Jebun Nessa1*, Israt Jahan2, Manira Sharmin Asha3, Sadia Afrin Anika4, AHM Zakir Hossain Shikder5, Gokul Chand Kundu6
1Chairman, Pedodontics Department, Faculty of Dentistry, BSMMU
2Phase B Resident of MS-Pedodontics, BSMMU
3Phase B Resident of MS-Pedodontics, BSMMU
4MS in Pedodontics (BSMMU), Assistant Professor of Pediatric Dentistry Department of Update Dental College
5Assistant Professor, Pedodontics Department BSMMU
6Medical Officer, Oral & Maxillo-Facial Surgery Department, BSMMU
*Corresponding Author: Jebun Nessa, Chairman, Pedodontics Department, Faculty of Dentistry, BSMMU.
Abstract
Background: Malignant leukemic disorder results in the clonal proliferation of lymphoid precursors with impeded maturation. B-cell or T- cell Leukemia or sometimes mixed lineage leukemia may occur as the disease can originate in lymphoid cells of different heredities. In children, predominantly Acute Lymphoblastic Leukemia (ALL) is the most prevalent type of neoplasm comprising about 30 % of all types of neoplasms. Over a period of 20 years, it has been reported that for ALL children with current treatment modalities, long-term survival (90 %) could be expected or most likely to be cured.
After proper and timely treatment most children with ALL (about 7-8 in 10 cases) can be recovered completely. But using aggressive chemotherapy and irradiation may considerably affect general health including oral and thus the quality of life of survivors. Following high-dose chemotherapy or radiotherapy increases the incidence of dental caries, gingival and periodontal diseases, candidiasis, oral mucositis, herpes simplex, loss of taste, and so on. Oral health is one of the most important health problems that viciously affect the other vital systems of the body. It is, therefore, fundamental to evaluate and eradicate potential sources of infection in the mouth.
Methods: 34 children of both sex (23 boys and 11 girls) between the age of 2-14 years, were selected as study sample using the purposive sampling technique who was diagnosed as ALL by the Pediatric Hematology Department of BSMMU and came to the Department of Pediatric Dentistry of BSMMU for seeking dental treatment.
Results: The mean age of the patients was 7.70±3.03. From data frequency, it was evident that 50 % of all study children were the 2nd child and also 50 % of the study children’s blood group was B positive. All the present study patients received chemotherapy for treatment purposes where the most frequent chemotherapies were Cyclophosphamide (47.1 %) and Vincristine (38.2 %) respectively. Following chemotherapy, 91.2 % of study participants developed some forms of oral manifestation and out of them, 55.9 % experienced dental caries, 32.4 % developed tooth sensitivity, and 2.9 % developed severe gum problems with tooth mobility. It was also observed that study children who received Cyclophosphamide were more susceptible to developing dental caries as well as tooth sensitivity than the other types of chemotherapy. Oral hygiene was assessed using Oral Hygiene Simplified Index, Plaque Index, Gingival Index, and dmft/DMFT.
Conclusion: Though the mortality rate in the pediatric leukemic population is declined radically with long-term survival rates (90 %) as a consequence, oral health complications are immensely a burning issue. To reduce the risk of such potential complications, the children who are to undergo chemotherapy and/or radiotherapy need to undertake early intervention through frequent and sharp observation of all pathological indications within the oral cavity. The pediatric dentist should have a vital role in taking preventive measures against dental caries, gum infections including other oral complications through meticulous and rigorous implementation of hygienic and care procedures that eliminate dental plaque as well as applying topical fluoride.
Objectives General Objective
To maintain the quality of life by taking appropriate preventive as well as curative measures against potential oral complications, especially for dental caries following high-dose aggressive chemotherapy or radiotherapy for ALL children.
Specific Objectives
- To reduce the incidence of dental caries as well as tooth sensitivity, prepare the mouth with prophylaxis cleaning followed by fluoride application before, during, and after ALL treatment regimen
- To decrease the incidence of gingival bleeding and swelling by establishing mouth preparation before, during, and after the treatment regime.
