Hanaa S. Bamefleh MBChB1*, Abdullah Almutham MBBS2
1Consultant of Anatomic Pathology, King Abdulaziz medical City, Riyadh, Kingdom of Saudi Arabia.
2Anatomic Pathology Resident, Prince Sultan Military Medical City, Riyadh, Kingdom of Saudi Arabia.
*Corresponding Author: Hanaa S. Bamefleh MBChB, Consultant of Anatomic Pathology, King Abdulaziz medical City, Riyadh, Kingdom of Saudi Arabia.
Abstract
The vast majority of breast malignancies are primary lesions. However, metastasis to the breast is unusual with melanoma and hematolymphoid malignancies being the most common. In addition, metastasis from lung cancer to breast can occur, with adenocarcinoma being the most common histological subtype to metastasize. We report a 77-year-old female who presented with bilateral breast lesions, initially was thought to be bilateral mammary carcinoma, after thorough imaging workup, it was diagnosed as secondary lesions from lung adenocarcinoma. This diagnosis was confirmed by proper immunohistochemical and molecular studies on the bilateral breast biopsies. The patient was managed by tyrosine kinase inhibitors and responded well. Identification of primary versus secondary breast lesions is a very essential step in inpatient management as treatment lines can differ entirely. Performing a proper immunohistochemical panel is essential in such cases to make this differentiation in addition to the clinical and imaging correlation.
Keywords: Lung cancer; Breast metastasis; Immunohistochemistry; Case report; Non-small cell; Lung; Breast; Metastatic carcinoma
Introduction
Lung cancer is the leading cause of cancer deaths among men and the second among women [1]. Lung cancer has a poor prognosis with a 5-year survival rate of around 15 %, with adenocarcinoma being the most common histological type [2]. Primary breast malignancy is the most common malignancy in women. However, metastasis to the breast from another primary location is quite uncommon with a prevalence of 0.4 %- 1.3 % [3–7]. If we include Malignant melanoma and hematolymphoid malignancies- as they are the most common to metastasize to the breast- the incidence will increase to 3 %. If we include autopsy studies the incidence can reach up to 1.7–6.6 % [8,9]. Agrawal et al. reported an incidence of 7.6 % for all metastatic malignancies of the breast [8,10–13]. Metastasis to the breast from solid organs includes lung, ovary, prostate, kidney, stomach, ileum, thyroid, and cervix [14]. Certainly, metastasis from the contralateral breast is the commonest among all others mentioned earlier [11,15]
followed by ovary then lung [8]. The largest series studied by William between 1983 and 1998 have identified 169 patients. All were confirmed by histopathologic examination to be metastasis to the breast from solid organs, he found that the most common metastasis to the breast is from malignant melanoma [9]
Most patients present with their primary malignancies followed after some time with breast metastasis, in approximately 25 % of patients the metastatic breast mass is the presenting symptom, and it may resemble benign disease of the breast such as fibroadenoma or primary breast malignancies [12,15].
We present a case with bilateral breast metastasis from a primary lung carcinoma, misdiagnosed as triple-negative invasive ductal carcinoma. Identifying the primary lesion in such cases is essential for proper management and to avoid unnecessary procedures.
