported could also be accounted for by the underlying illness. Furthermore, the inclusion of patients with missing data relating to opioid use before switch may have resulted in the overestimation of pain on analgesic treatment at the switch date. Similarly, the inclusion of patients with missing data relating to OXN PR administration and dose after the switch may have resulted in underestimation of the potential analgesic effect of OXN PR. However, these comprised a small proportion of patients overall (approximately 25 % and 15 %, respectively) and it is unlikely that this would account for a shift of more than two-thirds of the patients from pain scores > 6 (moderate-severe) before switch to ≤ 4 (mild to moderate) after switching. The reason for switching analgesia was not recorded. It seems most likely that the switch to OXN PR was made for inadequate pain control with previous therapy and this is borne out by the high pre-switch VAS pain rating. Indeed, more than half of the patients were treated with tramadol prior to switching, and pain control with this weak opioid is likely to have been inadequate.[6] Furthermore, as already mentioned, previous opioid use was not adequately recorded in approximately one-quarter of the patients before the switch and so the type and dose of opioid are unknown.
Consequently, our study may have been biased towards patients with poor pain control in whom switch to regular dosing with OXN PR would be most likely to improve pain control. Because of the diverse nature of the opioids used pre-switch, it is not possible to make conclusions about direct comparisons between OXN PR and any other specific opioid. Nevertheless, cancer pain is frequently poorly managed. In that respect, our study, which reflects results in real- world practice, is encouraging in that it demonstrates that when pain is poorly controlled, stepping up to a potent opioid with OXN PR can be done with significant improvement in pain control and without increasing the most bothersome opioid-related side effects.
We used a number of constipation episodes as a measure of constipation. We recognize that there are more accurate and specific tools to measure constipation that would be appropriate in a prospective study. However, our study is a retrospective review of case notes, and we used the information available to make an assessment of problematic constipation. A formal prospective, randomized study would be required to confirm our observations. It is notable, however, that our observations are in agreement with the results of previous prospective studies showing that OXN PR does not worsen bowel function in comparison with other opioids.
In conclusion, our study shows that in everyday clinical practice, switching patients with poorly controlled moderate to severe cancer pain to OXN PR can significantly improve pain control without increasing opioid-related constipation.
Funding and disclosures
The study and writing the report were supported by a non-restrictive grant from Mundipharma Middle East FZ‐LLC. The sponsor assisted with study design, and the study was conducted independently of the sponsor with assistance from a third-party contract research organization. All study documentation was maintained by Dr. AlDiri, who also was responsible for any decisions relating to the conduct of the study, interpretation of the results, and decision to submit the article for publication. Dr. Webb provided writing assistance for the first draft of the manuscript with financial support from Mundipharma Middle East FZ‐LLC. All of the authors reviewed, contributed to, and approved successive drafts and the final manuscript. The authors report no other conflicts of interest relating to this study.
References
- van den Beuken-van Everdingen MHJ, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, et al. (2007) Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Annal Oncol. 18(9): 1437-1449.
- Marcus DA (2011) Epidemiology of cancer pain. Curr Pain Headache Rep. 15: 231-234.
- Knaul FM, Farmer PE, Krakauer EL, De Lima L, Bhadelia A, et al. (2018) Alleviating the access abyss in palliative care and pain relief - an imperative of universal health coverage: the Lancet Commission report. Lancet. 391(10128): 1391-1454.
- Greco MT, Roberto A, Corli O, Deandrea S, Bandieri E, et al. (2014) Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 32(36): 4149–4154.
- Breivik H, Cherny N, Collett B, de Conno F, Filbet M, et al. (2009) Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol. 20(8): 1420-1433.
- Fallon M, Giusti R, Aielli F, Hoskin P, Rolke R, et al. (2018) Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann Oncol. 29(Suppl 4): iv166-iv191.
- Caraceni A, Hanks G, Kaasa S, Bennett MI, Brunelli C, et al. (2012) Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 13(2): e58-e68.
- World Health Organisation (WHO). WHO guidelines for the pharmacological and radiotherapeutic management of cancer pain in adults and adolescents. Geneva: World Health Organization; 2018.
- Swarm RA, Paice JA, Anghelescu DL, Are M, Bruce JY, et al. (2019) NCCN Clinical Practice Guidelines in Oncology: Adult Cancer Pain, Version 3.2019. J Natl Compr Canc Netw. 17(8): 977-1007.
- Pergolizzi JV, Gharibo C, Ho K-Y (2015) Treatment Considerations for Cancer Pain: A Global Perspective. Pain Pract. 15(8): 778-792.
- Deandrea S, Montanari M, Moja L, Apolone G (2008) Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 19(12): 1985-1991.
- Blanchard C, Chetty S, Ganca L, Gwyther E, Hodgson E, et al. (2015) Guide to the treatment of cancer pain in South Africa; 2015. Pretoria (SA): Medspec Publishing.
