Dimitrijevic Milovan, PhD1,2, Tomanovic Nada, PhD1,3, Arsovic Nenad, PhD1,2, Dudvarski Zoran, PhD1,2, Jovanovic Katarina, MD2, Tomic Ana-Marija, MD3, Jakovljevic Sasa, MD2*
1University of Belgrade, Faculty of Medicine, Belgrade, Serbia
2University Clinical Center of Serbia, Clinic of Otorhinolaryngology and Maxillofacial Surgery, Department of Maxillofacial Surgery, Belgrade, Serbia
3Institute of Pathology, Belgrade, Serbia; Department of Pathological Anatomy
*Corresponding Author: Jakovljevic Sasa, MD, University Clinical Center of Serbia, Clinic of Otorhinolaryngology and Maxillofacial Surgery, Department of Maxillofacial Surgery, Belgrade, Serbia.
Abstract
This study aimed to present a case of metastatic sinonasal mucosal melanoma in a 60-year-old female patient treated with immunotherapy and surgery. After several months of difficulty breathing and nosebleeds, the patient was diagnosed with mucosal melanoma. The treatment was started with immunotherapy. After partial regression, radical surgical treatment was performed, and then immunotherapy was continued. Treatment of sinonasal mucosal melanoma includes surgery, radiotherapy, chemotherapy, and immunotherapy, either alone or in combination. Failure in local control is associated with an increased risk of distant metastases and a significant reduction in survival. In conclusion, modern immunotherapy with monoclonal antibodies can significantly contribute to the locoregional control of the disease. In addition to early diagnosis, the goal of future research should be to develop the best possible chemotherapy regimens that would improve overall survival in these patients.
Keywords: Sinonasal mucosal melanoma, surgical treatment, mucosal melanoma immunotherapy, pembrolizumab
Introduction
Mucous melanoma of the head and neck is a rear entity that makes up about 10 % of melanoma originating in the head and neck, and approximately 1 % of all melanomas. [1,2] Sinonasal mucosal melanomas (SNMMs) are a very specific form of melanoma. [3] They are most often localized in the nasal cavity (81 %), and less often in the sinuses (19 %). [4-6] They mainly occur in the elderly and have a very poor prognosis due to the high rate of local recurrences and distant metastases. In addition, these tumors tend to grow slowly and therefore manifest relatively late. According to numerous authors, the incidence of SNMM is higher in geographical areas where cutaneous melanomas are less common. [4,6] Another feature of SNMM is the relatively low incidence of metastases in regional lymph nodes.
Local disease control with surgery and/or radiotherapy is the current mainstay of treatment. Local recurrences occur in about 50 % of cases. [5] It has been suggested that high rates of local recurrence are the result of the multifocal nature of the primary disease or lymphatic proliferation of mucosal melanoma cells. [4,5].
This study aimed to present a case of sinonasal mucosal melanoma with metastases in a 60-year-old patient who was treated with immunotherapy and surgery.
Case Report
A 60-year-old patient reported to the ENT and MFS Clinic due to difficulty breathing through the nose and occasional nosebleeds. The difficulties were present for ten months. Clinical and fiber endoscopic examination revealed a tumor mass of brownish-black color that completely obstructs the left nasal cavity. The mass was relatively hard and bled to the touch. (Figure 1) The other clinical findings were orderly.
Figure 1. Endoscopic appearance of the tumor mass in the left nasal passage.
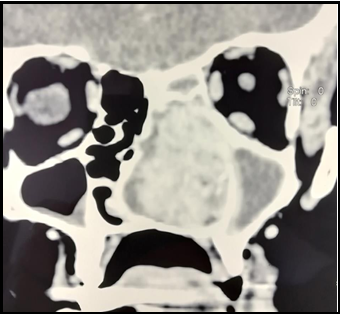
Computed tomography (CT) of the paranasal sinuses and neck showed that the entire left nasal passage was filled with a soft tissue tumor mass. The tumor was highly vascular, 45x25x33 mm in diameter. (Figure 2) Enlarged lymph nodes were found on both sides of the neck, the largest on the left with a diameter of 20 mm, and the right with 24 mm. Chest radiography and abdominal ultrasound were without pathological findings.