Case report
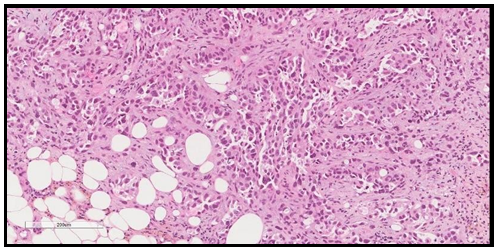
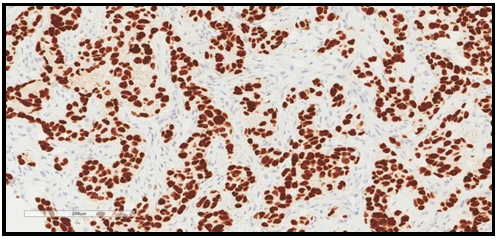
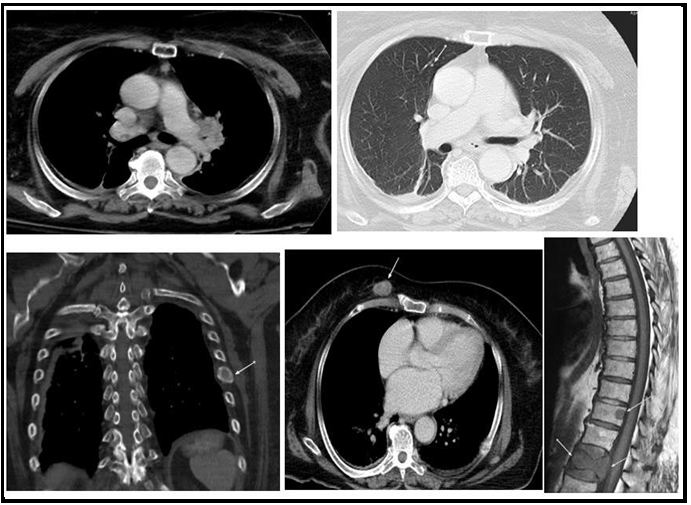
We report a 77-year-old lady with negative past medical history for malignancy, presented with bilateral breast lesions. A mammogram revealed highly suspicious masses in both breasts, bilateral BI-RADS 5. Computerized tomography scan ( CT Scan) Guided core needle biopsies from both breasts were done, they revealed fibro adipose tissue infiltrated by adenocarcinoma, the malignant cells are arranged in glands, trabeculae, and cords, no carcinoma in situ is seen and no lymphovascular invasion. No normal residual breast issue is seen in Figure 1. Immunohistochemical study showed the adenocarcinoma cells to be reactive for pan-cytokeratin (PCK) and cytokeratin 7 (CK7) While stains for estrogen, progesterone receptors (ER, PR), And Her2 Neu antibodies were all negative, with low Ki-67 index. The initial impression was triple-negative breast cancer, but when a CT scan chest was performed it revealed left hilar lung mass with a metastatic nodule in the right upper lobe. In addition, a metastatic lytic lesion in the rib was found Figure 3. Due to the CT scan findings, a TTF1 immunohistochemical stain was performed on the breast biopsy that turned out to be positive confirming primary lung adenocarcinoma with breast metastasis. Figure 2. A molecular study revealed epidermal growth factor (EGFR) Exon 19 mutation.
The patient was given a tyrosine kinase inhibitor and in spite of her high clinical stage of primary lung cancer she did well, however, after 6 months she was lost for follow-up due to travel outside the country.
Figure 1. H&E stained section showing malignant glandular formation consistent with adenocarcinoma. The power is 200X.
Figure 2. TTF-1 immunohistochemical stained section showing positive nuclear stain within malignant cells confirming lung adenocarcinoma. The power is 200X.
Figure 3: Metastatic disease including the left hilar mass, right upper lobe nodule, left rib lytic lesion, right anterior chest wall enhancing deposit and spinal deposits with lumbar vertebral collapse (MRI T1 weighted image)
Discussion
The most common malignancy worldwide is lung cancer with a high mortality rate of 16. Around 20 % of patients with primary lung cancer present with distant metastasis, the most frequent sites are brain, bone, liver, and adrenal glands. Breast metastasis is rare from a primary lung malignancy [3–6].
In breast metastasis, the lesion tends to be superficial within the subcutaneous fat without nipple or skin retraction, unlike the deep embedded primary breast lesions [6,17]. Mammographic features in breast metastasis usually demonstrate well-defined lesions that lack to express microcalcifications and speculations as is the primary breast malignancy, moreover the size of the lesion on physical exam is similar to the size on mammography due to lack of scares, this is opposite to primary breast cancer were the size of the primary lesion is smaller on mammography than on physical exam due to marked fibrosis [18,19]. The most commonly described mammographic presentation is usually single but may sometimes present as multiple well-circumscribed lesions with smooth margins [3,8,20]. Besides primary mammary carcinomas, microcalcifications are reported in metastatic serous ovarian papillary carcinoma [7].