- Kumar L, Barker C, Emmanuel A (2014) Opioid-Induced Constipation: Pathophysiology, Clinical Consequences, and Management. Gastroenterol Res Pract. 2014: 141737.
- Bell TJ, Panchal SJ, Miaskowski C, Bolge SC, Milanova T, et al. (2009) The Prevalence, Severity, and Impact of Opioid-Induced Bowel Dysfunction: Results of a US and European Patient Survey (PROBE 1). Pain Med. 10(1): 35-42.
- Morlion B, Clemens KE, Dunlop W (2015) Quality of Life and Healthcare Resource in Patients Receiving Opioids for Chronic Pain: A Review of the Place of Oxycodone/Naloxone. Clin Drug Investig. 35: 1-11.
- Caraceni A, Pigni A, Brunelli C (2011) Is oral morphine still the first-choice opioid for moderate to severe cancer pain? A systematic review within the European Palliative Care Research Collaborative guidelines project. Palliat Med. 25(5): 402-409.
- Morlion BJ, Mueller-Lissner SA, Vellucci R, Leppert W, Coffin BC, et al. (2018) Oral prolonged-release oxycodone/naloxone for managing pain and opioid-induced constipation: a review of the evidence. Pain Pract 2018; 18(5): 647-665.
- Ham O-K, Kang Y, Teng H, Lee Y, Im E-O (2015) Consistency and Accuracy of Multiple Pain Scales Measured in Cancer Patients from Multiple Ethnic Groups. Cancer Nurs. 38(4): 305- 311.
- Olsen MF, Bjerre E, Hansen MD, Tendal B, Hilden J, et al. (2018) Minimum clinically important differences in chronic pain vary considerably by baseline pain and methodological factors: systematic review of empirical studies. J Clin Epidemiol. 101: 87- 106.e2.
- Blanchard C, Chetty S, Ganca L, Gwyther E, Hodgson E, et al. Guide to the treatment of cancer pain in South Africa; 2015. Pretoria (SA): Medspec Publishing; 2015.
- Pérez-Hernández C, Blasco A, Gándara Á, Mañas A, Rodríguez- López MJ, et al. Prevalence and characterization of breakthrough pain in patients with cancer in Spain: the CARPE-DIO study. Sci Rep. 9(1): 17701.
- Ahmedzai SH, Leppert W, Janecki M, Pakosz A, Lomax M, et al. (2015) Long-term safety and efficacy of oxycodone/naloxone prolonged-release tablets in patients with moderate-to-severe chronic cancer pain. Support Care Cancer. 23: 823-830.
- Dupoiron D, Stachowiak A, Loewenstein O, Ellery A, Kremers W, et al. (2017) Long-term efficacy and safety of oxycodone- naloxone prolonged-release formulation (up to 180/90 mg daily): results of the open-label extension phase of a phase III multicenter, multiple-dose, randomized, controlled study. Eur J Pain. 21(9): 1485-1494.
- Woo A, Lechner B, Fu T, Wong S, Chiu N, et al. (2015) Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann Palliat Med. 4(4): 176-183.
- Amato F, Ceniti S, Mameli S, Pisanu GM, Vellucci R, et al. (2017) High dosage of a fixed combination oxycodone/naloxone prolonged release: efficacy and tolerability in patients with chronic cancer pain. Support Care Cancer. 25: 3051-3058.
- Ahmedzai SH, Nauck F, Bar-Sela G, Bosse B, Leyendecker P, et al. (2011) A randomized, double-blind, active-controlled, double- dummy, parallel-group study to determine the safety and efficacy of oxycodone/naloxone prolonged-release tablets in patients with moderate/severe, chronic cancer pain. Palliat Med. 26(1): 50-60.
- Dupoiron D, Stachowiak A, Loewenstein O, Ellery A, Kremers W, et al. (2017) A phase III randomized controlled study on the efficacy and improved bowel function of prolonged-release (PR) oxycodone-naloxone (up to 160/80 mg daily) vs oxycodone PR. Eur J Pain. 21(9): 1528-1537.
- Lazzari M, Greco MT, Marcassa C, Finocchi S, Caldarulo C, et al. (2015) Efficacy and tolerability of oral oxycodone and oxycodone/naloxone combination in opioid-naïve cancer patients: a propensity analysis. Drug Design, Development and Therapy. 2015: 9 5863-5872.
- Hjalte F, Tennvall GR, Welin K-O, Westerling D (2016) Treatment of Severe Pain and Opioid-induced Constipation: An Observational Study of Quality of Life, Resource Use, and Costs in Sweden. Pain Ther. 5: 227-236.
- Clemens KE, Quednau I, Klaschik E (2011) Bowel function during pain therapy with oxycodone/naloxone prolonged-release tablets in patients with advanced cancer. Int J Clin Pract. 65(4): 472-478.