- To prevent the incidence of periodontal disease by preparing the mouth before, during, and after the treatment regime.
- To lessen the incidence of candidiasis by meticulous mouth preparation before starting the treatment regimen
- To reduce the incidence of oral mucositis through mouth preparation before starting the treatment regimen.
- To diminish the incidence of herpes simplex, prepare the mouth before starting the treatment regimen.
- To reinforce education to the patients and their guardians regarding the importance of optimal oral health care.
Introduction
Background
Leukemia is the multiplication of a clone of abnormal blood cells with diminished differentiation, parameter, and programmed cell death. ALL is the most common childhood cancer constitutes almost 30 % of all childhood malignancies with a topmost incidence at 2-5 years. [1,2]
ALL is a cancer of blood-forming cells in the bone marrow while abnormal white blood cells called Lymphoblasts fill the bone marrow and escape into the bloodstream.
As a result, the production of normal blood cells is disturbed causing anemia, bleeding problems, and infections. [3,4]
There are different subtypes of ALL. For example, the abnormal lymphoblasts can be immature B or T lymphocytes. The abnormal lymphoblasts divide and multiply continuously but cannot be able to mature into proper lymphocytes. Since ALL develops very quickly and rapidly so becomes worse over a few weeks or so unless starting treatment. Though ALL can occur at any age, about 6 in 10 cases occur in children. Among the children, ALL is most commonly developed between the ages of 4 and 7 years.
Boys are more usually affected than girls.
Mostly the reason for ALL is not known though certain risk factors may increase the chance to develop it, for instance, previous exposure to radiotherapy for another condition as well as exposure to the chemical benzene. Some genetic conditions, most commonly Down’s Syndrome can increase the risk of having ALL in the future. But ALL is not a genetic condition and does not run in ancestors.
Clinical manifestations include flu-like symptoms, pain in the bone or joint caused by malignant marrow expansion, anorexia, irritability, lethargy, bleeding, petechiae, fever, lymphadenopathy, hepato- splenomegaly, loss of taste and so on.3
Oral features are in the form of gingival enlargement with bleeding, periodontal disease, dental caries, mucositis, candidiasis, herpes simplex and so.4
Over a period of 20 years, it has been reported that for ALL children with current treatment modalities, long-term survival could be expected or most likely to be cured.
After proper and timely treatment most children with ALL (about 7- 8 in 10 cases) can be recovered completely. But using aggressive chemotherapy and irradiation may considerably affect general health including the oral cavity and thus the quality of life of survivors. Oral
Methodology
Study Population
The study population consisted of children who were diagnosed with ALL and had been treated with chemotherapy at the Pediatric Hematology Department of BSMMU. A total of 34 children of both sexes between the age of 2 and14 years, who also came to seek oral treatment at the Pedodontics Department, BSMMU Shahbag, Dhaka- 1000, were selected as the study population.
Study Area
Pedodontics Department collaboration with Pediatric Hematology Department, BSMMU, Shahbagh, Dhaka-1000
Study Design
A hospital-based interventional study
Sampling Technique
A purposive sampling method was applied for this study.
All children, between the age of 2 and 14 years old, suffering from ALL and who came to this hospital for seeking treatment and to fulfill health is one of the most important health problems that viciously affect the other vital systems of the body. [5,6]
Early dental intervention can reduce oral health problem significantly. It is, therefore, fundamental to evaluate the oral health survey and to eradicate potential sources of infection in the mouth in these ALL groups of patients. [7]
Oral complications due to high-dose chemotherapy or radiotherapy include increased incidence of dental caries, gingival and periodontal diseases, candidiasis, oral mucositis, herpes simplex, loss of taste and so on. [2,7]
Therefore, taking an appropriate treatment plan of dental care including prophylaxis such as instruction about hygiene and rigorous implementation of hygienic and care procedures that eliminate dental plaque, is very important for patients with ALL. Therefore, those who are to undergo chemotherapy and/or radiotherapy needs to undertake early intervention through frequent and sharp observation of all pathological indications within the oral cavity [8] as well as taking preventive measure against dental caries and gum infection by applying fluoride gel topically before, during and after taking therapy (both for chemo- and radiotherapy).