Rola et.al have searched for metastatic lung cancer to breast between the years 1996 and 2017. Within the 21 years search, she has found 16 cases in their hospital files. Adenocarcinoma was the most common histologic type to metastasize to the breast. She summarized some histologic and prognostic points that help in suspecting or diagnosing these lesions; A triple-negative tumor with low-grade morphology, a tumor that lacks an in-situ component, with the absence of elastosis [3,5,6,14,21,22]. More features include a tumor presenting with the high stage but has negative axillary lymph node involvement and or not responding to standard breast cancer therapy [7,8]. More morphologic features on imaging studies include superficial location in subcutaneous tissue immediately adjacent to glandular breast, presence of multiple lymphatic tumor emboli, well- circumscribed tumor, smooth contour with multiple satellite foci, lack of architectural distortion, speculations and microcalcifications [14,23]. A predilection for the upper outer quadrant is noted by many authors [3,7,9,15]. An even very rare presentation reported by some authors is lung metastasis presenting as inflammatory carcinoma (IBC) of the breast [7,13,24,25].
This mode of presentation confirms that the tumor has reached the breast via the lymphatic route, a predilection for the ipsilateral breast, and upper outer quadrant have been noticed by many authors. Moreover, in patients with bilateral metastasis, both breasts can be affected again due to extensive lymphatic spread [7,14,24].
Ota et al described a 69 years old patient who underwent left lower lobectomy for primary adenocarcinoma of the lung that was epidermal growth factor (EGFR) exon 21 mutated. Initially, she responded well to tyrosine kinase inhibitors (TKI). But she had a recurrence 2 years later in the form of IBC. CT- Guided core needle biopsy from breast, confirmed lung primary as the tumor was TTF1 positive and harbour the same EGFR mutation. He also postulated that when metastatic tumor reaches the breast through blood supply it will form a single mass, but when it reaches the breast through lymphatics it tends to form multiple nodules [13].
Metachronous and synchronous presentation of metastasis are correlated with histologic type, initial staging at diagnosis, treatment histories, and outcomes, therefore it has clinical relevance [7,23].
Jennifer et al reported 39 cases of breast metastasis from lung cancer, 31 were adenocarcinoma, and 8 of which are small cell carcinoma (SCC), while 80 % of the SCC metastasized synchronously only 33 % of the adenocarcinoma did so [23].
In most situations, metastatic disease to the breast occurs after the diagnosis of the primary tumor i.e., metachronous. In approximately 25 % of patients, a breast mass is a cause for the initial presentation just like our patient. In these patients, metastases to the breast can mimic benign disease or primary breast malignancies. In the study by Babu all of his three cases, a breast mass was the initial mode of presentation [15]. This is further supported by the study of Williams who mentioned that 88.2 % of patients who presented with breast metastasis had a prior history of known malignancy [9].
Nicoletta et al reported a micropapillary adenocarcinoma that presented synchronously. This histologic type of primary lung adenocarcinoma is a high grade with a poor prognosis, so such a presentation is expected [24].
In Jennifer’s study 8/31 cases of metastatic lung cancer to the breast were SCC, 80 % of them presented synchronously, a similar observation noted by Alessandro and Nicoletta [7,23,24].
As the prognosis and management of primary mammary carcinoma versus metastatic carcinoma to the breast are different, all authors have agreed that an immunohistochemical panel is needed for differentiation between them; the recommended panel is displayed in Table 1. [8,13,14,26].
It is evident that the TTF-1 antibody is the most reliable antibody, it is positive in up to 85 % of lung carcinoma, the other tumor that can be positive is thyroid carcinoma, but breast metastasis of primary thyroid cancer is extremely rare [27]
Table 1: List the proper immunohistochemical panel that is recommended to differentiate between primary mammary carcinoma from metastatic lung adenocarcinoma
|
Antibody |
Lung adenocarcinoma |
Breast mammary carcinoma |
|
TTF1 |
73 %–88 % |
3 % week and focal |
|
Napsin A |
80 %-90 % |
less than 3 % |
|
CK7 |
|
|
|
Mammoglobin |
|
48 % to 72.1 % |
|
ER |
7.6 % to 27.2 %, |
80 % |
|
PR |
negative |
60 % |
|
Her2 Neu |
|
|
|
GCDFP-15 |
5.2 % to 15 % |
45 %-53 % |
|
GATA 3 |
less than 10 % |
67 %-95 % (43 %–73 %) In triple-negative cases |
The primary lung adenocarcinoma with metastasis to the breast in the series reported by Rola has unilateral metastasis in 11/13 while only 2 presented with bilateral metastasis [8]. Li Wang et.al have reported 2 patients with bilateral metastasis to the breast, but his patients had neuroendocrine carcinomas and not adenocarcinomas, moreover, his patients were found to have synchronous breast and lung masses at the time of presentation, unlike our patient who was found to have the lung mass discovered by CT-Scan later [28].
