B.I Patel1*, Nisha Patel2, Saumil Patel2, Alpesh Patel3, Shiva Shankaran Chettiar3, Devendrasinh Jhala4
1Fetal Medicine Expert, Gynob Sonoscan Center, Dev ART IVF- Test Tube Baby Center & Shachi women’s Hospital, Ahmedabad, Gujarat, India.
2Obstetrics & Gynaecologist, Gynob Sonoscan Center, Dev ART IVF- Test Tube Baby Center & Shachi women’s Hospital, Ahmedabad, Gujarat, India.
3GeneXplore diagnostics and research centre Pvt. Limited, Ahmedabad.
4Department of Zoology, BMT and Human Genetics, Gujarat University, Ahmedabad
*Corresponding Author: B.I Patel, Fetal Medicine Expert, Gynob Sonoscan Center, Dev ART IVF- Test Tube Baby Center & Shachi women’s Hospital, Ahmedabad, Gujarat, India.
Abstract:
Background: Early prenatal diagnosis and timely termination of the affected pregnancies can reduce medical and psychological stress and complications in affected couples. Chorionic villus sampling (CVS) is increasingly used nowadays for prenatal diagnosis.
Objective: To determine the procedural safety, efficacy, and outcome of transabdominal CVS for the prenatal diagnosis of genetic disorders
Methodology: Couples coming for prenatal diagnosis and undergoing CVS procedure were selected in analysis based on the inclusion and exclusion criteria. Route of CVS, the position of the placenta, reasons for opting for the transabdominal trans amniotic approach, reasons for delay and refusal of CVS procedures, different genetic diagnoses made, and complications of the procedure were analyzed.
Results: Out of the 2539 patients, most procedures (2387, 94 %) were done between 12-14 weeks of gestation. 1396 (55 %) had anterior placenta, while 1143 (45 %) had posterior placenta. Most patients had a routine transabdominal approach for CVS, while 663 patients had CVS through a trans-amniotic transabdominal approach. 26 patients refused for CVS procedure, the reasons being posterior low placenta with bleeding (12 patients), failure on the first attempt (5 patients), and patient refusal of counseling (25 patients). The most common diagnosis was found to be β-thalassemia in 2117 (83.38 %) cases. In 9 patients (0.1 %) in whom the aspiration was unsuccessful, they were called for an amniocentesis at 17 weeks. Common Complications of CVS were pain and discomfort (89 %), spotting (2 %), and rarely abortion (0.28 %).
Conclusion: CVS is an effective and safe procedure for early genetic diagnosis, but awareness is required in the Indian population to opt for this technique to avoid possible physical and psychological complications arising from genetic disorders.
Introduction
It is estimated that every year, around 8 million infants (6 % of worldwide births) are born with serious congenital disabilities, which are the leading cause of infant mortality worldwide. Most congenital disabilities arise from genetic disorders falling into three categories: chromosomal abnormalities, single-gene defects, and multifactorial influences. [1] Prenatal environment plays a leading role in developing infant genetic disorders. Most such disorders are either not treatable or the cost of treatment, if available, is out of the reach of the poor population. Early prenatal diagnosis and timely termination of the affected pregnancies have become an important rationale for overcoming socio-economic trauma in low-income people and all population categories. Previous studies have demonstrated the application of prenatal genetic counseling, which helped many couples have non-affected children with a higher risk of genetic disorders [2-4]. Prenatal diagnosis in the first trimester of pregnancy (11-14 weeks) reduces medical and psychological complications.
The global burden of Thalassemia contributes to about 5-10 % of total genetic disorders. Chorionic villus sampling (CVS) and amniocentesis are widely used in early prenatal diagnostic processes for detecting fetal congenital abnormalities like Thalassemia through which the birth rate of affected infants can be controlled. CVS is associated with greater diagnostic accuracy and low risk of complications. The miscarriage rate can be comparable to second- trimester amniocentesis as it involves a procedure to be performed during the first trimester [5].
