Steven P. Petrosino, Ph.D.*, Boaz Nyona Matende, MSci
Clinical Scientist and President, Nutrition Advisor LLC, 8815 Tayport Drive, Dublin OH 43017, USA
*Corresponding Author: Steven P. Petrosino, Ph.D., Clinical Scientist and President, Nutrition Advisor LLC, 8815 Tayport Drive, Dublin OH 43017, USA
Abstract
The COVID-19 pandemic is by far the deadliest pandemic since the catastrophic Spanish Flu pandemic of 1918-1919. Coronavirus disease 2019 (COVID-19), the highly infectious viral agent responsible for the COVID-19 pandemic began as a cluster of pneumonia cases reported in the Wuhan region of China in late 2019. This novel infectious disease spread rapidly to all parts of the world within a few months, and as of mid-2020, COVID-19 was fully established as a global pandemic. The search for urgent and effective treatments, as well as biomarkers of adverse disease outcomes are still ongoing. Current management of COVID-19 is supportive, and respiratory failure due to acute respiratory distress syndrome (ARDS) is the leading cause of mortality. A clear link between disease severity, hyper-inflammation, and major comorbidities, including hypertension, diabetes, and CVD has been established. This paper will seek to delineate the interplay of those markers of poor disease outcome with telomere length.
Keywords: COVID-19, SARS-COV-2, Telomere Length, Oxidative Stress, Inflammation, Metabolic Syndrome,
Abbreviations: COVID-19: Coronavirus Disease 2019, [SP1] NADPH: Nicotinamide Adenine dinucleotide phosphate, CFR: Case Fatality Rate, CRP: C-Reactive Protein, WHO: World Health Organization, MT: metallothionein, CVD: Cardiovascular disease, CKD: Chronic Kidney Disease, OS: Oxidative Stress, COPD: Chronic Obstructive Pulmonary Disease, ROS: Reactive Oxygen Species, GSH: Glutathione, BMI: Body Mass Index, CKD: Chronic Kidney Disease, LTL: Leukocyte Telomere Length, SOD: Superoxide
Introduction
COVID-19 Overview:
The novel coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread around the world at an unprecedented rate (Mahase, 2020). Due to its high infectiousness and rapid transmission rates, coupled with inadequate prevention and control measures, the pandemic continues to pose a serious global health emergency (Zheng et al., 2020). Hospitalized patients rely mainly on supportive care for various comorbidities, including acute respiratory distress syndrome (ARDS), which is the leading cause of mortality (Mehta et al., 2020). While the COVID-19 CFR rate is slowing down, the condition remains deadly in patients presenting with certain risk factors (Rajgor et al., 2020). A small proportion of patients are hospitalized with a serious COVID-19 disease, and almost all these patients present with pre-existing conditions that also predict mortality (Atkins et al., 2020). To date, the identification of presenting characteristics, comorbidities, and clinical outcomes in COVID-19 patients is well understood, but the factors or mechanisms behind the association are not well understood. The Centers for Disease Control and Prevention (CDC) website lists a number of conditions as key risk factors for a severe disease that requires special attention, including asthma; chronic kidney disease, (especially during dialysis); chronic lung disease; diabetes; hemoglobin disorders; an immunocompromised status; liver disease; being aged 65 years and above; being a resident in a nursing home or long-term care facility; the presence of serious heart conditions; and severe obesity (Centers for Disease Control and Prevention, 2020). Individuals with these risk factors are significantly more likely to experience poor clinical outcomes (Guan et al., 2020).
Telomere length and COVID-19 Overview:
Telomere is the repetitive DNA sequences located at the terminal end of chromosomes, (like the endcaps on shoelaces), and which are characterized by TTAGGG tandem repeats. These repeats cap the ends of chromosomes, protecting them from degradation (“fraying”), and end-to-end fusion, hence, maintaining genome integrity (Greider, 1996; Valdes et al., 2005). Chromosomal abnormalities have been associated with shortened telomeres, particularly in replicative senescence cells (Allsopp et al., 1992). In humans, telomerase is expressed all cell lines during the initial few weeks of embryogenesis, and subsequently the down-regulated most cell types (Herman et al., 2001). DNA loses telomeric repeats with each cell division, and these losses are estimated to amount to 25 base pairs per year, and eventually resulting in replicative cellular senescence (Revesz et al., 2014). Shortened telomere length is a marker of an advanced chronological age, which associates with the comparatively higher frequency of disease morbidity/mortality (Rizvi, Raza, & Mahdi, 2014), (Herman et al., 2001), reported that it was the shortest telomere and not the average telomere length that determined cell viability and chromosome stability. The clinical significance of telomeres has been extensively investigated, with evidence pointing to the association of shorter telomere length with conditions such as depression, poor health behaviors, age-related diseases, progeria, and premature mortality. Children with the rare condition progeria have much shorter telomeres than do healthy children. A team of pediatric researchers led by Cooke and co- workers used a new technology called RNA therapeutics, which delivers small molecules into cells to alter their gene expression. These researchers were able to get the cells from children with progeria to produce telomerase, which suggests that this devastating disease may be altered. (Walther BK, Li Y, Thandavarayan RA, Cooke JP. Progeria and accelerated cardiovascular aging. Cardiol Plus [serial online] 2018 [cited 2021 Feb 22]; 3:81-9. Available from: https://www.cardiologyplus.org/text.asp?2018/3/3/81/242082 [SP2] ) In addition, there is mounting evidence of a link between shortened telomere length and the development of various infectious diseases (Kiecolt-Glaser et al., 2013). The COVID-19 outbreak has presented immense challenges to the global community, leading to concerted research efforts to enhance knowledge and understanding of this viral respiratory disease [SP3]. As part of the efforts, this review presents data on the association between telomere length and COVID-19, with a focus on the known link between telomere length and COVID-19 comorbidities, the shared deficiencies and mechanisms, and proximate link through other respiratory ailments. The knowledge gained contributes to the wider strategy to control COVID-19, particularly in relation to the risk of severe COVID-19 disease that has been found to significantly associate with hospitalization and the risk of adverse disease outcomes.
Major Comorbidities in Hospitalized COVID-19 Patients:
Table 1: Major COVID-19 Comorbidities
|
Major COVID-19 Comorbidity in Hospitalized Patients |
Occurrence % (Richardson et al., 2020) |
|
Hypertension |
56.6 % |
|
Obesity |
41.7 % |
|
Diabetes |
33.8 % |
|
Cardiovascular Disease (coronary artery disease and congestive heart failure) |
18 % |
|
Chronic Respiratory Disease (asthma, and chronic obstructive pulmonary disease) |
17.3 %
|
|
Kidney disease (chronic and end-stage kidney disease) |
8.5 % |
|
Cancer |
6 % |
Source: Richardson et al., 2020.
Several of the listed comorbidities are prominently cited elsewhere in the COVID-19 literature, and include hypertension, diabetes, and cardiovascular disease (Fang, Karakiulakis, & Roth, 2020; Wang et al., 2020; Richardson et al., 2020).
Telomere Shortening and Health Risk:
A key function of telomeres is to oversee cell surveillance by preventing nucleolytic degradation and the irregular fusions and recombination’s (Beilfuss, Camargo, & Kamycheva, 2017). The enzyme telomerase maintains telomere length at equilibrium as telomeres shorten with each replication cycle (Blackburn, 1991). Shortened or dysfunctional telomeres is one way through which cells from organisms with renewable tissue can permanently withdraw from the cell cycle, other ways can include DNA damage, strong mitogenic signals, and disputed chromatin (Campisi, 2005). The observed gradual loss of telomeric DNA may lead to chromosome instability (Beilfuss, Camargo, & Kamycheva, 2017). Telomere length serves as a clock indicating the pace of cellular aging (Jeanclose et al., 2000). Findings from cross-sectional studies have demonstrated the role of advancing age in the steady decline of telomere length, highlighting how the decline is associated with various diseases that occur with aging, and how environmental factors may impact on differences in telomere length between individuals or ethnicities (Rizvi, Raza, & Mahdi, 2014; Mundstock et al., 2015). Generally, telomere shortening has been associated with cellular changes that usher in age-related changes and, hence, an increased susceptibility to age-related diseases (Beilfuss, Camargo, & Kamycheva, 2017).
COVID-19 Comorbidities and Telomere Length: Longevity studies conducted over three decades have shown a link between telomere length, aging, and death. Telomere length in replicating somatic cells has an inverse correlation with age (Fitzpatrick et al., 2007). The finite replicative capacity, as determined by telomere length, is associated with the onset of age- related chronic diseases (Rizvi, Raza, & Mahdi, 2014). Various COVID-19 comorbidities (hypertension, obesity, diabetes, cardiovascular disease [coronary artery disease and congestive heart failure] chronic respiratory disease [asthma, and chronic obstructive pulmonary disease], kidney disease [chronic and end-stage], and cancer) have been established to associate with telomere attrition.
Hypertension is the most prevalent of the COVID-19 comorbidities, and which has traditionally been associated with aging and cardiovascular disease mortality (Petrie, Guzik, & Touyz, 2018). Shorter telomere length is a consistent finding in hypertensive patients (Lung, Ku, & Kao, 2008; Fuster, Diez, & Andres, 2007; Yang et al., 2009). It is now hypothesized that mean leukocyte telomere length is predicts the risk of developing hypertension (Yang et al., 2009). Hypertension is also found to associate with cardiovascular disease, in fact, a significant proportion of mortalities in cardiovascular disease are attributable to hypertension (Wu et al., 2015), (Yang et al 2009) found that though both short telomere length and hypertension were implicated in coronary heart disease, the two were independent risk factors for developing the disease. Findings by Fitzpatrick et al., (2007) showed that shortened leukocyte telomere length was linked to cardiovascular disease and, therefore, supported the hypothesis that telomere shortening associated with age-related diseases. Telomere shortening may coincide with the beginning of the atherosclerotic process, either at the onset and development of arterial hypertension or during myocardial infarction (Saliques et al., 2010). Accelerated atherosclerotic disease leads to cardiovascular complications (Lobo et al., 2010). A systematic review and meta- analysis conducted by (Haycock et al., 2014) on the association between telomere length and CVD showed an inverse relationship between telomere length and the risk of coronary heart disease. (Salpea et al., 2010) showed a correlation between telomere shortening, consequential premature cellular senescence, and the development type 2 diabetes. Shorter-age-adjusted leukocyte telomere length (LTL) was noted in type 2 diabetes subjects compared to controls (Salpea et al., 2010). A meta-analysis utilizing nine cohorts with a total of 5759 cases and 6518 controls showed a statistically significant association between shortened telomere length and type 2 diabetes (OR: 1.291; 95 % CI: 1.112, 1.498; P < 0.001) (Zhao et al., 2013). The association between chronic respiratory disease and shortened telomere length has been evaluated in several studies (Savale et al., 2009; Albrecht et al., 2014). (Savale et al., 2009) showed that reduced lung function was linked to shortened length and, hence, an in increased risk of COPD. The findings are corroborated by a meta-analysis carried out by (Albrecht et al., 2014) that showed that telomere length in circulating leukocytes was associated with the pathogenesis of chronic conditions (such as COPD and asthma) and an overall decline in lung function that may reflect biological aging. The finding of telomere-shortening mutations in lung epithelial cells of patients suffering from COPD also provides evidence on the link between telomere shortening and chronic kidney disease (Fanner et al., 2012). Findings in two independent cohorts reinforced the evidence that relative telomere length is a marker and potentially a pathogenic factor for CKD progression (Raschenberger et al., 2015). Cellular senescence is a marker that underlies and influences the progression of nephropathy (Mazidi et al., 2017).