Biyuan reported a female patient who was discovered late to have primary lung adenocarcinoma with bilateral breast metastasis and skin deposits after being managed with many treatment lines [29]. Moreover, Xiao Wu reported two cases of primary adenocarcinoma with breast metastasis one of them had bilateral breast metastatic deposits [30]. Assi also reported a metastatic small cell carcinoma of the lung in a 52 years old smoker female patient, who presented with respiratory symptoms rather than breast mass, she received chemotherapy, after 6 months, at follow up visit she was found to have bilateral breast metastasis, manifesting as Inflammatory breast cancer with fixed breast mass, the left breast showed swelling redness and inverted nipple, the right breast had smaller masses [25].
Among the histologic types of primary lung cancer, adenocarcinoma is the commonest to metastasize to the breast. However, all histological subtypes may metastasize to the breast especially small cell carcinoma which tends to spread early, extensively through lymphatics, and is more likely to be synchronous with breast lesion in 80 % of cases [23]
The observation of bilateral breast metastasis from solid organ cancer is described in the Toombs review. He reported 21 metastatic lesions from solid organ cancer, he found 26 % of cases presenting initially with bilateral breast lesions [31].
The case presented by Dansin is very much similar to our patient in many aspects. She is a 52 years old patient who presented with IBC, diagnosed initially as triple-negative primary breast cancer. She received chemotherapy and progressed, later she was discovered to have a primary lung cancer during workup for cancer progression and examination of the pleural fluid proved a TTF-1 positive primary lung cancer with Exon 19 EGFR mutation. [32] This is the same as our patient’s scenario, however, our patient was found to have a lung mass on CT- Scan during workup for her disease progression, we did not have additional tissue, but we applied TTF-1 and Napsin on the first biopsy from the breast and it proved to be positive for these markers with even Exon 19 EGFR mutation. Breast carcinoma can acquire EFGR mutation, particularly the triple-negative type; an incidence of 1.4 % and 11.4 % is described in two Asian studies. Therefore, not all triple-negative breast cancer with EGFR mutation should be suspected to be metastasis from the lung, IHC panel confirmation is the gold standard [32]. Other case reports of breast metastasis from lung adenocarcinoma with EGFR mutation are available in the literature [23,33,34].
The age range of metastatic lung cancer to the breast as seen in the literature is 40-77 years. The two young patients below 30 years reported in the literature are for Diego & Wang SC [14,26]. Diego reported a 29-year-old patient, diagnosed with breast and lung cancer at the same time, she survived for 20 months after first and second-line chemotherapy for lung cancer, in his literature search since 2000 he found 62 cases, eight of them were for male patients (12.7 %) [14]. Kannan et al. Reported a male patient with lung cancer metastasized to the breast after 4 years [35]. This literature review showed approximately seven hundred cases Table 2. [23,7,9,8] More individual case reports are available that are not included in those big series, which means more cases are available and attention to this problem has to be paid when dealing with patients known to have malignancy and presenting with breast lesion.