Figure 2. Computed tomography of paranasal cavities (coronal section). Large tumor mass in the left nasal passage that destroys the hard palate and remodels the nasal septum.
After completing diagnostic processing, a biopsy of the tumor lesion was performed, with moderate bleeding. Pathohistological and immunohistochemical findings showed that it was mucosal melanoma. Tumor tissue is made up of tumor cells of epithelioid morphology whose cytoplasm contains the pigment melanin. Tumor cells were positive for S100, MITF, HMB45, Melanie, and SOX10 (Figure 3a, b, c).
Figure 3. Tumor cells showed positivity for HMB45 (a), Melan A (b), and SOX10 (c). Magnification x20 (a, b) and x10 (c).
A test for the detection of mutations in the BRAF gene was also performed, which showed the absence of V600 mutations in the BRAF gene. The patient was presented to the Medical Board for Melanoma, which decided to start treatment with immunotherapy.
Thirteen cycles of pembrolizumab were administered, followed by partial regression of the disease. It was decided to then start radical surgical treatment. A lateral rhinotomy and medial maxillectomy were performed and the tumor was completely removed. (Figure 4)
Figure 4. Tumor mass from the left nasal passage removed en block.
Since postoperative CT showed no signs of residual tumor and enlarged neck lymph nodes, it was decided to continue treatment with pembrolizumab. The patient subsequently received 5 more cycles of therapy. After three years after the diagnosis, it was stated that there were no signs of recurrence of the disease.
Discussion
SNMM is a neuroectodermal tumor arising from melanocytes, which may be present in the mucosa, superficial and deep stroma of the nasal conchae and septum, and secretory glands, and are especially prevalent around the deflector fissure. relative to other regions of the head and neck may explain the relative incidence of mucosal melanoma at this site. [5] SNMM accounts for 0.2-8 % of all primary mucosal melanomas in Europe and the United States and 0.03 % of all malignancies. [2,7] It accounts for less than 1 % of all melanomas and about 4-8 % of sinonasal malignancies. [2,5,8,9]
Most tumors are located on the lateral wall of the nose, while in the paranasal sinuses it is most often localized in the maxillary sinus.
They are mainly manifested by non-specific symptoms, which include nasal obstruction, epistaxis, facial pain, and rhinorrhea, while in the advanced stage symptoms such as diplopia and proptosis may occur. [2,4-7,10] Therefore, there is usually a long interval between the appearance of symptoms and the diagnosis of the disease, primarily with localization in the paranasal sinuses.
SNMM are known to have a specific pattern of chromosomal changes. Recent data on immunohistochemical and genetic profiling indicate potentially significant changes in the KIT gene and a minor role for BRAF gene mutations in the pathogenesis of mucosal melanomas. [1,2,9] According to various studies, the somatic mutations that characterize SNMM are KIT, 0–40 %, NRAS 10–50 %, and BRAF 0–10 %. [9] The tyrosine kinase inhibitor (Imatinib) is effective in patients with c-KIT mutations. [2,9]
Classical immunohistochemical criteria for mucosal melanoma are negative epithelial, endocrine, lymphoid, and muscle markers associated with one or more positive melanocyte markers: protein S100, HMB-45, and Melan-A. [3-6,10,11] Current guidelines state that ≥ 2 positive markers are required for the diagnosis of mucosal melanoma. [3] In addition, SNMMs are more likely to show vascular invasion, probably due to numerous arterial anastomoses in the nasal passages. [4]
The differential diagnosis of SNMM includes olfactory neuroblastoma, lymphoma, plasmacytoma, undifferentiated carcinoma, adenocarcinoma, rhabdomyosarcoma, malignant fibrous histiocyte, leiomyosarcoma, angiosarcoma, malignant fibrous histiocyte, and neurogenic sarcoma. [6,11]
SNMM treatment includes surgery, radiotherapy, chemotherapy, and immunotherapy, either alone or in combination.