Nutrient Deficiencies linked to Telomere Length and COVID-19:
Vitamin D Deficiency
Prolonged exposure to the sunlight degrades both pre-vitamin D3 and vitamin D3 into inactive photoproducts (Holick, 2007). The synthesis of vitamin D in the skin is also affected by factors such as latitude, total ozone, altitude, ground, cover, cloud thickness and skin color (Engelsen et al., 2005). Vitamin D2 and D3 sources from the diet are incorporated into chlyomicrons and transported by the lymphatic system into the venous circulation. Only a small fraction of foods naturally has vitamin D and include oily fish such as mackerel, salmon, herring, and oils obtained from fish, including cod liver oil (Holick & Chen, 2008). Circulating vitamin D is bound to the vitamin D binding protein and transported to the liver, where it is converted by vitamin D-25-hydroxylase to 25-hydroxyvitamin D [25(OH)D]. The vitamin 25-hydroxyvitamin D [25(OH)D] is a major circulating vitamin that is used by clinicians to determine vitamin D status (Holick, 2007). Most countries around the world have a relatively low supply of foods rich in vitamin D and a deficient natural exposure to natural UBV radiation from the sunlight and, hence, a high prevalence of vitamin D deficiency (> 20 % prevalence of 25(OH)D < 30 nmol/L) or the risk of vitamin D deficiency is identified around the globe (Roth et al., 2018). The high prevalence of vitamin D deficiency relates with high rates of prevalence of morbidity and mortality in vitamin D correlated diseases in health outcomes.
Vitamin D Deficiency and Telomere Shortening: Changes in vitamin D metabolism and activity occur during aging, including intestinal resistance to dihydroxyvitamin D (1, 25(OH)2D), which affects intestinal calcium uptake, and a decrease in the population of vitamin D receptors (de Jongh, van Schoor, & Lips, 2017). A national survey conducted on the association between telomere length and serum levels of 25(OH)2D in the United States adult population reported a possible association between 25(OH)D levels and telomere length (Mazidi, Michos, & Banach,2017). A study examining the relationship between plasma 25(OH)2D and relative leukocyte telomere length by sex and race in the US partly supported the findings, whereby, a significant association between vitamin D deficiency (defined as 25(OH)D < 30 nmol/L) and short leukocyte telomere length in whites only was identified, but no association was established between continuous 25(OH)2D and long leukocyte telomere length in all participants (Liu et al., 2018). A general population study, found that age, sex, BMI, and non-Hispanic black race/ethnicity were significant predictors of leukocyte telomere length (Beifuss, Camargo, & Kamycheva, 2017). Notably, a cross- sectional study failed to show any association between 25(OH)D and 1,25(OH)2D and relative leukocyte telomere length (Julin et al., 2017).
As highlighted previously in the paper, DNA telomere length may be influenced by pro-inflammatory cytokines and oxidative stress (vonZglinicki, Burke, & Kirkwood, 2001; Elks & Scotts, 2014). It is believed that the release of reactive oxygen species (ROS) and pro- inflammatory cytokines, which are both induced by increased oxidative stress, damage telomere DNA eventually leading to telomere length shortening (Zhou et al., 2015). Vitamin D as an antioxidant has been shown to ameliorate injury caused by oxidative stress through suppression of the ROS-generating enzyme NADPH oxidase activity (Husain et al., 2015). In the absence of vitamin D, an accumulation of oxidative stress will occur and be likely associated with conditions such as insulin resistance, hypertension, and leukocyte telomere length in the longer term (Demissie et al., 2006). The up regulation of the antioxidant defense may also be involved in regulation of inflammatory mediators.
Evidence shows that 1,25(OH)2D may have a role in several genomic activities, biochemical and enzymatic reactions, whereas 25(OH)D concentrations are critical in overcoming inflammation, destruction of invading pathogens, and the minimization of oxidative stress that occurs through exposure to toxic agents (Wimalawansa, 2019). For instance, optimal concentration of 25(OH)D enhances the expression of the nuclear factor, erythroid-2(Nf-E2)-related factor 2(Nrf2) and enhances the Klotho (a hormone involved in phosphate regulation and also serves as an anti-aging protein), which regulates cellular signaling systems, including the formation of antioxidants (Razzaque, 2012). Vitamin D also reduces the expression of the inflammatory mediator’s interleukin-2 and interferon gamma, effectively limiting the turnover of cells and hence, potentially reducing telomere length (Paul, 2011). A randomized double-blind placebo-controlled clinical trial showed that in vitamin D deficient and overweight women with polycystic ovary syndrome (PCOS), the supplementation of calcium and vitamin D influenced inflammatory and oxidative stress biomarkers (Foroozanfard et al., 2015).
Vitamin D Deficiency and COVID-19
A significant proportion of COVID-19 research has been devoted to understanding the role played by vitamin D. Findings by (Merzon et al., (2020) showed that low plasma 25(OH) vitamin D level was associated with an increased risk of COVID-19 infection. A possible link between vitamin D deficiency and latitude, ethnicity, alongside the impact on cytokines, ACE2, and thrombosis was demonstrated (Rhodes et al., 2020). Optimization of the vitamin D status has also been used widely to enhance immune-protection against COVID-19 (McCartney & Byrne, 2020). Therefore, there is a strong link between COVID-19 and vitamin D deficiency.
Vitamin D Deficiency and COVID-19 Comorbidities
COVID-19 is new phenomenon and, therefore, its direct link to vitamin D deficiency is currently not well understood. A proximate association between vitamin D and COVID-19 can be established through the already understood association between vitamin D and COVID-19 comorbidities. The findings can be used to develop a hypothesis between vitamin D and COVID-19.
Vitamin D deficiency is associated with metabolic syndrome and morbid obesity (Botella-Carretero et al., 2007). A US study that identified a high prevalence of vitamin D deficiency among overweight and obese children (Turer, Lin, & Flores, 2013). In an attempt to explain the link between vitamin D deficiency and obesity, an animal model study suggested that the involvement of vitamin D receptor (VDR) in energy metabolism, implying that certain VDR polymorphisms are associated with obesity (Wong et al., (2011). Experimental data has suggested that vitamin D deficiency could be causing greater adiposity by promoting parathyroid hormone levels and an overflow of calcium into adipocytes and, hence, increasing lipogenesis (Yao et al., 2015). The link between vitamin D deficiency and hypertension and CVD is explained by the fact that vitamin D is an effector of the conversion of angiotensinogen via renin to angiotensin-I and the further conversion to angiotensin-II by the angiotensin-converting enzyme (ACE) (Kota et al., 2011; El MAATY & Gad, 2013). Transgenic mice studies have demonstrated the important role of vitamin D as a negative regulator of renin activity (El MAATY & Gad, 2013). Additionally, vitamin D deficiency may be involved due to the fact that vitamin D plays a role in the regulation of vascular endothelium, as evidenced by its ability to modulate the nitric oxide (NO) system; and through the cardiovascular effects of secondary hyperparathyroidism, which occurs when vitamin D deficiency is left untreated (Lee et al., 2008; El MAATY & Gad, 2013). Vitamin D deficient status is also widely associated with type 2 diabetes or insulin resistance, with various molecular mechanisms put forth in an attempt to explain the association (Ozfirat & Chowdhury, 2010). Evidence suggests that vitamin D may play a role in the insulin secretion, first through the finding of VDR in β cells and vitamin D-dependent calcium-binding proteins (DBP) in pancreatic tissue, secondly due to the fact that vitamin D has been found to play a role in normal insulin release in response to glucose and maintenance of tolerance against glucose and, hence, the finding by several studies that hypovitaminosis D might be playing an important role in the pathogenesis of type 2 diabetes in human beings (Palomer et al., 2008; Szymczak-Pajor & Sliwinska, 2019). In relation to chronic respiratory disease, vitamin D has increasingly been recognized as a prevalent problem. Patients with chronic lung diseases such as asthma, cystic fibrosis, chronic obstructive pulmonary disease (COPD), and intestinal pneumonia are found to be at an increased risk for vitamin D deficiency (Gilbert, Arum, & Smith, 2009; Finklea, Grossmann, & Tangpricha, 2011). Besides evidence showing that vitamin D deficiency is associated with chronic respiratory diseases such as COPD, other studies have reported on the potential beneficial effect of high doses of vitamin D in reducing exacerbations in COPD (Lethouck et al., 2012). Studies in murine model of bone metastasis provide evidence that vitamin D deficiency plays a role in the promotion of cancers, such as breast cancer (Crew et al., 2009). Vitamin D exerts antiproliferative, prodifferentiation, and proapoptotic effects on nonclassic target tissues such as breast and, hence, its deficiency is thought to promote the growth of breast cancer, as demonstrated in the bones of nude mice by (Ooi et al., (2010). Studies conducted in patients suffering from chronic kidney disease also show low levels of circulating vitamin D, in addition to Parathyroid hormone (PTH), calcium, and phosphorous (Levin et al., 2007).
Zinc Deficiency:
Zinc is an essential trace element that has attracted a lot of medical attention due to its role in human nutrition and health. Zinc has three major roles, including catalytic, regulatory, and structural (Myers, Nield, &Myers, 2012). Besides functioning as a structural component in proteins, Zinc is essential for other cellular functions, including cell proliferation, DNA and RNA synthesis, stabilization of cell structures/membranes, redox regulation, and apoptosis (Maywald, Wessels, & Rink, 2017). Zinc is the second most abundant trace element in the human body after iron, but the body lacks the capacity and, hence, the need for regular intake through food or supplementation (Jarosz et al., 2017). The physiological role of zinc is best explained through its association with metallothioneins (MTs), which are cysteine-rich proteins that bind to metal ions such as zinc. The MT as a protein isoform with unique amino acid sequence has affinity to different ions, including Zn2+ and Cu+, mainly functioning as an antioxidant or a radical scavenger (Gammoh & Rink, 2017). Several MTs, including 14 zinc importers (SLC39/ZIPs), and 10 zinc exporters (SLC30/ZnTs) have been described in mammals (Myers, Nield, & Myers, 2012). Mild cellular oxidants readily oxidize MTs, followed by a concomitant release of Zn2+ in a mechanism whereby changes to a more oxidizing conditions leads to the release of zinc, while changes to a more reducing environment results into zinc binding (Krezel & Maret, 2012). Evidence shows that MTs themselves act as potent electrophilic scavengers and cytoprotective agents against inflammation and inflammatory injury, with ability to capture a wide range of ROS, such superoxide, hydroxyl radicals, hydrogen peroxide, and nitric acid (Jarosz et al., 2017). MT traduce signals through the release of zinc ions, which in turn activate intracellular response to antioxidant stress. Zinc deficiency has an inverse relationship with cellular oxidative stress and has also been demonstrated to increase the production of ROS in various cells, such as mouse 3T3 cells, rat C6 glioma cells, human fibroblasts, and epithelial and neuronal cells (Choi, Liu, & Pan, 2018).