Prenatal diagnosis by CVS is a promising source of genetic information about the fetus. It can be performed through the transabdominal or trans-cervical route in early gestational age compared to amniocentesis, performed later than 17 weeks of pregnancy with the same complications [6,7]. The transabdominal way involves the insertion of a needle or forceps into the uterus through the abdominal wall under aseptic conditions and is safer and more convenient for the patient than the trans-cervical route, which has a higher risk of infection [8]. Compared to other methods like amniocentesis, early diagnosis is possible with CVS, and early termination of pregnancy can be carried out if required, which helps minimize the mental trauma to the mother and the whole family.
This study aimed to determine the procedural safety, efficacy, and outcome of transabdominal (TA)-CVS for the prenatal diagnosis of genetic disorders.
Materials and Methods
Prenatal diagnosis to identify various genetic disorders, especially Beta thalassemia, was carried out in 2539 cases attending Gynob Son's can center, Ahmedabad, India. Before giving an appointment for TA-CVS sampling, all couples were counseled about the procedure's risks, selection complications, errors in diagnosis, and risk of miscarriage. Before the procedure, written informed consent was obtained from all participants. A preliminary first-trimester scan determined viability, gestational age, number of fetuses, and placental position. Nuchal translucency, nasal bone, and gross anomalies were assessed during this USG scan.
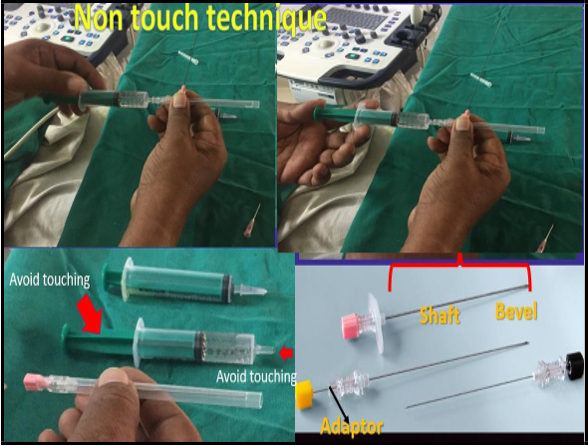
All the procedures were performed under local anesthesia and ultrasound sonography (USG) guidance. The ultrasound scanning was done on Voluson E8 Radiance or Logic V5 using a 5MHz convex probe. The placental position and thickness of the placenta were ascertained, and a suitable site for introducing the needle on the anterior abdominal wall was selected. After proper hand sanitization, the abdominal skin in a 10-15 cm radius was cleaned with betadine solution. Approximately 8-10 ml of 2 % xylocaine was infiltrated with a 23G needle at the site selected for aspiration. The whole tract of the CVS needle from the skin to the uterine fundus was injected with the local anesthetic. While standing on one side of the patient, holding the 18G spinal needle (Figure 1) without touching its tip was introduced from the puncture site of the local anesthetic.
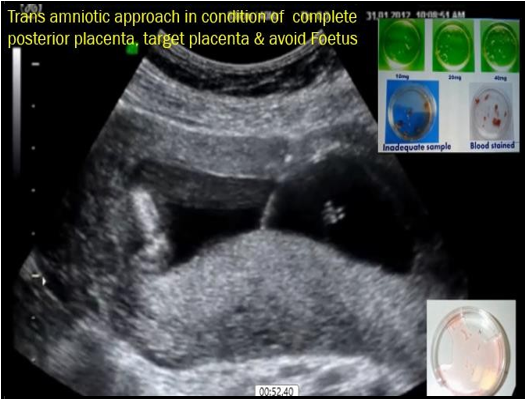
After that, the USG probe was held in one hand, and the needle was maneuvered with the other hand. An important step in the procedure was to always keep the needle tip visible on the sonography monitor to avoid damage to the internal organs and tissues of the mother as well as to avoid any harm to the fetus and for proper forward movement. Forward movement of the needle can be more properly felt using the hand without glowing; adequate sanitization of the writing is a prerequisite. After piercing the abdominal skin and uterine walls, the needle was pushed with a jerky movement to enter the placenta in its longitudinal or tangential plane, depending upon the position of the placenta. The uneven forward push helped avoid any placental separation at the needle entry site. The needle was kept in a tangential direction for an anterior placenta, while the hand was kept vertically placed for a posterior placenta. While approaching a fundo posterior placenta, special care was required to avoid injury to the intestinal loops. If a direct approach to the posterior placental is not possible, then the amniotic cavity will be helpful (Trans amniotic cavity approach) (Figure 2). The most important in this trans amniotic approach is to keep the proper alignment of the needle with the probe point, and it should not deviate more than 0.5 mm from this probe point. The spine needle ring with heparinized media was kept ready. By taking this care, there was hardly any problem encountered.