Table 2: Summary of major literature reviews [23,7,9,8]
|
Author |
Search Years |
Cases Found |
Number of Cases with Known Histology |
Histologic Type |
|||
|
Adenocarcinoma Lung |
Other NSCLC* |
Others including SCC** |
Other primaries |
||||
|
Alvaetal |
1855- 1998 |
78 |
|||||
|
William |
1983-1998 |
169 |
|||||
|
Ali |
1999- 2017 |
159 |
99 |
36 |
33 |
30 |
|
|
Ali |
1996-2017 |
14 |
14 |
11 |
2 |
1 |
|
|
Mirrielees |
1965-2013 |
179 |
41 |
18 |
10 |
13 |
|
|
Alessandro |
(1990-2010 |
83 |
30 |
12 |
18 |
||
|
Total |
682 |
||||||
NSCLC: Non-small cell lung cancer, SCC: Small cell carcinoma
Conclusion
Metastasis to the breast is a rare incidence, in a patient with a known history of malignancy presenting with breast mass, it is important to rule out metastasis before initiating any treatment. With the advancement of imaging studies more cases of metastatic breast lesions are being reported, in good, experienced hands mammography can distinguish breast metastasis from primary breast cancer. However, the best way to reach a final diagnosis is by applying a comprehensive IHC panel on the tissue or cytology samples that are available rather than getting a new biopsy, this will expedite the correct diagnosis and save cost. This approach is important in adenocarcinomas that are negative for ER, PR, and HER2 Neu, this will help to initiate timely proper management and avoid unnecessary surgical producers.
The prognosis of those patients is usually poor, knowing this might affect the patient’s life and management decision
In summary clinical, imaging, histological, immunohistochemical, and molecular features must be taken into account to optimize the accurate diagnosis and management of these rare lesions.
Disclaimer
We confirm that there are no conflicts of interest and no financial support. The manuscript has been read and approved by the two authors. No one has participated and denied being an author. We agreed on the order that our names appeared in the manuscript. We respect that the corresponding author is the sole contact for the Editorial process.
References
- Bernard WS, Christopher PW. World cancer report 2014. World Heal Organ. 2014: 630.
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA EB (Eds). SEER Cancer Statistics Review 1975-2008 National Cancer Institute SEER Cancer Statistics Review 1975- 2008 National Cancer Institute. Cancer. 2011: 1975-2008.
- Hajdu SI, Urban JA (1972) Cancers metastatic to the breast. Cancer. 29(6): 1691-1696.
- Vizcaíno I, Torregrosa A, Higueras V, Morote V, Cremades A, et al. (2001) Metastasis to the breast from extrammary malignancies: A report of four cases and a review of literature. Eur Radiol. 11(9): 1659-1665.
- Georgiannos SN, Chinaleong J, Goode AW, Sheaff M (2001) Secondary neoplasms of the breast: A survey of the 20th century. Cancer. 92(9): 2259-2266.
- Klingen TA, Klaasen H, Aas H, Chen Y, Akslen LA (2009) Secondary breast cancer: A 5-year population-based study with review of the literature. Apmis. 117(10): 762-767.
- Sanguinetti A, Puma F, Lucchini R, Santoprete S, Cirocchi R, et al. (2012) Breast metastasis from a pulmonary adenocarcinoma: Case report and review of the literature. Oncol Lett. 5(1): 328- 332.
- Ali RH, Taraboanta C, Mohammad T, Hayes MM, Ionescu DN (2018) Metastatic non-small cell lung carcinoma a mimic of primary breast carcinoma—case series and literature review. Virchows Arch. 472(5): 771-777.
- Williams SA, Ehlers RA, Hunt KK, Yi M, Kuerer HM, et al. (2007) Metastases to the breast from nonbreast solid neoplasms: Presentation and determinants of survival. Cancer. 110(4): 731- 737.
- Kumar J (1996) Cytodiagnosis of Metastasis in the Breast - A report of eight cases. J Cytol. 13(1): 51-54.
- Akçay MN (2002) Metastatic disease in the breast. Breast. 11(6): 526-528.
- Gupta D, Merino MI, Farhood A, Middleton LP (2001) Metastases to breast simulating ductal carcinoma in situ: Report of two cases and review of the literature. Ann Diagn Pathol. 5(1): 15-20.
- Ota T, Hasegawa Y, Okimura A, Sakashita K, Sunami T, et al. (2018) Breast metastasis from EGFR-mutated lung adenocarcinoma: A case report and review of the literature. Clin Case Reports. 6(8): 1510-1516.
- Enrico D, Saucedo S, Bravo I (2019) Breast metastasis from primary lung adenocarcinoma in a young woman: A case report and literature review. World J Clin Oncol. 10(7): 269-278.
- Babu KS, Roberts F, Bryden F, McCafferty A, Downer P, et al. (2009) Metastases to breast from primary lung cancer. J Thorac Oncol. 4(4): 540-542.