Zinc Deficiency and Telomere Length:
The role of zinc in the maintenance of optimal health and genomic stability has been widely studied. Zinc plays a critical role in the activation of poly (ADP-ribose) polymerase that is involved in the repair at DNA damage sites (Paul, 2011). In humans, zinc deficiency has been shown to cause DNA damage. DNA damage may cause telomere shortening by triggering the fusion between chromosomes, which often takes place following telomere cap attrition associated with DNA damage (Paul, 2011). In older individuals, the percentage of cells of with critically shortened telomeres and a decrease in telomere length has been associated with the reduction in the concentration of intracellular labile zinc and zinc-binding protein MT in peripheral blood mononuclear cells (Cipriano et al., 2009). Shortened telomeres may create physiological conditions that mimic old age, possibly leading to the impairment of zinc homeostasis and MT expression, which may in turn contribute to further shortening of telomeres due to the role of zinc in the modulation of telomerase activity (Siren & Siren, 2010). Maternal diet that is deficient in zinc causes chromosomal abnormalities, including a demonstration of fusion between different chromosomes in rat models (Bell et al., 1975). The loss of telomere cap through attrition following DNA damage associated with zinc deficiency has been suggested to be one of the reasons as to why the fusion takes place.
The protective role of zinc against oxidative stress is well-understood. While no direct evidence has been found in relation to the role of zinc in the removal of ROS or free radicals, it is known that dietary zinc deficiency is associated with oxidative damage, which can be reduced through zinc supplementation, which is in turn associated with a decreased incidence of infections in the elderly (Prasad et al., 2007). Experiment in purified systems shows zinc binding decreases the susceptibility of sulfhydryl groups to oxidation (Bray & Bettger, 1990). A continuous correlation between oxidative stress and telomere shortening is fibroblasts has previous established (Richter & von Zglinicki, 2007), with evidence showing that oxidative stress may accelerate shortening (Serra et al., 2000), in addition to shortened telomere length functioning as a marker for chronic oxidative stress (Houben et al., 2008). An experiment conducted by (Serra et al., (2000) showed that the expression of antioxidant enzymes (cGPX and CuZnSOD) in messenger RNA (mRNA) was associated with slower telomere shortening, while lower expression of these enzymes was associated with higher rates of oxidative protein damage, measured in terms of protein carbonyl content. Therefore, lower levels of the enzymes as result of zinc deficiency may directly contribute to oxidative stress, which will in turn lead to telomere shortening.
Zinc Deficiency and COVID-19:
Zinc is known to modulate antibacterial and antiviral immunity, besides regulating the inflammatory response (Skalny et al., 2020). Even with limited evidence, Zinc supplementation was considered as one of the therapeutic approaches in the prevention in COVID-19. The use was based on availability of evidence regarding zinc status and respiratory tract infections (Wessels, Rolles, & Rink, 2020). A study conducted by (Jothimani et al., (2020) showed that zinc deficient COVID-19 patients developed more complications compared to those with a normal zinc status (70.4 % vs 30.0 %, p = 0.009) and, therefore, providing evidence that zinc deficiency played a role in COVID-19.
Zinc Deficiency and Covid-19 Comorbidities:
Zinc deficiency is a common comorbidity in many chronic diseases, including major COVID-19 comorbidities in which the association is mainly described in terms of inflammatory and oxidant induction (Devirgilis et al., 2007). Epidemiological studies show that low zinc intake and low serum levels of zinc are linked to increased prevalence of obesity and its comorbid conditions, such as diabetes and CVD (Garcia, Long, & Rosado, 2009). In addition to the association with a high prevalence of obesity, zinc deficiency has also been associated with increased oxidative stress and the inflammatory response in obese individuals, which includes a link between low superoxide dismutase activity and obese individuals (Tungtrongchitr et al., 2003).As reported by (Chen et al., (1998) fasting plasma zinc concentrations have an inverse relationship to BMI and plasma glucose levels, a finding that also points to a possible role of zinc in diabetes (another key COVID-19 comorbidity). In relation to diabetes, essential dietary zinc and proteins that modulate zinc metabolism play a key role in metabolic homeostasis in peripheral tissues that respond to insulin (Myers, Nield, & Myers, 2012). Mounting evidence shows that insulin resistance may be associated with zinc deficiency, with some of the mechanisms proposed to explain insulin resistance during zinc deficiency including the following: zinc deficiency causes impairment in insulin secretion by the pancreas; zinc deficiency may interfere with insulin receptor binding; zinc deficiency may also lead to decreased insulin receptor synthesis; and that zinc deficiency may cause abnormal glucose carrier structure and/or translocation inside the cell due to increased lipid peroxidation (Salgueiro et al.,2001). Research findings also show that diabetic patients have significantly lower mean serum zinc levels compared with healthy controls, and that zinc supplementation for type-2 diabetics had a beneficial effect of elevating serum zinc level (Al-Maroof& Al-Sharbatti, 2006).
Zinc is also thought to play a role in the development and presentation of hypertension and CVD. Findings show that low dietary intake of zinc correlates with the pathogenesis of hypertension (Chiplonkar et al., 2004). A study conducted by (Williams et al., (2019) found that dietary restriction of zinc reduced the renal bioavailability of Zn2+, effectively promoting the uptake of sodium ions (Na+), which in turn induces hypertension. Zinc deficiency associates with impaired zinc homeostasis through direct effect on levels of intracellular free zinc, which may exacerbate oxidative stress and play a role in the pathogenesis of CVD and other chronic diseases, including diabetes mellitus (Foster & Samman, 2010). Older patients with hypertension and CVD may also present with enhanced inflammatory agents, which may trigger the influx of zinc within cells mediated by specific zinc importers (Liuzzi et al., 2005).
Zinc deficiency may also be linked to chronic kidney disease (CKD). A possible association is through the inflammatory response in obesity, which often includes the loss of zinc from some tissues, such as plasma, and accumulation of zinc in some tissues such as the liver (Lobo et al., 2010). Zinc metabolism abnormalities have long been documented in chronic renal disease, particularly in association with uremia and nephrotic disease (Mahajan, 1989). In cancer, zinc up- regulates telomerase activity, which is associated with unlimited proliferation of cancer cells (Nemoto et al., 2000). Physiologically, tumors require zinc to survive and grow, but the provision of excess zinc may be associated with tumor cell apoptosis, although with a possible variation in sensitivity in different types of tumors (Siren& Siren, 2010).
Glutathione Deficiency:
Glutathione (γ-glutamyl-L-cysteinyl glycine, GSH) refers to the non- protein cell molecule with the most abundance of all sulfhydryl groups, and which is essential for both direct (chemical) and enzymatic neutralization of toxic reactive oxygen species (ROS), specifically ensuring cellular protection against oxidants (Wu et al., 2004). Glutathione is an in vivo synthesized antioxidant, which results from the action of gamma-glutamyl cysteine and GSH synthetase enzymes on glutamate, cysteine, and glycine (Griffith, 1999). Reduced GSH is the most common non-protein thiol in animal cells and has been identified to be a major intracellular antioxidant, facilitating the detoxification of electrophilic compounds and peroxides via glutathione-S-transferases (GST) catalysis (Fraternale et al., 2009). In an oxidative environment, two GSH molecules have their sulfur atoms donating one electron each, a process that results in the conversion of GSH to glutathione disulfide (GSSG), which can in turn be reduced back to the initial GSH molecule through the action of GSSG reductase (GR) (Giustarini et al., 2016). Glutathione deficiency may lead to chronic oxidative stress, which has been associated with the acceleration of senescence (Kurz et al., 2004). Antioxidant activity in plasma is mainly regulated by glutathione peroxidase 3 (GPx3) (Upchurch et al., 1998). Glutathione deficiency, which may be marked by a decrease in the levels of GSH or GSSG in blood may be common in patients with chronic diseases, including cancer, genitourinary, gastrointestinal, cardiovascular disease, and musculoskeletal diseases (Beeh et al., 2002).
Glutathione deficiency and Telomere shortening:
Levels of GSH have traditionally been used as a marker of oxidative status of a cell, which already shows an indirect link between GSH deficiency and telomere length. A study conducted by (Ibanez- Cabellos et al., (2018) showed that reduced levels of GSH and GSSG triggered oxidative stress. Chronic oxidative stress compromises telomere integrity, accelerating the onset of senescence in human endothelial cells (Kurz et al., 2004). (Hadi, Smaism, & Albayati (2016) identified a link between significantly increased levels of glucose, telomerase enzyme, and glutathione peroxidase, suggesting an association between glutathione deficiency and telomere shortening.
Glutathione deficiency and COVID-19:
As COVID-19 is an entirely new phenomenon, not a lot of research evidence is available on the relationship between COVID-19 and glutathione deficiency. Limited evidence indicates that glutathione supplementation enhances redox homeostasis, which improves outcomes in COVID-19 patients (Polonikov, 2020). Glutathione deficiency is therefore a common finding in both telomeres shortening and COVID-19.
Glutathione Deficiency and COVID-19 Comorbidities: A decrease GSH levels is generally observed in chronic diseases (Lang et al., 2000; Kurz et al., 2004). The fact that glutathione deficiency associates with increased oxidative stress, a well- recognized etiological factor in the chronic diseases, leads to implication of possible glutathione deficiency in the different COVID-19 comorbidities. GPx3 is shown to be highly expressed in adipose tissue, and a reduction in its expression is found in obese subjects, which further contributes to local and systematic oxidative stress (Lee et al., 2008). Other studies have shown that the progression of morbid obesity may be associated with glutathione peroxidase 1 (GPx1) (Carazo et al., 2011). Glutathione plays a role in nitric oxide metabolism, which facilitates the control of blood pressure, in glutathione deficient states an increase in oxidative is found and is well-recognized etiological factor in the developmentof hypertension (Robaczewska et al., 2016). In glutathione-deficient CVD patients, a significant reduction in antioxidant status is found, with a concomitant increase in the concentration of lipid peroxidation products, providing evidence that the glutathione antioxidant system plays an important role in the prevention of development and progression of CVD (Karajibani et al., 2009). In patients suffering from type 2 diabetes, altered glycemic mechanisms have been associated accelerated glutathione utilization and depletion (Lutchmansingh et al., 2018). The findings suggest that glutathione deficiency may have a role to play in the development and progression of diabetes. In relation to chronic lung disease, oxidant/antioxidant imbalance is regarded as the major cause of cell damage, which is forms the hallmark for lung inflammation, with alterations alveolar and lung GSH metabolism recognized as a central feature in numerous chronic inflammatory lung diseases (Rahman, 2005).