Figure 1: 18G needle for CVS procedure
Figure 2: Transabdominal Transamniotic approach for posterior placenta
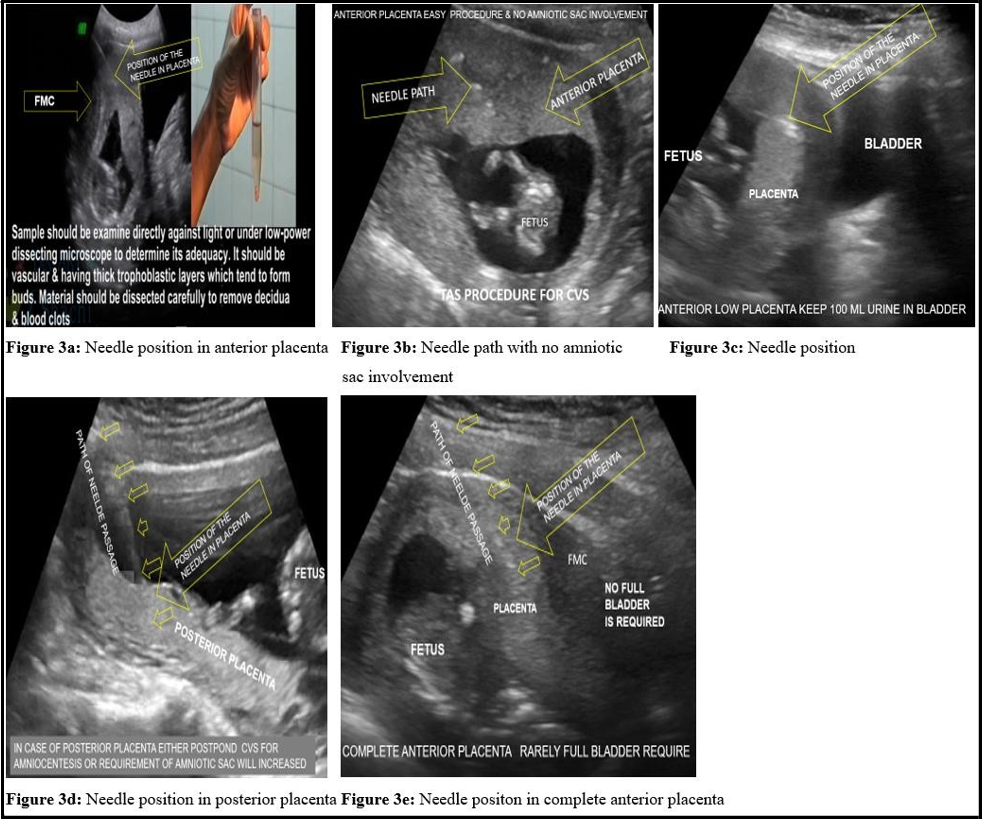
A loss of resistance appreciated the needle’s entry into the placenta. Once the needle was in the placenta, it was sufficiently advanced to leave at least 1 cm of the placental tissue ahead of the needle tip stylet was removed (figure 3). A 10 ml disposable syringe having 4-5 ml heparinized media attached to the spinal needle was rinsed with a heparinized press and used for TA-CVS. The syringe plunger was pulled back to about the 8 ml mark to create a negative suction force, and the fingers maintained the position. The aspiration syringe and needle in placental function were jiggled and performed to and fro movement about 5-7 times, with maintaining simultaneous negative suction force created as mentioned at the beginning of the procedure. In this way, the disrupted villi were sucked into the syringe through the needle. The aspiration needle with the syringe was removed with simultaneous negative suction force, and aspirated villi in the syringe were transferred to a test tube or Petri dish. The adequate amount (> 15-25 mg) of grayish-white chorionic villi confirmed a successful aspiration. A second or rarely third aspiration attempt was performed in case of a poor sample yield. After removing the spinal needle, apply betadine ointment to the puncture mark, which was sealed with a sterile elastic bandage. The sample was immediately sent for prenatal genetic diagnosis.