Preferably, all dental care should be completed before cancer therapy is initiated and if it is not practicable, priority treatments should be provided for the abolition of acute infection sources like extraction of grossly decayed teeth or infected teeth and so on. Therefore, the investigator is particularly interested in initial evaluation of oral and dental health as a mouth preparation before starting treatment that includes the following steps:
- Reviewing the child's medical history
- Reviewing the current hematological status
- Reviewing the proposed chemotherapy/radiation protocol
- Completing a thorough head, neck, and dental examination including panoramic and bitewing radiographs
the study eligibility criteria were invited to participate in the present study until a satisfactory number of participants was obtained (N=34). So, a total of 34 patients of both sexes (23 boys and 11 girls) were measured to gather the information.
The Principal Investigator informed the guardian of the study children about their involvement in this study. Written consent and confidentiality were considered and highlighted during recruitment procedures. All potential participants with their guardians had given a study instrument and allowed time to consider their participation in the study.
Inclusion Criteria
The study population consisted of children who were diagnosed with ALL and had been treated with chemotherapy at the Pediatric Hematology Department of BSMMU and came for seeking dental help in the department of Pedodontics department of BSMMU.
Exclusion Criteria
There were no exclusion criteria in this age group of children suffering from ALL and interested to participate in the study.
Sample Size
The sample size was 34 (N) who was registered and treated in the Department of Pediatric Hematology as well as Pedodontics Department, BSMMU.
Study Period
It was 12 months: From 01-01-2019 to 31.12.2019 though because of the Corona pandemic, timely data collection was tremendously hampered during that allocated period. So, the investigator extended the study period with the permission of the concerned authority.
Data Collection Tools
Using a standardized form, a semi-structured questionnaire was collected by the resident of the same department who was trained by the principal investigator in connection with patients’ care to obtain accurate data as well as to protect patient confidentiality.
Data Collection Procedure
The sociodemographic data of the patients were collected via a semi-structured questionnaire. One parent for each patient filled out a questionnaire with the help of one of the selected residents of the Pedodontics department of BSMMU.
A clinical examination and face-to-face interview were conducted by the Principal Investigator itself. In the clinical study, oral health was assessed using Oral Hygiene Index Simplified (OHI-S), Dental Plaque was assessed using Plaque Index (PI), and gingival health using Gingival Index (GI) whereas caries history was assessed using dmft/DMFT. Data from each study participant was linked using a confidential, exclusive ID number. Confidentiality was maintained by storing all data securely and electronic data were password-protected. Data were only identified by an identification code and stored individually and just accessible by the Principal Investigator.
Data Analysis
The data from the questionnaire and the clinical examination were entered and analyzed using SPSS version 21.0. Descriptive statistics (Frequency, percentage, mean with standard deviations) were reported in the result section. The Chi-square test was used to test the statistical significance of observed associations at P > 0.05.
This study was approved by the IRB Ethics Committee of the BSMMU.
Result Obtained
Table I: Distribution of Age of Study Population (N=34)
|
Age Group (Years) |
Frequency |
Percent (%) |
|
<10 |
26 |
76.5 |
|
>10 |
8 |
23.5 |
|
Total |
34 |
100.0 |
Table I shows that the mean age of the study patients was 7.70 3.03 years, 76.5 % (26) of children were in the age group of 2-9 years and 23.5 % (8) were in the age group of 10-14 years. The age range was 2-14 years.
Figure 1: Showing Gender Distribution of Study Children (N=34)
Figure 1 presenting the distribution of study children (N=34) according to gender type where 23 (68%) are boys and 11(32%) are girls.