Key Mechanisms Involved in COVID-19, its Comorbidities, and Shortened Telomere Length
A number key COVID-19 comorbidities included in the present paper have comparable pathophysiological pathways that are suspected to associate with poor prognosis in COVID-19 disease. Some of the mechanisms are also evident in shortened telomere length. Common mechanisms that contribute to the pathophysiology of age-related diseases involve oxidative stress and inflammation.
Oxidative Stress
The production of oxygen reactive species (ROS) usually occurs as an aspect of aerobic metabolism, and environmental factors such as cigarette smoking, and inhalation of air pollutants. Aerobic metabolism to generate ATP, includes oxidative phosphorylation (OXPHOS) that has ROS as an inevitable byproduct (Schiavi & Ventura, 2014). ROS are potent reactive molecules that can alter the function of or cause damage to cell structures, including carbohydrates, lipids, nucleic acids, proteins (Birben et al., 2012). In healthy aerobic organisms, the production of ROS is usually balanced with the antioxidant defense system. Oxidative stress can be described as a shift in the balance between oxidants and antioxidants in the favor of oxidants (Birben et al., 2012). The regulation of the redox (oxidizing) state is critical for the viability, activation, and proliferation of cells and, hence, overall organ function. While an integrated antioxidant system is present in aerobic organisms, including enzymatic and non-enzymatic antioxidants that effectively block the effects of ROS, pathological conditions may cause the overwhelming of the antioxidant system (Birben et al., 2012). In fact, oxidative stress, may modulate different health conditions that will negatively impact on telomere length and associated with chronic diseases (Mundstock et al., 2015).
Oxidative Stress, COVID-19 and COVID-19 Comorbidities:
Studies on the link between COVID-19 and oxidative stress are still on-ongoing. However, published scientific evidence shows that changes observed in COVID-19 patients show involvement of oxidative stress, including coagulopathy, cell hypoxia, and amplification and perpetuation of the cytokine storm (Cechini & Cecchini, 2020). Nevertheless, it is fairly understood that COVID-19 comorbidities, including diabetes, hypertension, cancer, and cardiovascular diseases, and severe asthma may involve oxidative stress and inflammation. Demissie et al., (2006) reported on the link between insulin resistance, oxidative stress, hypertension, and leukocyte telomere length. Using terminal restriction fragment (TRF) length, the authors explored how leukocyte telomere length was associated with insulin resistance, hypertension, and oxidative stress. Based on their findings, the researchers reported that hypertension, insulin resistance, and oxidative stress were associated with shorter leukocyte telomere length (Demissie et al., 2006). A study showed that arterial telomere uncapping could be playing a role in hypertension through tumor suppressor protein P53 (P53)/ cyclin- dependent kinase inhibitor 1A (P21) induced senescence (Morgan et al., 2014). Further logistic regression conducted to compare significant predictors of a hypertension status, and the results showed that localized telomeres and BMI status were both significant predictors of a hypertension status (Morgan et al., 2014). An increase in pulse pressure and stiffness of the central arteries are found associated with aging, and also serve as independent indicators of cardiovascular risk (Jeanclose et al., 2000; Benetos et al., 2001). (Fyhrquist et al., (2011), investigated the relationship between leukocyte telomere length and risk of cardiovascular diseases in high- risk hypertensive populations. The findings of the study showed that leukocyte telomere length was associated with risk factors for type 2 diabetes and served as a predictor of cardiovascular disease in elderly patients with hypertension (Fyhrquist et al., 2011). Studies have also shown that short telomere length was associated with obesity parameters, with some findings indicating that there was a gender difference in relation how the obesity phenotype was related to telomere length (Nordfjall et al., 2008). Chronic asthma, another key COVID-19 comorbidity, has significantly been associated with other chronic conditions in older adults, including obstructive pulmonary disease, cardiovascular disease, and cancer, in addition to creating a substantial risk for early mortality (Iribarren et al., 2012; Belsky et al., 2014). Using a cohort of 203, 595 Northern California adults with asthma and a parallel asthma-free cohort, (Iribarren et al., (2012) showed that asthma was prospectively associated with an increased risk of major cardiovascular disease (CVD). Obesity, a key COVID- 19 comorbidity is known to alter metabolic process in a manner that leads to the establishment of the other COVID-19 comorbidities. A study conducted by (Cattan et al., (2008) showed that chronic oxidative stress induced through the depletion of tissue glutathione resulted into telomere length attrition, and thus contributed to the understanding of the link between glutathione deficiency, oxidative stress, and telomere length.
Link Between Oxidative Stress, Metabolic Syndrome, and COVID-19 Comorbidities:
A dysfunction in the nutrient and pathogen-sensing mechanisms often leads to a cluster of chronic metabolic disorders, particularly obesity, type 2 diabetes, and cardiovascular disease (Hotamisligil, 2006). Obesity has been identified as a key common risk factor for many conditions, including hypertension, diabetes, and cardiovascular diseases. People suffering from obesity have been found to have increased levels of oxidative stress and inflammation (Furukawa et al., 2013; Monteiro & Azevedo, 2010). According to (Furukawa et al., (2013) fat accumulation correlates with oxidative stress in both humans and mice, which is augmented by the expression of NADPH oxidase and the decreased expression of antioxidative enzymes. According to (Lobo et al., (2010) adipose tissue is an important producer of several hormones, growth factors, and cytokines (such as leptin, adipsin, resistin, angiotensinogen, TNF-α, plasminogen activator inhibitor type-1, and IL-6). As such, obese individuals may experience challenges associated with the regulation of adipose- tissue-generated hormones, growth factors, and cytokines. Obesity has been identified to be an important risk factor for metabolic syndrome; a cluster of conditions that occur together, increasing the risk of heart disease, stroke, and type 2 diabetes, among other conditions.
Figure 1: Metabolic Syndrome and Oxidative stress
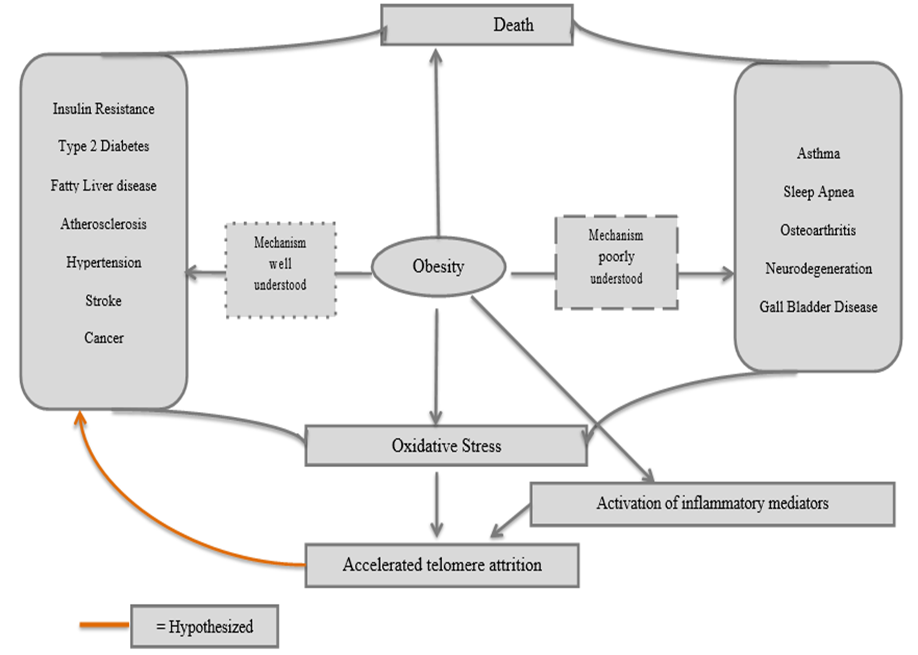
*With obesity at the center, the relationship between severe COVID- 19 comorbidities, oxidative stress and inflammatory mediators, and telomere length is clarified.
As hypothesized by (Furukawa et al. (2004) and confirmed in later studies, obesity is causative agent in the development of metabolic syndrome. Key disturbances implicated in metabolic syndrome include glucose intolerance, central obesity, dyslipidemia (hypertriglyceridemia, elevated nonesterified fatty acids [NEFAs], and decreased high-density lipoprotein [HDL] cholesterol), and hypertension (Azevedo et al., 2009). While the origin of metabolic syndrome is not yet clarified, there is increasing evidence that obesity (as shown in the flow chart above) may be one of the factors (Monteiro & Azevedo, 2010). Evidence shows that phagocytic NADPH oxidase overactivity accounts for oxidative stress in metabolic syndrome (Fortuna et al., 2006). This is coupled with the decreased production of antioxidative enzymes and elevated levels of fatty acids as a result of dysregulated production of adipocytokines (fat-derived hormones) such as adiponectin, plasminogen activator inhibitor–1, IL-6, and monocyte chemotactic protein–1, causes a further increase in oxidative stress (Furukawa et al. 2014). The resultant oxidative stress is then thought to play a critical role in the pathogenesis of different diseases (Brownlee, 2001). In the diabetic condition, evidence shows that oxidative stress leads to impaired glucose uptake in muscles and fat (Maddux et al., 2001; Rudich et al., 1998). As described by Matsuoka et al., (1997), oxidative stress also leads to decreased insulin secretion from pancreatic β cells and is also underlies the pathophysiology of other conditions such as hypertension and atherosclerosis as explained by (Vasdev, Gill, & Singal, (2006).
Inflammatory Response, COVID-19, and COVID-19 Comorbidities:
Inflammatory and oxidative processes are considerably involved in cellular homeostasis, and the two biomarkers may have a concomitant Figure 1: Graphic representation of the cytokine storm or independent relationship with telomere attrition and COVID-19 comorbidities. The inflammatory response initially involves the acute phase response (APR), which describes how the body responds to any tissue damage and infection through a series of specific physiological reactions in its attempt to repair the damage caused by offending organisms, promote wound healing, and recruit host defense mechanisms, including the innate immune response (Black, 2003). Acute inflammation involves the recruitment of leucocytes from the circulation, described as the initial trafficking of polymorphonuclear granulocytes and monocytes, followed by local differentiation into macrophages (Lawrence & Gilroy, 2007). Acute phase proteins (APPs) include “positive” APPs that are up-regulated in response to injury or infection or “negative” acute phase proteins that are down- regulated in response to in response to injury or infection. Cytokines, and particularly IL-6, are the primary inducers of APR. Studies on inflammatory markers, such as C-reactive protein (CRP) and the proinflammatory cytokines TNFa and IL-6, have implicated the inflammatory pathway as one of the underlying pathogenic mechanisms in many diseases (Arida et al., 2018).