A post-aspiration USG was done to observe fetal well-being and any hematoma formation or placental separation. The patients were allowed to go home after 2 hours and advised to take bed rest for 3-5 days. Prophylactic antibiotics like Azithromycin, Cefixime, and progesterone were given for five days.
Figure 3: Different Needle positions in the CVS procedure and sample collection for diagnosis.
Results
During the study, 2539 patients meeting inclusion and exclusion criteria underwent the CVS procedure. Table I shows the position of the placenta and the CVS procedure performed on the patients. Of 2539 patients, 1396 (55 %) had anterior placenta while 1143 (45 %) had posterior placenta. As per the Physician’s knowledge, 762 patients have gone through the fundal anterior, and 634 have gone through the anterior route for CVS. In the posterior position, 172 patients had fundal posterior placenta where the Transabdominal technique was employed. 308 patients had posterior placenta in which the CVS technique started with the Transabdominal approach, but due to the involvement of the amniotic cavity, the trans-amniotic process was followed. In posterior placenta trans-amniotic approach was followed in 663 patients. The Amniocentesis CVS procedure was performed on 9 patients.
Table I: Position of Placenta and Selection of CVS route.
|
Position of the Placenta |
No. of patients |
Type of CVS Approach selected |
|
|
Anterior |
Fundal Anterior |
762 |
Transabdominal (TA) |
|
Anterior |
634 |
Transabdominal (TA) |
|
|
Posterior |
Fundal only |
172 |
Transabdominal (TA) |
|
Posterior |
308 |
Started with complete Transabdominal approach but shifted to Transabdominal Trans-amniotic approach to avoid involvement of amniotic cavity |
|
|
Complete posterior |
663 |
Transabdominal Trans-amniotic approach |
|
|
Total |
2539 |
1568 – Transabdominal and 971 - Transabdominal transamniotic |
|
During the screening of participants for the study, 42 patients refused to perform the CVS procedure. The reasons for refusal were posterior low placenta with bleeding (12), failure on the first attempt and rejection on the second attempt (5), and patient refusal at the time of counseling (25). Table II Shows reasons for the denial of CVS.
Table II: Reasons for refusal of CVS procedure
|
Reason for refusal for patients for CVS |
No of patients |
|
Reasons related to doctors’ perception |
|
|
Low posterior placenta with high risk of bleeding |
12 |
|
Reasons related to Patients’ perception |
|
|
Failure at first attempt so refuses the second attempt |
5 |
|
Refusal at the time of counselling |
25 |
A total of 2539 CVS procedures were carried out and processed for diagnosis. It was found that 2117 (83.38 %) cases were of β- thalassemia and 422 (16.61 %) cases for other genetic indications like Down syndrome, cystic fibrosis, Duchene muscular dystrophy, etc. (Table III). The gestation at CVS ranged from 12-14 weeks. Most procedures (2387,94 %) were done between 12 and 14 weeks, and only 24 (0.96 %) were done between 14-15 weeks. Only 128 (5 %) were carried out between 14-15 weeks. Reasons for delayed CVS were illiteracy and unawareness on the side of the patient, delayed referral from the remote, counseling not followed properly, etc.
Table III: Results of prenatal genetic diagnosis for β-thalassemia and other genetic disorders (n=2539).
|
Prenatal Genetic Diagnosis (PGD) (n = 2539) |
PGD for β-thalassemia (n = 2117) |
Normal |
462 |
|
Minor |
1052 |
||
|
Major |
583 |
||
|
Complicated |
20 |
||
|
PGD for diseases other than β- thalassemia (n = 422) |
|
422 |
The factors associated with a difficult aspiration were obesity, previous cesarean section scar, fibroids, retroverted uterus, and thin chorionic plate. In 2437 (96 %) cases, the sample yield was excellent (> 20-25 mg). However, the sample was adequate (10-20 mg) for a genetic diagnosis in the remaining cases. In the vast majority, 2530 (99.99 %) aspiration was successful in the first attempt. In the remaining 9 patients (0.1 %) in whom aspiration was unsuccessful, a second attempt was required, and two were called for an amniocentesis at 17 weeks. The overall success rate was 99.99 %.