Birth order
Table II: Distribution of the Study Patients by Birth Order (N=34)
|
Birth order |
Frequency |
Percentage (%) |
|
Only Child |
4 |
11.8 |
|
1st child |
8 |
23.5 |
|
2nd child |
17 |
50.0 |
|
3rd Child |
2 |
5.9 |
|
4th Child |
2 |
5.9 |
|
6th Child |
1 |
2.9 |
|
Total |
34 |
100.0 |
According to Table II, in terms of birth order, it was evident that among this study population (N=34), the 2nd child showed the highest frequency 17 (50 %).
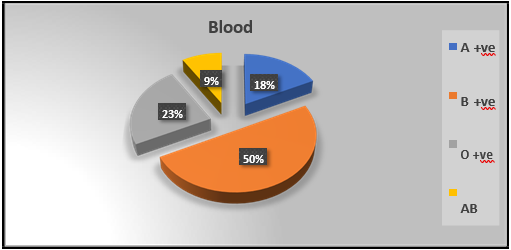
Figure 2: Distribution of the study patients by blood group (N=34)
Figure 2: displays 50 % (17) of the present study population (N=34) had B positive blood group.
Table III: Distribution of ALL study patients by oral and dental manifestation (N=34)
|
Oral and dental manifestation |
Frequency |
Percentage (%) |
|
Yes |
29 |
85.3 |
|
No |
5 |
14.7 |
|
Total |
34 |
100.0 |
Table III expresses that among all ALL study patients (N=34), 85.3 % (29) came with oral and dental manifestation while 14.7 % (5) had not such manifestation before receiving therapy.
Table IV: Types of chemotherapy received by the study patients (N=34)
|
Type of chemotherapy |
Frequency |
Percentage (%) |
|
L-Asparaginase, Vincristine |
2 |
5.9 |
|
Cyclophosphamide |
16 |
47.1 |
|
Vincristine |
13 |
38.2 |
|
Doxorubicin |
2 |
5.9 |
|
Mitoxantrone |
1 |
2.9 |
|
Total |
34 |
100.0 |
Table IV exhibits the data about ALL therapy received by the present study population (N=34) where the most frequent ALL therapy were chemotherapy Cyclophosphamide (47.1 %) and the Vincristine (38.2 %) respectively.
Table V: Frequency distribution of oral manifestation of the study patients following chemotherapy (N=34)
|
Oral Manifestation |
Frequency |
Percentage (%) |
|
None |
3 |
8.8 |
|
Sensitivity |
11 |
32.4 |
|
Caries |
19 |
55.9 |
|
Gum infection with tooth mobility |
1 |
2.9 |
|
Total |
34 |
100.0 |
Table V showed that among all study subjects (N=34) 8.8 % patient did not develop any oral manifestation but the rest 91.2 % developed some kind of oral manifestation. It had been observed that among all study population (N=34) 55.9 % of study children experienced dental caries after receiving chemotherapy, 32.4 % developed tooth sensitivity and only 2.9 % study patient developed severe gum problem with tooth mobility.
Table VI: Association of Oro-Dental Problem after Receiving Chemotherapy (N=34)
|
Types of Chemotherapy |
Oral and Dental Complication after Therapy |
||||
|
None |
Gum Infection |
Sensitivity |
Caries |
Total |
|
|
L-Asparaginase, Vincristine |
1(33.3 %) |
1(100.0 %) |
0(0.0 %) |
0(0.0 %) |
2.0 |
|
Cyclophosphamide |
0(0.0 %) |
0(0.0 %) |
5(45.5 %) |
11(57.9 %) |
16.0 |
|
Vincristine |
1(33.3 %) |
0(0.0 %) |
6(54.5 %) |
6(31.6 %) |
13.0 |
|
Doxorubicin |
1(33.3 %) |
0(0.0 %) |
0(0.0 %) |
1(5.3 %) |
2.0 |
|
Mitoxantrone |
0(0.0 %) |
0(0.0 %) |
0(0.0 %) |
1(5.3 %) |
1.0 |
|
Total |
3.0 |
1.0 |
11.0 |
19.0 |
34 |
Table V & VI depicted that among all study patients (N=34) most ALL patients (47.1 %) received Cyclophosphamide for the treatment of ALL and the next frequent chemotherapy was Vincristine (38.2 %). Study data also revealed that among those patients who were received Cyclophosphamide, mostly developed dental caries (57.9 %) as oral and dental complication.