Acute inflammation in COVID-19:
Following a COVID-19 infection, an inflammatory response is mounted within 7 to 10 days to limit inflection spread (Manjili & Manjili, 2020). Uncontrolled inflammation has been directly implicated in COVID-19 and is currently regarded as a key predictor of severe disease and adverse COVID-19 outcomes (Burke et al., 2020). Unmodulated acute inflammatory response in COVID-19 results in “cytokine storm”, often seen in sepsis, COVID-19, and other severe respiratory disease caused by coronaviruses (Manjili & Manjili, 2020). In COVID-19, cytokine storm is manifested as a hyperproduction of pro-inflammatory cytokines, including IL-1, IL- 6, IL-12, IFN-γ, and TNF-α, which are found in significantly higher levels in intensive care unit (ICU) admitted COVID-19 patients (Costela-Ruiz et al., 2020).
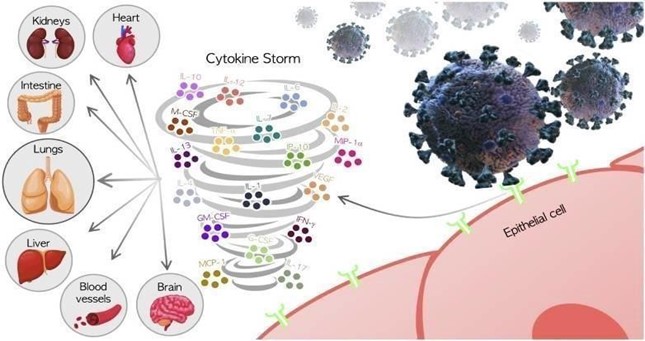
Source: Costela-Ruiz, 2020.
Chronic Inflammation Metabolic Syndrome, Shortened Telomere length, and COVID-19 Comorbidities
Chronic inflammation can be described as a failure in the resolution of acute inflammation, especially in relation to the clearance of macrophages from inflamed site (Lawrence & Gilroy, 2007). During the chronic inflammation phase, cytokine interactions result in monocyte chemotaxis to the cite of inflammation, where macrophage activating factors, including IFN-γ, MCP-1, activate the macrophages while migration inhibition factors (MIF), such as GM- CSF (38) and IFN-γ, retain them at the inflammatory site (Feghali & Wright, 1997). Oxidative stress may lead to inflammation through the activation of several transcription of factors, which result into the differential expression of various genes (Hussain et al., 2016). Chronic inflammation may also be associated with telomere shortening (Shin & Shin, 2019).
Obesity and the metabolic syndrome are also accompanied by a peculiar inflammatory state that is not accompanied by infection, autoimmunity, or massive tissue loss (Monteiro & Azevedo, 2010). A section of researchers has attempted to coin the name “metaflammation” to refer to the fact that the inflammation is triggered metabolically. Recent studies have shown a relationship between obesity indices and inflammatory markers, such as C - reactive protein (CRP), where a high level of CRP in the blood is used as a marker of inflammation. According to, obesity, insulin resistance, and type 2 diabetes are closely associated with chronic “inflammation”, which is often characterized by abnormal cytokine production, increased acute-phase reactants and other mediators, in addition to the activation of a network of inflammatory signaling pathways (Wellen, 2005). According to (Lobo et al., (2010), during the inflammatory response associated with obesity and chronic kidney disease (CKD), internal redistribution of zinc occurs, including the loss of zinc from some tissues (such as plasma) and accumulation of zinc in some tissues such as the liver. The changes in redistribution may present serious structural and functional challenges in zinc-dependent physiological processes. A link between zinc deficiency, oxidative stress, and inflammation can be established in certain individuals suffering from obesity.
Figure 2: Association between Obesity, Zinc Deficiency, Oxidative Stress and Inflammation
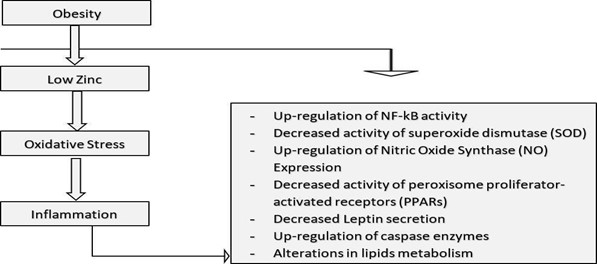
It is not well understood whether telomere length predicts metabolic alterations over an extended period of time as most studies have tended to examine cross-sectional and longitudinal relationships between telomere length and components of metabolic syndrome. Even so, there is a general agreement that shorter telomere length at baseline, cross-sectionally associates with a worse metabolic profile and with worse predictable trajectories for metabolic syndrome (Revesz et al., 2014).
Telomere Length and Respiratory Infections:
COVID-19 is one of the many infectious diseases that affect the respiratory system. The nature of COVID-19 infection and pathogenesis in the lower and upper respiratory systems is largely comparable to other pathogenic respiratory infections and, hence, existing evidence on association between telomere length and respiratory infections may be indicative on the role of telomere length in COVID-19. The relationship between telomere length and respiratory infections has been explored by a number of studies. An investigation on the risk of experimentally induced upper respiratory infection in healthy adults and leukocyte telomere length showed that shorter leukocyte telomere length, particularly in CD8 and CD28 cells, was associated with an increased risk of infection and clinical illness (Cohen et al., 2013). The hypothesis was partly supported by Kiecolt-Glaser et al., (2013) by showing that telomere length acted as a limited mediator in the association between childhood socioeconomic status susceptibility to upper respiratory infections.
Evidence Summary: COVID-19 and Telomere Length: Based on the analysis conducted in the previous sections, there is evidence of an association between telomere shortening and COVID- 19, and includes shared deficiencies, mechanisms, finding.
Table 3: Biomarkers of Short Telomere Length (STL) and Association with COVID-19
|
STL Biomarker |
Evidence |
Findings in COVID-19 |
|
Oxidative Stress |
Correlation between telomere shortening in fibroblasts and oxidative stress established; shortened telomere length long-identified as a marker of oxidative stress; experimental data shows that reduced expression of antioxidant enzymes (cGPX and CuZnSOD) in messenger RNA (mRNA) increases rate of telomere shortening (Richter & von Zglinicki, 2007; Houben et al., 2008; Serra et al.,2000). Oxidative stress is also implicated in major COVID-19 comorbidities, particularly through metabolic syndrome trajectories (Revesz et al., 2014). |
Preliminary studies show that oxidative stress may be involved in the pathogenesis of COVID-19, and its effect ameliorated through Vitamin D supplementation (McCartney & Byrne, 2020; Cocchini & Cochini, 2020). |
|
Inflammation (Chronic and Acute) |
There is no evidence on direct link between acute inflammation and telomere length. Chronic inflammation is however suggested to contribute to inflammation, besides being associated with oxidative stress (Hussain et al., 2016; Shin & Lee, 2019). |
Uncontrolled inflammation is widely reported in COVID-19 (Burke et al., 2020). Numerous hyperinflammatory cytokines observed in COVID-19, including pro- inflammatory cytokines, including IL-1, IL-6, IL-12, IFN-γ, and TNF-α. Chronic conditions such as COPD, that are characterized by chronic lung inflammation also contribute to adverse COVID-19 outcomes. |
|
Glutathione Deficiency |
Reduced levels of the GSH is a key cellular oxidative stress. Chronic oxidative stress compromises telomere integrity (Kurz et al., 2004). Correlated changes in the levels of telomerase enzyme, glutathione peroxidase, and glucose point to potential role for GSH deficiency in telomere shortening (Hadi, Smaism, & Albayati, 2016). GSH deficiency observed in major COVID-19 comorbidities, including obesity, diabetes, CVD, inflammatory lung disease (Lee et al., 2008; Karajibani et al., 2009; Lutchmansingh et al., 2018; Rahman, 2005) |
Glutathione deficiency suggested to be the likely cause of serious manifestations and death in COVID-19 (Polonikov, 2020) |
|
Zinc Deficiency |
Zinc Deficiency causes DNA damage, which results into telomere shortening ((Paul, 2011). In older adults, decreased telomere length is associated with reduction in the concentration of intracellular labile zinc and zinc- binding protein MT in peripheral blood mononuclear cells (Cipriano et al.,2009). Zinc deficiency promotes ROS production in mouse 3T3 cells, rat C6 glioma cells, human fibroblasts, and epithelial neural cells (Choi, Liu, & Pan, 2018). The link between ROS and shortened telomere length is well established. |
Zinc is directly involved in the modulation of antiviral immunity through regulation of inflammatory response (Skalny et al., 2020). Zinc- deficient COVID-19 patients developed |
|
Vitamin D Deficiency |
Studies have largely failed to show a relationship between telomere shortening and both 25(OH)D and 1,25(OH)D (Julin et al., 2017). Vitamin D deficiency is however correlated with major COVID-19 comorbidities, including obesity (Botella-Carretero et al., 2007; Turer, Lin, & Flores, 2013), type 2 diabetes (Ozfirat & Chowdhury, 2010; Palomer et al., 2008; Szymczak-Pajor & Sliwinska, 2019), hypertension and CVD (Kota et al., 2011; El MAATY & Gad, 2013), COPD (Gilbert, Arum, & Smith, 2009; Finklea, Grossmann, & Tangpricha, 2011), cancer (Ooi et al.,2010), and chronic kidney disease (phosphorous (Levin et al., 2007). Most of the comorbidities are correlated with shortened telomere length. |
Vitamin D improves immune protection against COVID-19, implying aa relationship between vitamin D deficiency and COVID- 19, likely through the antioxidant pathway (McCartney & Byrne,2020). |
|
Respiratory Infections |
Increased susceptibility to respiratory infections in shortened telomere length (Kiecolt-Glasser et al., 2013; Cohen et al., 2013) |
Besides COVID-19 being a respiratory disease, chronic respiratory diseases, including asthma and chronic obstructive pulmonary disease, are comorbidities for severe COVID-19 disease (Richardson et al., 2020). |
Conclusion
The reviewed literature shows that COVID-19 comorbidities may be triggered by predictable trajectories of metabolic disturbances that occur in patients with shortened telomere length. The findings show that various biomarkers, including vitamin D deficiency, Zinc deficiency, glutathione deficiency, oxidative stress, and chronic inflammation are directly or indirectly correlated with both shortened telomere length and severity of COVID-19.