Most patients (89 %) felt pain and discomfort lasting up to 24-36 hours after the procedure, and that was relieved by simple analgesics like Buscopan or Ibuprofen. Few patients developed spotting shortly after the process (2 %), but all recovered. No post-procedure infection was observed, and procedure-related risk of abortion was present in 7 patients (0.28 %). The remaining 9.28 % of patients did not develop any post-procedure complications.
Discussion
Prenatal diagnosis through early fetal sampling has played a pivotal role in preventing thalassemia and other genetic disorders. Ultrasound guidance adds to the safety of the fetus and the mother. Chorionic villus sampling (CVS) was introduced in the early 80s, and since then, it has given a new dimension to prenatal diagnosis. The procedure for CVS can be done earlier than amniocentesis [9]. In this study the majority of the CVS was done between 12-14 weeks of gestation. At this stage, the placenta is of adequate size and can be easily sampled. Though there is no lower limit for doing the CVS procedure, the best time for it is around 12-14 weeks of gestation because early aspiration is difficult due to a thin placental plate, as well as limb reduction defects have been related to procedures undertaken before 10 weeks gestation, the results of which were found to be consistent with previous studies [10,11].
Moreover, late aspiration creates more difficulty in the decision- making of medical termination of pregnancy due to the high risk of complications later if an abnormality is detected in the growing fetus. The CVS is done either by trans-abdominal or trans-cervical route, but the disadvantage of the trans-cervical course is the possibility of transmitting infection from the contaminated cervical canal. The trans-abdominal way has an obvious advantage of mechanical similarity to amniocentesis that makes CVS easier and familiar to perform [12]. In this study, practically all placenta positions were sampled through the trans-abdominal route without much difficulty, making it the most feasible choice for use in routine practice.
The choice of needle for CVS may vary from a simple 18G spinal needle to the co-axial chorion biopsy needles (20G). The latter is costlier, and availability could be better. Due to the larger gauze size, the chance of placental disruption and separation is higher with higher pain in the mother.
CVS is a safer procedure in experienced hands. Mild and transient post-procedure pain is common due to uterine cramps. Bacterial contamination is rare and serious complications can be avoided by taking utmost care to prevent injury to intestinal loops. This study's overall fetal loss rate is 0.28 % (7 out of 2539). The failure rate in CVS in this study is meager compared to the trans-cervical route (5- 8 %), with the risk of ascending infection, premature abortion, placental separation, bleeding, spotting, etc., as shown in previous literature. [8] Comparative analysis of CVS and amniocentesis reveals that the rate of miscarriages was higher by 0.5 % in amniocentesis, which was observed as a major finding from previous studies along with an earlier diagnosis of genetic deformities, which proves to be a better advantage of CVS procedures [13]. A significant difference in total losses was also observed among patients with transcervical CVS and transabdominal CVS, 8.6 % and 6.5 %, respectively, in previously reported studies, and a higher resampling requirement in transcervical CVS compared to transabdominal CVS, indicating superiority of transabdominal CVS which was found to be consistent with results of the current study [14].
Prenatal diagnosis is an essential recommendation for all couples with recessive genetic disorders like thalassemia with up to 25 % recurrence risk. Prenatal diagnosis is also offered to women aged 35 years or above or who are found by screening to be at a higher risk of having an infant with Down syndrome or another chromosomal abnormality. Chorionic villus sampling has a great advantage over mid-trimester amniocentesis in producing early results. Moreover, rapid analytic techniques have significantly reduced the waiting time between selection and diagnosis and help make earlier decisions on the medical termination of pregnancy if any abnormality is detected [15-17].
Conclusion
Ultrasound-guided transabdominal CVS is a useful outdoor fetal sampling and prenatal diagnosis procedure. It can play an important role in avoiding live birth with genetic disorders that are otherwise incurable. A placenta in almost any position can be approached without much difficulty. The procedure is also safe, having minimal risk of infection and abortion. Technical limitations are only Focal myometrial contraction, fibroids or retroplacental separation, and multiple scars. Still, awareness about CVS is less in rural areas of India, which need the attention of all health care stakeholders.