Figure 3: Improvement of Dental Complication after Fluoride Application
Figure 3 displaying that among all study patients (N=34), after fluoride therapy, 25 (74 %) cases showed improvement of oral and dental problem that is no more caries or caries progression, and no sensitivity as well and 9 (26 %) showed no change in their complication and this difference is statistically significant (P > 0.05).
Conclusion
Because of dramatic improvements in the understanding of biology and pathogenesis of ALL, physicians are capable to resolve and plan the appropriate therapy for ALL patients. Therefore, throughout the world, now the disease prognosis is improved greatly as the mortality rate in the pediatric leukemic population is declined drastically. Though survival periods have become longer their quality of life is negatively affected by complications of chemotherapy and radiotherapy as well as the disease itself, with troubles in social and intellectual functioning.
As the oral manifestation would sometimes be the key manifestation of ALL, a dentist can diagnose a case of ALL in regular dental practice. So, the pediatric dentist has an important role in the prevention, stabilization, and treatment of oral and dental problems that can compromise the child’s health and quality of life during and follow-up of the ALL treatment.
Recommendation
In the mouth, many different classes of bacteria are residing that are helpful for us.
Chemotherapy and radiation therapy may cause changes in the lining of the mouth and the salivary glands, which make saliva, due to high mitotic activity. This can slow or stop the growth of new cells as well as upset the healthy balance of bacteria thus creating a harmful environment and may lead to mouth sores, infections, and tooth decay. Moreover, any dormant existing lesions, such as cavities, broken teeth, loose crowns or fillings, and gum disease can flare up and become life-threatening once the child is immune suppressed. Since oral complications may be triggered by receiving high-dose chemotherapy, radiation therapy, or stem cell transplant, to minimize oral problems and discomfort pediatric dentists should have an oral care plan in place before, during, and after treatment. Following chemotherapy or radiotherapy, if there occur fewer complications, cancer treatment may work better and the patient may have a better quality of life. A multidisciplinary approach involving oncologists, hematologists, nurses, dieticians, pediatric dentists, and other related health professionals is essential to work closely in caring for the child before, during, and after ALL cancer therapy. Prevention of oral complications includes a healthy diet, good oral care, and dental check-ups as well as routine fluoride application.
Limitation
Because of the Corona pandemic, the investigator challenged tremendous barriers to conducting the present study in terms of data collection as well as operating the procedure taken to combat the initiation of potential complications of chemotherapy in this study.
References
- Smith MA, Ries LAG. Childhood cancer: incidence, survival, and mortality. In: Principles and Practice of Pediatric Oncology. Pizzo PA, Poplack DG (eds). 4th edition. Philadelphia, Pa, USA: Lippincott Williams & Wilkins; 2002, pp. 1–12.
- Balis FM, Holcenberg JS, Blaney SM. General principles of chemotherapy. In: Principles and Practice of Pediatric Oncology. Pizzo PA, Poplack DG (eds). 4th edition. Philadelphia, Pa, USA: Lippincott Williams & Wilkins; 2002, pp. 237–308.
- Javed F, Utreja A, Bello Correa FO, Al-Askar M, Hudieb M, et. al. (2012) Oral Health Status in Children with Acute Lymphoblastic Leukemia. Crit Rev Oncol Hematol. 83(3): 303-9.
- Nasim VS, Shetty YR, Hegde AM (2007) Dental Health Status in Children with Acute Lymphoblastic Leukemia. J Clin Pediatr Dent. 31(3): 210-3.
- Margolin JF, Steuber CP, Poplack DG. Acute lymphoblastic leukemia. In: Principles and Practice of Pediatric Oncology. Pizzo PA, Poplack DG (eds). 4th edition. Philadelphia, Pa, USA: Lippincott Williams & Wilkins; 2002, pp. 489–544.