References
- Albrecht, E., Sillanpää, E., Karrasch, S., Alves, A. C., Codd, V., Hovatta, I., ... & Mangino, M. (2014). Telomere length in circulating leukocytes is associated with lung function and disease. European Respiratory Journal, 43(4), 983-992.
- Al-Maroof, R. A., & Al-Sharbatti, S. S. (2006). Serum zinc levels in diabetic patients and effect of zinc supplementation on glycemic control of type 2 diabetics. Saudi medical journal, 27(3), 344.
- Allsopp, R. C., Vaziri, H., Patterson, C., Goldstein, S., Younglai, E. V., Futcher, A. B., ... & Harley, C. B. (1992). Telomere length predicts replicative capacity of human fibroblasts. Proceedings of the National Academy of Sciences, 89(21), 10114-10118.
- Arida, A., Protogerou, A. D., Kitas, G. D., & Sfikakis, P. P. (2018). Systemic inflammatory response and atherosclerosis: the paradigm of chronic inflammatory rheumatic diseases. International journal of molecular sciences, 19(7), 1890.
- Atkins, J.L., Masoli, J.A., Delgado, J., Pilling, L.C., Kuo, C.L., Kuchel, G.A. and Melzer, D., 2020. Preexisting comorbidities predicting COVID-19 and mortality in the UK Biobank community cohort. The Journals of Gerontology: Series A.
- Azevedo, A., Santos, A. C., Ribeiro, L., & Azevedo, I. (2009). The metabolic syndrome. In Oxidative stress, inflammation and angiogenesis in the metabolic syndrome (pp. 1-19). Springer, Dordrecht.
- Beeh, K. M., Beier, J., Haas, I. C., Kornmann, O., Micke, P., & Buhl, R. (2002). Glutathione deficiency of the lower respiratory tract in patients with idiopathic pulmonary fibrosis. European Respiratory Journal, 19(6), 1119-1123.
- Beilfuss, J., Camargo Jr, C. A., & Kamycheva, E. (2017). Serum 25-hydroxyvitamin D has a modest positive association with leukocyte telomere length in middle-aged US adults. The Journal of Nutrition, 147(4), 514-520.
- Bell, L. T., Branstrator, M., Roux, C., & Hurley, L. S. (1975). Chromosomal abnormalities in maternal and fetal tissues of magnesium‐orzinc‐deficient rats. Teratology,12(3), 221-226.
- Belsky, D. W., Shalev, I., Sears, M. R., Hancox, R. J., Lee Harrington, H., Houts, R., ... & Caspi, A. (2014). Is chronic asthma associated with shorter leukocyte telomere length at midlife?. American journal of respiratory and critical care medicine, 190(4), 384-391.
- Benetos, A., Okuda, K., Lajemi, M., Kimura, M., Thomas, F., Skurnick, J., ... & Aviv, A. (2001). Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension, 37(2), 381-385.
- Black, P. H. (2003). The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain, behavior, and immunity, 17(5), 350-364.
- Blackburn, E. H. (1991). Structure and function of telomeres. Nature, 350(6319), 569-573.
- Botella-Carretero, J. I., Alvarez-Blasco, F., Villafruela, J. J., Balsa, J. A., Vázquez, C., & Escobar-Morreale, H. F. (2007). Vitamin D deficiency is associated with the metabolic syndrome in morbid obesity. Clinical Nutrition, 26(5), 573-580.
- Bray, T. M., & Bettger, W. J. (1990). The physiological role of zinc as an antioxidant. Free Radical Biology and Medicine, 8(3), 281-291.
- Brownlee, M. (2001). Biochemistry and molecular cell biology of diabetic complications. Nature, 414(6865), 813-820.
- Burke, H., Freeman, A., Cellura, D. C., Stuart, B. L., Brendish, N. J., Poole, S., ... & Spalluto, C. M. (2020). Inflammatory phenotyping predicts clinical outcome in COVID-19. Respiratory research, 21(1), 1-9.
- Campisi, J. (2005). Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell, 120(4), 513- 522.
- Carazo, A., León, J., Casado, J., Gila, A., Delgado, S., Martín, A., ... & Ruiz-Extremera, A. (2011). Hepatic expression of adiponectin receptors increases with non-alcoholic fatty liver disease progression in morbid obesity in correlation with glutathione peroxidase 1. Obesity surgery, 21(4), 492-500.
- Cattan, V., Mercier, N., Gardner, J. P., Regnault, V., Labat, C., Mäki-Jouppila, J., ... & Lacolley, P. (2008). Chronic oxidative stress induces a tissue-specific reduction in telomere length in CAST/Ei mice. Free Radical Biology and Medicine, 44(8), 1592- 1598.
- Cecchini, R., & Cecchini, A. L. (2020). SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Medical hypotheses, 143, 110102.
- Centers for Disease Control and Prevention (2020). Groups at Higher Risk for Severe Illness. CDC. Retrieved from https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/groups-at-higher-risk.html
- Chen, M. D., Liou, S. J., Lin, P. Y., Yang, V. C., Alexander, P. S., & Lin, W. H. (1998). Effects of zinc supplementation on the plasma glucose level and insulin activity in genetically obese (ob/ob) mice. Biological Trace Element Research, 61(3), 303-311.
- Chiplonkar, S. A., Agte, V. V., Tarwadi, K. V., Paknikar, K. M., & Diwate, U. P. (2004). Micronutrient deficiencies as predisposing factors for hypertension in lacto-vegetarian Indian adults. Journal of the American College of Nutrition, 23(3),239-247.
- Cipriano, C., Tesei, S., Malavolta, M., Giacconi, R., Muti, E., Costarelli, L., ... & Vera, E. (2009). Accumulation of cells with short telomeres is associated with impaired zinc homeostasis and inflammation in old hypertensive participants. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 64(7), 745-751.
- Choi, S., Liu, X., & Pan, Z. (2018). Zinc deficiency and cellular oxidative stress: prognostic implications in cardiovascular diseases. Acta Pharmacologica Sinica, 39(7), 1120-1132.
- Cohen, S., Janicki-Deverts, D., Turner, R. B., Casselbrant, M. L., Li-Korotky, H. S., Epel, E. S., & Doyle, W. J. (2013). Association between telomere length and experimentally induced upper respiratory viral infection in healthy adults. Jama, 309(7), 699-705.
- Costela-Ruiz, V. J., Illescas-Montes, R., Puerta-Puerta, J. M., Ruiz, C., & Melguizo-Rodríguez, L. (2020). SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine & Growth Factor Reviews.
- Crew, K. D., Shane, E., Cremers, S., McMahon, D. J., Irani, D., & Hershman, D. L. (2009). High prevalence of vitamin D deficiency despite supplementation in premenopausal women with breast cancer undergoing adjuvant chemotherapy. Journal of Clinical Oncology, 27(13), 2151.
- de Jongh, R. T., van Schoor, N. M., & Lips, P. (2017). Changes in vitamin D endocrinology during aging in adults. Molecular and cellular endocrinology, 453, 144-150.
- Demissie, S., Levy, D., Benjamin, E. J., Cupples, L. A., Gardner, J. P., Herbert, A., ... & Aviv, A. (2006). Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging cell, 5(4), 325-330.
- Devirgiliis, C., Zalewski, P. D., Perozzi, G., & Murgia, C. (2007). Zinc fluxes and zinc transporter genes in chronic diseases. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 622(1-2), 84-93.
- Elks, C. E., & Scott, R. A. (2014). The long and short of telomere length and diabetes. Diabetes, 63(1), 65-67.
- EL MAATY, M. A. A., & Gad, M. Z. (2013). Vitamin D deficiency and cardiovascular disease: potential mechanisms and novel perspectives. Journal of nutritional science and vitaminology, 59(6), 479-488.
- Engelsen, O., Brustad, M., Aksnes, L., & Lund, E. (2005). Daily duration of vitamin D synthesis in human skin with relation to latitude, total ozone, altitude, ground cover, aerosols and cloud thickness. Photochemistry and photobiology, 81(6), 1287-1290.
- Fang, L., Karakiulakis, G., & Roth, M. (2020). Are patients with hypertension and diabetes mellitus at increased risk for COVID- 19 infection?. The Lancet. Respiratory Medicine, 8(4), e21.
- Faner, R., Rojas, M., MacNee, W., & Agustí, A. (2012). Abnormal lung aging in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. American journal of respiratory and critical care medicine, 186(4), 306-313.
- Feghali, C. A., & Wright, T. M. (1997). Cytokines in acute and chronic inflammation. Front Biosci, 2(1), d12-d26.
- Finklea, J. D., Grossmann, R. E., & Tangpricha, V. (2011). Vitamin D and chronic lung disease: a review of molecular mechanisms and clinical studies. Advances in Nutrition, 2(3), 244- 253.
- Fitzpatrick, A. L., Kronmal, R. A., Gardner, J. P., Psaty, B. M., Jenny, N. S., Tracy, R. P., ... & Aviv, A. (2007). Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. American journal of epidemiology, 165(1), 14-21.
- Foroozanfard, F., Jamilian, M., Bahmani, F., Talaee, R., Talaee, N., Hashemi, T., ... & Esmaillzadeh, A. (2015). Calcium plus vitamin D supplementation influences biomarkers of inflammation and oxidative stress in overweight and vitamin D‐deficient women withpolycysticovarysyndrome:arandomizeddouble‐blindplacebo ‐ controlled clinical trial. Clinical endocrinology, 83(6), 888-894.
- Fortuño, A., San José, G., Moreno, M. U., Beloqui, O., Díez, J., & Zalba, G. (2006). Phagocytic NADPH oxidase overactivity underlies oxidative stress in metabolic syndrome. Diabetes, 55(1), 209-215.
- Foster, M., & Samman, S. (2010). Zinc and redox signaling: perturbations associated with cardiovascular disease and diabetes mellitus. Antioxidants & redox signaling, 13(10), 1549-1573.
- Fyhrquist, F., Silventoinen, K., Saijonmaa, O., Kontula, K., Devereux, R. B., de Faire, U., ... & Dahlöf, B. (2011). Telomere length and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. Journal of human hypertension, 25(12), 711-718.
- Fraternale, A., Paoletti, M. F., Casabianca, A., Nencioni, L., Garaci, E., Palamara, A. T., & Magnani, M. (2009). GSH and analogs in antiviral therapy. Molecular aspects of medicine, 30(1-2), 99-110.
- Furukawa, S., Fujita, T., Shimabukuro, M., Iwaki, M., Yamada, Y., Nakajima, Y., ... & Shimomura, I. (2017). Increased oxidative stress in obesity and its impact on metabolic syndrome. The Journal of clinical investigation, 114(12), 1752-1761.
- Fuster, J. J., Díez, J., & Andres, V. (2007). Telomere dysfunction in hypertension. Journal of hypertension, 25(11), 2185- 2192.