- World Health Organization. Cancer Fact Sheets. Globocan 2012. 2012: 0-5.
- Chun-Nan Y, Cheng-Hung L, Chen M-F (2004) Clinical and ultrasonographic characteristics of breast metastases from extramammary malignancies. Am Surg. 70(4): 287-90.
- Noguera JJ, Martínez-Miravete P, Idoate F, Díaz L, Pina L, et al. (2007) Metastases to the breast: A review of 33 cases. Australas Radiol. 51(2): 133-138.
- Lee SK, Kim WW, Kim SH, Hur SM, Kim S, et al. (2010) Characteristics of metastasis in the breast from extramammary malignancies. J Surg Oncol. 101(2): 137-140.
- Ji FF, Gao P, Wang JG, Zhao J, Zhao P (2012) Contralateral breast metastasis from pulmonary adenocarcinoma: Two cases report and literature review. J Thorac Dis. 4(4): 384-389.
- Lee AHS (2007) The histological diagnosis of metastases to the breast from extramammary malignancies. J Clin Pathol. 60(12): 1333-1341.
- Verger E, Conill C, Velasco M, Sole M (1992) Metastasis in the Male breast lfrom a lung adenocarcinoma. Acta Oncol (Madr). 31(4): 479.
- Mirrielees JA, Kapur JH, Szalkucki LM, Harter JM, Salkowski LR, et al. (2014) Metastasis of primary lung carcinoma to the breast: A systematic review of the literature. J Surg Res. 188(2): 419-431.
- Maounis N, Chorti M, Legaki S, Ellina E, Emmanouilidou A, et al. (2010) Metastasis to the breast from an adenocarcinoma of the lung with extensive micropapillary component: A case report and review of the literature. Diagn Pathol. 5(1): 82.
- Assi HA, Khoury KE, Mouhieddine TH, Khalil LE, Kanj A, et al. (2014) Small Cell Lung Cancer with Metastasis to the Breast : A Case Report and Review of the Literature. J Cancer Biol Res. 2(1): 1025.
- Wang SC, Tseng JC, Yu CP, Cheng MF, Perng WC CC. Breast Metastasis from Lung Adenocarcinoma in a 26-year-old Woman: A Case Report.
- Song HJ, Xue YL, Xu YH, Qiu ZL, Luo QY (2011) Rare metastases of differentiated thyroid carcinoma: Pictorial review. Endocr Relat Cancer. 18(5): 165-174.
- Wang L, Wang SL, Shen HH, Niu FT, Niu Y (2014) Breast metastasis from lung cancer: a report of two cases and literature review. Cancer Biol Med. 11(3): 208-215.
- Luo B, Rao L, Ma F, Liu X (2020) Individualized comprehensive treatment for lung adenocarcinoma with multiple skin and bilateral breast metastasis : A case report. 45(1): 102-108.
- Wu X, Wang H, Fang M, Li C, Zeng Y, et al. (2019) ALK or ROS1-rearranged breast metastasis from lung adenocarcinoma: a report of 2 cases. Tumori. 105(6): NP67-NP71.
- Toombs BD, Kalisher L (1977) Metastatic disease to the breast: clinical, pathologic, and radiographic features. Am J Roentgenol. 129(4): 673-676.
- Dansin E, Carnot A, Servent V, Daussay D, Robin YM, et al. (2015) EGFR-mutated breast metastasis of lung adenocarcinoma: A case report. Case Rep Oncol. 8(1): 164-168.
- Fukumoto K, Usami N, Okasaka T, Kawaguchi K, Okagawa T, et al. (2011) Late breast metastasis from resected lung cancer diagnosed by epidermal growth factor receptor gene mutation. Lung Cancer. 74(2): 352-353.
- Sato K, Takeyama Y, Yoshihara M, Kato T, Hashimoto H, et al. (2012) CBDCA + pemetrexed + bevacizumab and its maintenance chemotherapy in a case of solitary breast metastasis from a lung adenocarcinoma resistant to gefitinib. Case Rep Oncol. 5(3): 546-553.
- Ramar K, Pervez H, Potti A, Mehdi S (2003) Breast metastasis from non-small-cell lung carcinoma. Med Oncol. 20(2): 181-184.