Extensive surgical excision with clear resection lines is the main therapeutic option, as it provides the best chance of long-term survival in these patients. [1,2,5,8,9] The surgical approach and extent of resection depend on the location and size of the tumor. Some authors suggest that endoscopic resection may be associated with better clinical outcomes. [1,12] Proponents of this approach argue that it has lower complication rates, although the tumor is resected in parts rather than en bloc. The advantage of endoscopic surgery lies in the potential reduction of morbidity. In a review of 58 patients treated for SNMM at the MD Anderson Cancer Center, the endoscopic approach resulted in a better two-year overall survival than the open approach. [1] However, these results should be considered because the endoscopic approach is most commonly used for smaller tumor lesions.
The role of neck dissection in SNMM which is an intern as a NO is clearly defined. Numerous authors do not recommend prophylactic neck dissection in SNMM, given the low rate of regional metastases. [1,2,4,5,9] The sentinel lymph node biopsy technique used in cutaneous melanoma is currently being investigated in mucosal melanoma. The reported rates of regional lymph node metastases in SNMM are 8-11 %, and hematogenous metastases are detected in 6 % of patients (lungs, brain, bones, liver). [2] Our patient had multiple metastases in the lymph nodes of the neck at the time of presentation, but she had no distant metastases.
Adjuvant postoperative radiotherapy provides significant benefits for loco-regional disease control but does not affect overall survival. Radiotherapy as the only modality has not shown a significant advantage for local control or survival, and as such, in most cases, it is applied mainly to large tumors that are not operable. [1] Successful control of the hematogenous spread of the disease requires aggressive systemic therapy. However, the effects of systemic therapy have so far been unsatisfactory, as it has largely failed to provide significant survival benefits. [1] The clinical benefit of immunotherapy with ipilimumab, a monoclonal antibody associated with cytotoxic T lymphocytes, in the treatment of unresectable melanoma has recently been demonstrated in a large, randomized study. The authors observed a significant improvement in overall survival in patients receiving ipilimumab. [1,2,9] In one study of patients with metastatic melanoma resistant to ipilimumab, nivolumab showed a higher overall survival rate (72.9 %) than dacarbazine (42 (1 %). [9] The use of other agents, such as pembrolizumab, also showed a higher survival rate without progression and toxicity. [7] Immunotherapy with interleukin 2 or interferon-alpha (IFNα) is currently under evaluation, either alone or in combination with chemotherapy. [2]
Bartell et al. have used a chemotherapy regimen that includes vincristine, cisplatin, and dacarbazine with biological agents (such as IFN-α and IL-2) in patients with mucosal melanoma and metastatic disease. A good clinical response was observed in 47 % of patients, with 27 % of patients showing a complete response. The authors suggested that chemotherapy should be considered in patients with metastatic disease or inoperable tumors. [4]
The most common cause of death in SNMM is distant metastases. Reported five-year overall survival ranges from 20-60 %, with an average of 27 %. [1,5,6,9] Poor survival in patients with SNMM is mainly attributed to aggressive local and hematogenous spread of the disease.