- Gammoh, N. Z., & Rink, L. (2017). Zinc in infection and inflammation. Nutrients, 9(6), 624.
- García, O. P., Long, K. Z., & Rosado, J. L. (2009). Impact of micronutrient deficiencies on obesity. Nutrition reviews, 67(10), 559- 572.
- Gilbert, Christopher R., Seth M. Arum, and Cecilia M. Smith. "Vitamin D deficiency and chronic lung disease." Canadian respiratory journal 16 (2009).
- Giustarini, D., Tsikas, D., Colombo, G., Milzani, A., Dalle- Donne, I., Fanti, P., & Rossi, R. (2016). Pitfalls in the analysis of the physiological antioxidant glutathione (GSH) and its disulfide (GSSG) in biological samples: An elephant in the room. Journal of Chromatography B, 1019, 21-28
- Greider, C. W. (1996). Telomere length regulation. Annual review of biochemistry, 65(1), 337-365.
- Griffith, O. W. (1999). Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radical Biology and Medicine, 27(9-10), 922-935.
- Hadi, D. M., Smaism, M. F., & Albayati, A. H. (2016) Telomerase enzyme and glutathione peroxidase 1gene as a risk factor in diabetes mellitus type 1 patients in Babylon province. International Journal of ChemTech Research. 9(12) pp 668-680,
- Haycock, P. C., Heydon, E. E., Kaptoge, S., Butterworth, A. S., Thompson, A., & Willeit, P. (2014). Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta- analysis. Bmj, 349.
- Hemann, M. T., Strong, M. A., Hao, L. Y., & Greider, C. W. (2001). The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell, 107(1), 67-77.
- Holick, M. F. (2007). Vitamin D deficiency. New England Journal of Medicine, 357(3), 266-281.
- Holick, M. F., & Chen, T. C. (2008). Vitamin D deficiency: a worldwide problem with health consequences. The American journal of clinical nutrition, 87(4), 1080S-1086S.
- Houben, J. M., Moonen, H. J., van Schooten, F. J., & Hageman, G. J. (2008). Telomere length assessment: biomarker of chronic oxidative stress? Free radical biology and medicine, 44(3), 235-246.
- Husain, K., Hernandez, W., Ansari, R. A., & Ferder, L. (2015). Inflammation, oxidative stress and renin angiotensin system in atherosclerosis. World journal of biological chemistry, 6(3), 209.
- Hussain, T., Tan, B., Yin, Y., Blachier, F., Tossou, M. C., & Rahu, N. (2016). Oxidative stress and inflammation: what polyphenols can do for us? Oxidative medicine and cellular longevity, 2016.
- Ibáñez-Cabellos, J. S., Pérez-Machado, G., Seco-Cervera, M., Berenguer-Pascual, E., García-Giménez, J. L., & Pallardó, F. V. (2018). Acute telomerase components depletion triggers oxidative stress as an early event previous to telomeric shortening. Redox biology, 14, 398-408.
- Iribarren, C., Tolstykh, I. V., Miller, M. K., Sobel, E., & Eisner, M. D. (2012). Adult asthma and risk of coronary heart disease, cerebrovascular disease, and heart failure: a prospective study of 2 matched cohorts. American journal of epidemiology, 176(11), 1014- 1024.
- Jarosz, M., Olbert, M., Wyszogrodzka, G., Młyniec, K., & Librowski, T. (2017). Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology, 25(1), 11-24.
- Jeanclos, E., Schork, N. J., Kyvik, K. O., Kimura, M., Skurnick, J. H., & Aviv, A. (2000). Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension, 36(2), 195-200.
- Jothimani, D., Kailasam, E., Danielraj, S., Nallathambi, B., Ramachandran, H., Sekar, P., ... & Rela, M. (2020). COVID-19: Poor outcomes in patients with Zinc deficiency. International Journal of Infectious Diseases, 100, 343-349.
- Jordan, R. E., Adab, P., & Cheng, K. K. (2020). Covid-19: risk factors for severe disease and death. BMJ , 368:m1198.
- Julin, B., Shui, I. M., Prescott, J., Giovannucci, E. L., & De Vivo, I. (2017). Plasma vitamin D biomarkers and leukocyte telomere length in men. European journal of nutrition, 56(2), 501-508.
- Karajibani, M., Hashemi, M., Montazerifar, F., Bolouri, A., & Dikshit, M. (2009). The status of glutathione peroxidase, superoxide dismutase, vitamins A, C, E and malondialdehyde in patients with cardiovascular disease in Zahedan, Southeast Iran. Journal of nutritional science and vitaminology, 55(4), 309-316.
- Kiecolt-Glaser, J. K., Jaremka, L. M., Derry, H. M., & Glaser, R. (2013). Telomere length: a marker of disease susceptibility?. Brain, behavior, and immunity, 34, 29.
- Krężel, A., & Maret, W. (2017). The functions of metamorphic metallothioneins in zinc and copper metabolism. International journal of molecular sciences, 18(6), 1237.
- Kurz, D. J., Decary, S., Hong, Y., Trivier, E., Akhmedov, A., & Erusalimsky, J. D. (2004). Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. Journal of cell science, 117(11), 2417-2426.
- Lang, C. A., Mills, B. J., Mastropaolo, W., & Liu, M. C. (2000). Blood glutathione decreases in chronic diseases. Journal of Laboratory and Clinical Medicine, 135(5), 402-405.
- Lawrence, T., & Gilroy, D. W. (2007). Chronic inflammation: a failure of resolution? International journal of experimental pathology, 88(2), 85-94.
- Li, Y., Guo, F., Cao, Y., Li, L., & Guo, Y. (2020). Insight into COVID‐2019forpediatricians.Pediatricpulmonology,55(5),E1-E4.
- Lee, J. H., O'Keefe, J. H., Bell, D., Hensrud, D. D., & Holick, M. F. (2008). Vitamin D deficiency: an important, common, and easily treatable cardiovascular risk factor? Journal of the American College of Cardiology, 52(24), 1949-1956.
- Lee, Y. S., Kim, A. Y., Choi, J. W., Kim, M., Yasue, S., Son, H. J., ... & Kim, J. B. (2008). Dysregulation of adipose glutathione peroxidase 3 in obesity contributes to local and systemic oxidative stress. Molecular endocrinology, 22(9), 2176-2189.
- Lehouck, A., Mathieu, C., Carremans, C., Baeke, F., Verhaegen, J., Van Eldere, J., ... & Janssens, W. (2012). High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Annals of internal medicine, 156(2), 105- 114.
- Liu, J. J., Cahoon, E. K., Linet, M. S., Little, M. P., Dagnall, C. L., Higson, H., ... & Freedman, D. M. (2018). Relationship between plasma 25-hydroxyvitamin D and leukocyte telomere length by sex and race in a US study.
- Liuzzi, J. P., Lichten, L. A., Rivera, S., Blanchard, R. K., Aydemir, T. B., Knutson, M. D., ... & Cousins, R. J. (2005). Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proceedings of the National Academy of Sciences, 102(19), 6843- 6848.
- Lobo, J. C., Torres, J. P. M., Fouque, D., & Mafra, D. (2010). Zinc deficiency in chronic kidney disease: is there a relationship with adipose tissue and atherosclerosis? Biological trace element research, 135(1-3), 16-21.
- Lung, F. W., Ku, C. S., & Kao, W. T. (2008). Telomere length may be associated with hypertension. Journal of human hypertension, 22(3), 230-232.
- Lutchmansingh, F. K., Hsu, J. W., Bennett, F. I., Badaloo, A. V., McFarlane-Anderson, N., Gordon-Strachan, G. M., ... & Boyne, M. S. (2018). Glutathione metabolism in type 2 diabetes and its relationship with microvascular complications and glycemia. PloS one, 13(6), e0198626.
- McCartney, D. M., & Byrne, D. G. (2020). Optimisation of vitamin D status for enhanced Immuno-protection against Covid-19. Ir Med J, 113(4), 58.
- Maddux, B. A., See, W., Lawrence, J. C., Goldfine, A. L., Goldfine, I. D., & Evans, J. L. (2001). Protection against oxidative stress—induced insulin resistance in rat L6 muscle cells by micromolar concentrations of α-lipoic acid. Diabetes, 50(2), 404-410.
- Mahase, E. (2020). Coronavirus: covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate.
- Manjili, R. H., Zarei, M., Habibi, M., & Manjili, M. H. (2020). COVID-19 as an Acute Inflammatory Disease. The Journal of Immunology.
- Matsuoka, T. A., Kajimoto, Y., Watada, H., Kaneto, H., Kishimoto, M., Umayahara, Y., ... & Yamasaki, Y. (1997). Glycation- dependent, reactive oxygen species-mediated suppression of the insulin gene promoter activity in HIT cells. The Journal of clinical investigation, 99(1), 144-150.
- Maywald, M., Wessels, I., & Rink, L. (2017). Zinc signals and immunity. International journal of molecular sciences, 18(10),2222.
- Mazidi, M., Rezaie, P., Covic, A., Malyszko, J., Rysz, J., Kengne, A. P., & Banach, M. (2017). Telomere attrition, kidney function, and prevalent chronic kidney disease in the United States. Oncotarget, 8(46), 80175.
- Mehta, P., McAuley, D. F., Brown, M., Sanchez, E., Tattersall, R. S., Manson, J. J., & HLH Across Speciality Collaboration. (2020). COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England), 395(10229), 1033.
- Monteiro, R., & Azevedo, I. (2010). Chronic inflammation in obesity and the metabolic syndrome. Mediators of inflammation, 2010.
- Morgan, R. G., Ives, S. J., Walker, A. E., Cawthon, R. M., Andtbacka, R. H., Noyes, D., ... & Donato, A. J. (2014). Role of arterial telomere dysfunction in hypertension: relative contributions of telomere shortening and telomere uncapping. Journal of hypertension, 32(6), 1293.
- Mundstock, E., Sarria, E. E., Zatti, H., Mattos Louzada, F., Kich Grun, L., Herbert Jones, M., ... & Barbé‐Tuana, F. M. (2015). Effect of obesity on telomere length: systematic review and meta‐ analysis. Obesity, 23(11), 2165-2174.
- Mazidi, M., Michos, E. D., & Banach, M. (2017). The association of telomere length and serum 25-hydroxyvitamin D levels in US adults: the National Health and Nutrition Examination Survey. Archives of medical science: AMS, 13(1), 61.
- Merzon, E., Tworowski, D., Gorohovski, A., Vinker, S., Golan Cohen, A., Green, I., & Frenkel‐Morgenstern, M. (2020). Low plasma 25 (OH) vitamin D level is associated with increased risk of COVID‐19 infection: an Israeli population‐based study. The FEBS journal, 287(17), 3693-3702.