- Barberia B, Hernandez C, Miralles V, Maroto M. (2008) Paediatric Patients Receiving Oncology Therepy: Review of the Literature and Oral Management Guidelines. Europ J Paediatr Dent. 9(4): 188-194.
- Azher U, Shiggaon N (2013) Oral Health Status in Children with Acute Lymphoblastic Leukemia Undergoing Chemotherapy. Indian J Dent Res. 24(4): 523.
- Elzbieta P, Maria MB (2012) Oral hygiene in Children Suffering from Acute Lymphobastic Leukemia Living in Rural and Urban Regions. Annals of Agri and Environ Med. 19(3): 529-533.
- Benson RE, Rodd HD, North S, Loescher AR, Farthing PM, et al. (2007) Leukaemic infiltration of the mandible in a young girl. Int. J paediatr Dent. 17(2): 145-150.
- Cem D, Cenk H, Bolent A, lgen P, Atila T (2001) Oral Health Status in Children with Acute Lymphoblastic Leukemia and Lymphoma Turk J Haematol. 18(3): 179-183.
- Collard MM, Hunter ML (2001) Oral and Dental Care in Acute Lymphobastic Leukemia: a Survey of United Kingdom Children’s Cancer Study Group Centres. Int J Paediatr Dent. 11(5): 347-351.
- Millns B, Martin MV, Williams MC (1999) Raised Salivary Endotoxin Cncentration As a Predictor of Infection in Pediatric Leukemia Patients. Oral Surg Oral Med Oral pathol Oral Radiol Endod. 88(1): 50-55.
- Winkler O, Hadnagy W, Idel H (2001) Cytokines Detectable in Saliva of Children as Appropriate Markers of Local Immunity of the Oral Cavity-an Approach for the Use in Air Pollution Studies. Int. J Hyg Environ Health. 204(2-3): 181-184.
- Bonnaure- Mallet M, Bunetel L, Tricot-Doleux S, Guearin J, Bergeron C, et al. (1998) Oral Complications During Treatment of Malignant Diseases in Childhood: Effects of Tooth Brushing. Eur J Cancer. 34(10): 1588-1591.
- Vaughan MD, Rowland CC, Tong X, Srivastava DK, Hale GA, et al. (2005) Dental Abnormalities in Children Preparing forPediatric Bone Marrow Transplantation. Bone marrow Transplant. 36(10): 863-866.
- Vissink A, Burlage FR, Spijkervet FK, Jansma J, Coppes RP (2003) Prevention and Treatment of the Consequences of Head and Neck Radiotherapy. Crit Rev Oral Biol Med. 14(3): 213-225.
- Venkatesh Babu B NS, Kavyashree BS (2015) Comparative Evaluation of Oral Health Status in Children with Acute Lymphoblastic Leukemia. Int J Scien Stud. 2(10): 52-55.
- Hegde AM, Joshi S, Rai K, Shetty S (2011) Evaluation of Oral Hygiene Status, Salivary Characteristics and Dental Caries Experience in Acute Lymphoblastic Leukemia (ALL) Children. J Clin Pediatr Dent. 35(3): 319-23.
- Ponce-Torres E, Ruiz- Rodriguez MS, Alejo- Gonzalez F, Hernandez-Sierra JF, Pozos –Guillen AJ (2010) Oral Manifestations in Pediatric Patients Receiving Chemotherapy for Acute Lymphoblastic Leukemia. J Clin Pediatr Dent. 34(3): 275- 9.
- Al-Mashhadane FA (2007) Oral Health Status Among Children Receiving Chemotherapy. Dent J. 7(1): 96-100.
- Farber S, Diamond LK, Mercer RD, Sylvester RF, Wolff JA (148) Temporary Remissions in Acute Leukemia in Children Produced by Folic Acid Antagonist, 4-Aminopteroyl-Glutamic Acid (Aminopterin). The New Eng J Med. 238(23): 787-793.
- George SL, Aur RJ, Mauer AM, Simone JV (1979) A Reappraisal of the Results of Stopping Therapy in Childhood Leukemia. The New Eng J Med. 300(6): 269-73.