Certain histopathological features of SNMM are predictors of poor survival and they include vascular invasion, necrosis, and polymorphic populations of tumor cells. [6] Tumor localization has also been shown to influence prognosis. Patients with nasal tumors have a better prognosis than those with localization in the paranasal sinuses. [9] Failure in local disease control is associated with an increased risk of distant metastases and a significant reduction in overall survival. However, more than 50 % of patients will develop distant metastases despite good local tumor control. [2] Thus, Nandapalan et al. reported a local recurrence rate of 52 %. [4] Finally, Kalogirou EM et al. reported a case of SNMM recurrence 10 years after treatment, suggesting that this tumor carries a long-term risk of recurrence. [10]
Conclusion
The prolonged presence of nonspecific symptoms delays the timely diagnosis of SNMM. The complex anatomy of the sinonasal region and the often, advanced stage of the disease complicates and often prevent radical surgical resection of the tumor. Despite numerous advances in surgical treatment, radiotherapy, and chemotherapy, SNMM remains an aggressive malignant disease with a very poor prognosis. Surgical treatment is the method of choice in the treatment of these patients, and modern immunotherapy with monoclonal antibodies can significantly contribute to the locoregional control of the disease. Therefore, in addition to early diagnosis, the goal of future trials should be to develop the best possible chemotherapy regimens that would improve overall survival in these patients.
Conflict of interest: The authors declare no conflicts of interest.
Author contributions:
D.M. and A.N. designed the study. N.T. T.A. and S.J. collected the data. M.D., N.T., and J.S. analyzed the data. D.Z., J.K. and J.S. wrote the manuscript.
References
- Lazarev S, Gupta V, Hu K, Harrison LB, Bakst R (2014) Mucosal Melanoma of the Head and Neck: A Systematic Review of the Literature. Int J Radiat Oncol Biol Phys. 90(5): 1108-18.
- Green B, Elhamshary A, Gomez R, Rahimi S, Brennan PA (2017) An update on the current management of head and neck mucosal melanoma. J Oral Pathol Med. 46(7): 475-479.
- Letievant JC, Poupart M, Ambrun A, Colin C, Pignat JC (2016) Single-center retrospective series of fourteen patients with mucosal melanoma of the nasal cavity and paranasal sinuses. Eur Ann Otorhinolaryngol Head Neck Dis. 133(6): 387-391.
- Breik O, Sim F, Wong T, Nastri A, Iseli TA, et al. (2016) Survival Outcomes of Mucosal Melanoma in the Head and Neck: Case Series and Review of Current Treatment Guidelines. J Oral Maxillofac Surg. 74(9): 1859-71.
- Gilain L, Houette A, Montalban A, Mom T, Saroul N (2014) Mucosal melanoma of the nasal cavity and paranasal sinuses. Eur Ann Otorhinolaryngol Head Neck Dis. 131(6): 365-369.
- Clifton N, Harrison L, Bradley PJ, Jones NS (2011) Malignant melanoma of nasal cavity and paranasal sinuses: report of 24 patients and literature review. J Laryngol Otol. 125(5): 479-85.
- Dreno M, Georges M, Espitalier F, Ferron C, Charnole A, et al. (2017) Sinonasal mucosal melanoma: A 44-case study and literature analysis. Eur Ann Otorhinolaryngol Head Neck Dis. 134(4): 237-242.
- Karim MU, Khan K, Ali N, Ikram M (2015) Sino-nasal mucosal malignant melanoma. BMJ Case Rep. 2015: bcr2014206745.
- Na'ara S, Mukherjee A, Billan S, Gil Z (2020) Contemporary Multidisciplinary Management of Sinonasal Mucosal Melanoma. Onco Targets Ther. 13: 2289-2298.
- Kepekci AH, Kig C, Gundogan GI (2018) The evaluation of malignant mucosal melanoma of nasal cavity with a rare occasion. Int J Physiol Pathophysiol Pharmacol. 10(4): 139-143.
- Nakaya M, Mochiki M, Takeuchi S, Yuge T, Nakao K, et al. (2004) Malignant melanoma of nasal cavity: report of 16 Japanese patients. Auris Nasus Larynx. 31(3): 233-7.
- Miglani A, Patel SH, Kosiorek HE, Hinni ML, Hayden RE, et al. (2017) Endoscopic resection of sinonasal mucosal melanoma has comparable outcomes to open approaches. Am J Rhinol Allergy. 31(3): 200-204.