- Myers, S. A., Nield, A., & Myers, M. (2012). Zinc transporters, mechanisms of action and therapeutic utility: implications for type 2 diabetes mellitus. Journal of nutrition and metabolism, 2012.
- Nemoto, K., Kondo,Y., Himeno, S.,Suzuki, Y., Hara, S., Akimoto, M., (2000). Modulation of telomerase activity by zinc in humanprostatic and renal cancer cells. Biochem Pharmacol.2(59),401–5.
- Nordfjäll, K., Eliasson, M., Stegmayr, B., Melander, O., Nilsson, P., & Roos, G. (2008). Telomere length is associated with obesity parameters but with a gender difference. Obesity, 16(12), 2682-2689.
- Ooi, L. L., Zhou, H., Kalak, R., Zheng, Y., Conigrave, A. D., Seibel, M. J., & Dunstan, C. R. (2010). Vitamin D deficiency promotes human breast cancer growth in a murine model of bone metastasis. Cancer research, 70(5), 1835-1844.
- Ozfirat, Z., & Chowdhury, T. A. (2010). Vitamin D deficiency and type 2 diabetes. Postgraduate medical journal, 86(1011), 18-25.
- Palomer, X., González‐Clemente, J. M., Blanco‐Vaca, F., & Mauricio, D. (2008). Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes, Obesity and Metabolism, 10(3), 185-197.
- Paul, L. (2011). Diet, nutrition and telomere length. The Journal of nutritional biochemistry, 22(10), 895-901.
- Petrie, J. R., Guzik, T. J., & Touyz, R. M. (2018). Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Canadian Journal of Cardiology, 34(5), 575- 584.
- Polonikov, A. (2020). Endogenous Deficiency of Glutathione as the Most Likely Cause of Serious Manifestations and Death in COVID-19 Patients. ACS Infectious Diseases.
- Prasad, A. S., Beck, F. W., Bao, B., Fitzgerald, J. T., Snell, D. C., Steinberg, J. D., & Cardozo, L. J. (2007). Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. The American journal of clinical nutrition, 85(3), 837-844.
- Rajgor, D. D., Lee, M. H., Archuleta, S., Bagdasarian, N., & Quek, S. C. (2020). The many estimates of the COVID-19 case fatality rate. The Lancet Infectious Diseases, 20(7), 776-777.
- Rahman, I. (2005). Regulation of glutathione in inflammation and chronic lung diseases. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 579(1-2), 58-80.
- Raschenberger, J., Kollerits, B., Ritchie, J., Lane, B., Kalra, P. A., Ritz, E., & Kronenberg, F. (2015). Association of relative telomere length with progression of chronic kidney disease in two cohorts: effect modification by smoking and diabetes. Scientific reports, 5, 11887.
- Razzaque, M. S. (2012). FGF23, Klotho and Vitamin D Interactions. In Endocrine FGFs and Klothos (pp. 84-91). Springer, New York, NY.
- Révész, D., Milaneschi, Y., Verhoeven, J. E., & Penninx, B.W. (2014). Telomere length as a marker of cellular aging is associated with prevalence and progression of metabolic syndrome. The Journal of Clinical Endocrinology & Metabolism, 99(12), 4607-4615
- Rhodes, J. M., Subramanian, S., Laird, E., Griffin, G., & Kenny, R. A. (2020). Perspective: Vitamin D deficiency and COVID‐--__ 19 severity–plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2 and thrombosis. Journal of internal medicine.
- Richardson, S., Hirsch, J. S., Narasimhan, M., Crawford, J. M., McGinn, T., Davidson, K. W., ... & Cookingham, J. (2020). Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. Jama.
- Richter, T., & von Zglinicki, T. (2007). A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Experimental gerontology, 42(11), 1039-1042.
- Rizvi, S., Raza, S. T., & Mahdi, F. (2014). Telomere length variations in aging and age-related diseases. Current aging science, 7(3), 161-167.
- Robaczewska, J., Kedziora-Kornatowska, K., Kozakiewicz, M., Zary-Sikorska, E., Pawluk, H., Pawliszak, W., & Kedziora, J. (2016). Role of glutathione metabolism and glutathione-related antioxidant defense systems in hypertension. J Physiol Pharmacol, 67(3), 331-337.
- Roth, D. E., Abrams, S. A., Aloia, J., Bergeron, G., Bourassa, M. W., Brown, K. H., ... & Jefferds, M. E. (2018). Global prevalence and disease burden of vitamin D deficiency: a roadmap for action in low-and middle-income countries.
- Rudich, A., Tirosh, A., Potashnik, R., Hemi, R., Kanety, H., & Bashan, N. (1998). Prolonged oxidative stress impairs insulin- induced GLUT4 translocation in 3T3-L1 adipocytes. Diabetes, 47(10), 1562-1569.
- Salgueiro, M. J., Krebs, N., Zubillaga, M. B., Weill, R., Postaire, E., Lysionek, A. E., ... & Boccio, J. (2001). Zinc and diabetes mellitus. Biological Trace Element Research, 81(3), 215- 228.
- Saliques, S., Zeller, M., Lorin, J., Lorgis, L., Teyssier, J. R., Cottin, Y., ... & Vergely, C. (2010). Telomere length and cardiovascular disease. Archives of cardiovascular diseases, 103(8- 9), 454-459.
- Salpea, K. D., Talmud, P. J., Cooper, J. A., Maubaret, C. G., Stephens, J. W., Abelak, K., & Humphries, S. E. (2010). Association of telomere length with type 2 diabetes, oxidative stress and UCP2 gene variation. Atherosclerosis, 209(1), 42-50.
- Savale, L., Chaouat, A., Bastuji-Garin, S., Marcos, E., Boyer, Maitre, B., ... & Le Corvoisier, P. (2009). Shortened telomeres in circulating leukocytes of patients with chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine, 179(7), 566-571.
- Serra, V., Grune, T., Sitte, N., Saretzki, G., & von Zglinicki, T. (2000). Telomere length as a marker of oxidative stress in primary human fibroblast cultures. Annals of the New York Academy of Sciences, 908(1), 327-330.
- Schiavi, A., & Ventura, N. (2014). The interplay between mitochondria and autophagy and its role in the aging process. Experimental gerontology, 56, 147-153.
- Shin, D., Shin, J., & Lee, K. W. (2019). Effects of inflammation and depression on telomere length in young adults in the United States. Journal of Clinical Medicine, 8(5), 711.
- Siren, P. M., & Siren, M. J. (2010). Systemic zinc redistribution and dyshomeostasis in cancer cachexia. Journal of cachexia, sarcopenia and muscle, 1(1), 23-33.
- Skalny, A. V., Rink, L., Ajsuvakova, O. P., Aschner, M., Gritsenko, V. A., Alekseenko, S. I., ... & Tsatsakis, A. (2020). Zinc and respiratory tract infections: Perspectives for COVID-19. International Journal of Molecular Medicine, 46(1), 17-26.
- Szymczak-Pajor, I., & Śliwińska, A. (2019). Analysis of association between vitamin D deficiency and insulin resistance. Nutrients, 11(4), 794.
- Tungtrongchitr, R., Pongpaew, P., Phonrat, B., Tungtrongchitr, A., Viroonudomphol, D., Vudhivai, N., & Schelp, F. P. (2003). Serum copper, zinc, ceruloplasmin and superoxide dismutase in Thai overweight and obese. Journal of the Medical Association of Thailand= Chotmaihet thangphaet, 86(6), 543.
- Turer, C. B., Lin, H., & Flores, G. (2013). Prevalence of vitamin D deficiency among overweight and obese US children. Pediatrics, 131(1), e152-e161.
- Upchurch, G. R., Ramdev, N., Walsh, M. T., & Loscalzo, J. (1998). Prothrombotic consequences of the oxidation of fibrinogen and their inhibition by aspirin. Journal of thrombosis and thrombolysis, 5(1), 9-14.
- Valdes, A. M., Andrew, T., Gardner, J. P., Kimura, M., Oelsner, E., Cherkas, L. F., ... & Spector, T. D. (2005). Obesity, cigarette smoking, and telomere length in women. The lancet, 366(9486), 662-664.
- Vasdev, S., Gill, V. D., & Singal, P. K. (2006). Modulation of oxidative stress-induced changes in hypertension and atherosclerosis by antioxidants. Experimental & Clinical Cardiology, 11(3), 206.
- von Zglinicki, T., Bürkle, A., & Kirkwood, T. B. (2001). Stress, DNA damage and ageing—an integrative approach. Experimental gerontology, 36(7), 1049-1062.
- Wang, B., Li, R., Lu, Z., & Huang, Y. (2020). Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY), 12(7), 6049.
- Wellen, K. E. (2005). hotamisligil GS. Inflammation, stress, diabetes. J Clin Invest, 115, 1111-1119.
- Wessels, I., Rolles, B., & Rink, L. (2020). The potential impact of zinc supplementation on COVID-19 pathogenesis. Frontiers in immunology, 11, 1712.
- Williams, C. R., Mistry, M., Cheriyan, A. M., Williams, J. M., Naraine, M. K., Ellis, C. L., ... & Cai, H. (2019). Zinc deficiency induces hypertension by promoting renal Na+ reabsorption. American Journal of Physiology-Renal Physiology, 316(4), F646-F653.
- Wu, C. Y., Hu, H. Y., Chou, Y. J., Huang, N., Chou, Y. C., & Li, C. P. (2015). High blood pressure and all-cause and cardiovascular disease mortalities in community-dwelling older adults. Medicine, 94(47).
- Wu, Y. C., Chen, C. S., & Chan, Y. J. (2020). The outbreak of COVID-19: An overview. Journal of the Chinese Medical Association, 83(3), 217
- Yang, Z., Huang, X., Jiang, H., Zhang, Y., Liu, H., Qin, C., ...& Ju, Z. (2009). Short telomeres and prognosis of hypertension in a Chinese population. Hypertension, 53(4), 639-645.
- Yao, Y., Zhu, L., He, L., Duan, Y., Liang, W., Nie, Z., ... & Fang, Y. (2015). A meta-analysis of the relationship between vitamin D deficiency and obesity. International journal of clinical and experimental medicine, 8(9), 14977.
- Zhao, J., Miao, K., Wang, H., Ding, H., & Wang, D. W. (2013). Association between telomere length and type 2 diabetes mellitus: a meta-analysis. PloS one, 8(11), e79993.
- Zheng, Y. Y., Ma, Y. T., Zhang, J. Y., & Xie, X. (2020). COVID-19 and the cardiovascular system. Nature Reviews Cardiology, 17(5), 259-260.
- Zhou, M., Zhu, L., Cui, X., Feng, L., Zhao, X., He, S., ... & Li, Y. (2015). Influence of diet on leukocyte telomere length, markers of inflammation and oxidative stress in individuals with varied glucose tolerance: a Chinese population study. Nutrition Journal, 15(1), 39.