Acknowledgements: Indian Red Cross Society (Gujarat Branch) has created awareness regarding thalassemia and provided monitoring support to poor patients during this study.
Declaration
Funding: No funding was received for this study.
Conflict of interest: The authors have no competing interests to declare.
Ethics Approval: The study protocol was reviewed and approved by the Institutional Ethics Committee. The study followed the clinical research standards in Schedule Y and the new drugs and clinical trial act 2020.
Consent to Participate: All the participants were explained clearly about the nature and purpose of the study in the language they understood, and written informed permission was obtained from them. All the participants were ensured that their identity would not be revealed at any study stage.
Availability of data and Material: If required, the details of patients and complete data can be obtained from the corresponding author.
References
- Christianson A, Howson CP, Modell B (2006) March of Dimes Global Report of Birth Defects: The Hidden Toll of Dying and Disabled Children.
- Milunsky A, Atkins L (1974) Prenatal diagnosis of genetic disorders. An analysis of experience with 600 cases. JAMA. 230(2): 232-5.
- Hirschhorn K, Lucas M, Wallace I (1973) Precise identification of various chromosomal abnormalities. Ann Hum Genet. 36(4): 375-9.
- Hsu LY, Dubin EC, Kerenyi T, Hirschhorn K (1973) Results and pitfalls in prenatal cytogenetic diagnosis. J Med Genet. 10(2): 112-9.
- Rhoads GG, Jackson LG, Schlesselman SE, de la Cruz FF, Desnick RJ, et al. (1989) The safety and efficacy of chorionic villus sampling for early prenatal diagnosis of cytogenetic abnormalities. N Engl J Med. 320(10): 609-17.
- Randomised trial to assess safety and fetal outcome of early and midtrimester amniocentesis. The Canadian Early and Mid- trimester Amniocentesis Trial (CEMAT) Group. Lancet. 351(9098): 242-7.
- Alfirevic Z (2000) Early amniocentesis versus transabdominal chorion villus sampling for prenatal diagnosis. Cochrane Database Syst Rev. (2): CD000077.
- Hallak M, Johnson MP, Pryde PG, Isada NB, Zador IE, et al. (1992) Chorionic villus sampling: transabdominal versus transcervical approach in more than 4000 cases. Obstet Gynecol. 80(3 Pt 1): 349-52.
- Copeland KL, Carpenter RJ Jr, Fenolio KR, Ledbetter DH (1989) Integration of the transabdominal technique into an ongoing chorionic villus sampling program. Am J Obstet Gynecol. 161(5): 1289-94.
- Saxena R, Jain PK, Thomas E, Verma IC (1998) Prenatal diagnosis of beta-thalassaemia: experience in a developing country. Prenat Diagn. 18(1):1-7.
- Old JM, Ward RH, Petrou M, Karagözlu F, Modell B, et al. (1982) First-trimester fetal diagnosis for haemoglobinopathies: three cases. Lancet. 2(8313): 1413-6.
- Jackson LG, Zachary JM, Fowler SE, Desnick RJ, Golbus MS, et al. (1992) A randomized comparison of transcervical and transabdominal chorionic-villus sampling. The U.S. National Institute of Child Health and Human Development Chorionic- Villus Sampling and Amniocentesis Study Group. N Engl J Med. 327(9): 594-8.
- Meaney FJ, Riggle SM, Cunningham GC, Stern KS, Davis JG (1993) Prenatal genetic services: toward a national data base. Clin Obstet Gynecol. 36(3): 510-20.
- Smidt-Jensen S, Philip J (1991) Comparison of transabdominal and transcervical CVS and amniocentesis: sampling success and risk. Prenat Diagn. 11(8): 529-37.
- Wade RV, Young SR (1989) Analysis of fetal loss after transcervical chorionic villus sampling--a review of 719 patients. Am J Obstet Gynecol. 61(3): 513-8.
- Saxena R, Jain PK, Thomas E, Verma IC (1998) Prenatal diagnosis of beta-thalassaemia: experience in a developing country. Prenat Diagn. 18(1):1-7.
- Agarwal S, Gupta A, Gupta UR, Sarwai S, Phadke S, et al. (2003) Prenatal diagnosis in beta-thalassemia: an Indian experience. Fetal Diagn Ther. 18(5): 328-32.