Vlad Teodor Berbecar1*, Gener Ismail2, Gabriel Mircescu3
1MD, University of Medicine and Pharmacy “Carol Davila”, Bucharest, Romania
2Assoc. Prof., MD, Ph.D., University of Medicine and Pharmacy “Carol Davila”, Head of Nephrology Department, Fundeni Clinical Institute, Bucharest, Romania.
3Prof., MD, Ph.D., University of Medicine and Pharmacy “Carol Davila”, Head of Nephrology Department, “Carol Davila Nephrology Hospital”, Bucharest, Romania.
*Correspondence: Berbecar Vlad Teodor, MD, University of Medicine and Pharmacy “Carol Davila”, Bucharest, Romania
Abstract
Introduction
Cardiovascular disease (CVD) is the most common cause of morbidity and mortality worldwide, and hypertension is the most important modifiable risk factor for CVD. Although Romania has one of the highest CVD mortalities in Europe, it is underestimated in rural areas where access to healthcare is limited.
Methods
Data from 2988 subjects were collected during health campaigns aimed at providing free medical care in rural, remote areas of Romania. Rural residents underwent medical examinations and blood tests to evaluate the prevalence CVDs and of their major risk factors, i.e., hypertension (HT), obesity, smoking, diabetes, and dyslipidemia.
Results
The overall prevalence of CVD was 14 %: coronary heart disease (9 %), stroke (2.9 %), peripheral artery disease (1.3 %), and atrial fibrillation (3.2 %). The prevalence of HT was unexpectedly high (72.8 %) as was the proportion of newly diagnosed HT (33.3 %). Of those aware, 65 % were treated, but only 17.2 % were on target. Other CV risk factors prevalence was obesity (31.3 %), diabetes mellitus (12.6 %), dyslipidaemia (64.7 %), and smoking (16.2 %). Obesity, smoking, and diabetes increased the likelihood of developing CVD by 1.7 times, with HT being the leading risk factor by 2.7-fold. The 10-year risk of a cardiovascular event (Framingham score) was high (over 20 %) in one-third of the subjects, while the risk of a fatal CV event in the following 10 years (SCORE) was above 5 % in a quarter (22 %) of the studied population.
Conclusion
This is the first study that focused on the health of the rural population in Romania and used data collected from mobile health caravans, a concept that is in continuous growth. The results showed an unexpectedly high prevalence of HT, as well as a high risk of developing cardiovascular disease, pointing to the need for strategies to improve medical care.
Keywords: rural health, hypertension, cardiovascular disease, cardiovascular risk, mobile healthcare.
Introduction
With more than one-third of the global adult population diagnosed, hypertension (HT) is the most frequent modifiable risk factor for cardiovascular diseases (CVDs), which are responsible for over 30 % of deaths worldwide [1,2]. In Europe, CVDs accounted for 45 % of all deaths in 2016, mostly being attributable to coronary heart disease (CHD) and stroke [3]. In recent years, CVD prevalence has slightly declined in western societies. Meanwhile, more than 80 % of the global burden of CVD occurs in developing countries, where cardiovascular (CV) mortality is 2-to-5 times higher than that attributable to infectious disease, and where HT is more prevalent [4,5].
Data from the European Registry of Cardiovascular Disease showed that Romania had high CV mortality and is the leader in stroke mortality [6]. Previous studies evaluating CV risk factors in the Romanian general population reported prevalence of45.1 %, 31.9 %, 11.6 %, and 67.1 % for HT, obesity, diabetes, and dyslipidaemia, respectively [7,8,9,10].
Primary prevention of CVD remains a major challenge, although starting from the Framingham study (1961), it has been demonstrated that hypertension, obesity, smoking, diabetes, and dyslipidemia represent major risk factors for developing CVD [11,12]. Since then, many studies have shown that management of these modifiable risk factors combined with healthy lifestyles decreases the occurrence of CVD [13,14,15,16,17].
Population studies focusing on the rural residents in Romania are scarce. Rural areas cover 87.1 % of the country’s surface and include approximately 45 % of its total population [18]. Most of the population in these areas is confronted with severe poverty and poor living conditions [19]. Some of Romania’s towns are also considered “rural”, struggling with the same quality of living problems as the rural villages [20].
Providing healthcare to rural and remote areas is challenging. Geographic access and transportation to medical services is an important factor for reduced healthcare usage in rural areas, especially because of spatial isolation from metropolitan areas or urban centers [21]. Inadequate availability or supply of rural healthcare services is the most important barrier to accessing services at times of need [22]. There is a shortage of general practitioners (GPs) especially in rural areas and there is a growing concern that healthcare systems will not be able to provide sufficient and close-to-home care to meet the future needs of an increasingly aging society [23].
The differences between rural and urban residents have been highlighted in many studies. Waist and hip circumference, body mass index, and total cholesterol levels were higher in rural areas than in urban areas [24]. Having only primary education was more common in rural areas than in urban areas [25]. Cultural differences affect healthcare and can influence the threshold of ill health, below which individuals choose not to seek medical intervention. Studies have also shown that rural areas exhibit lower levels of hospital usage and have poorer health outcomes than urban areas [26]. Also, rural residents had fewer overall visits and saw fewer medical specialists and more generalists for their care than their urban counterparts [27]. Previous social studies done on the Romanian rural population revealed a high correlation between the quality of living and the frequency of visits to the doctor [28].
The current paper assesses the prevalence of cardiovascular disease and cardiovascular risk factors, with emphasis on HT, and uses risk assessment models to estimate the risk of CVD disease in the rural population of Romania.
Materials and methods
Setting and subjects
Information regarding the health of rural residents was collected during campaigns organized by the “Doctors’ Caravan Association”- a non-governmental organization composed of physicians and medical students volunteering to travel to Romania’s rural regions and offer free medical services. Because of the large number of rural inhabitants (almost half of the country’s population) that strive with poor infrastructure and reduced access to healthcare, a model of mobile health caravans was designed by the “Doctors’ Caravan Association”, that managed to create a fully functioning team of physicians equipped with adequate medical tools that could supply mobile healthcare where it was most needed.

Figure 1. Study sites
The study analyzed data recorded from 2015 - 2017 in 20 villages/small towns located mostly in the South and East of Romania [Figure 1]. The selection of the settlements was done according to the association’s objectives, aimed at providing basic medical care to people in distant rural areas with low accessibility to medical services. These settlements are representative of the economic disparities between urban and rural Romania, where a large proportion of the marginalized rural population resides. A total of 2988 patients were examined by the volunteering physicians.
Methods
All patients signed a consent form agreeing to be examined by the physicians and agreeing to participate in the epidemiologic study. The inclusion criteria consisted of (> 18 years) of age and willingness to sign the consent form. All the patients who did not meet these criteria were excluded from the database, even though they were examined by the physicians in the health campaigns.
Initially, blood tests were drawn from the inhabitants willing to be examined. The blood panel included a complete blood count, lipid profile (cholesterol, triglycerides, HDL), glucose and glycated hemoglobin, as well as markers for liver and kidney function (alanine transaminase and creatinine) and chronic hepatitis B and C markers. On a second visit, a team of medical doctors recorded a standardized medical history, measured the blood pressure (BP), performed a full physical exam, and gave treatment recommendations based on the clinical findings and the laboratory tests.
Due to several factors, including patients’ large number, multiple visits to the study locations and the variability of physicians who performed the examination, the extent of data that entered in the analysis was uneven: some patients performed blood tests but did not subsequently undergo a physical examination, while others benefited only from the clinical examination.
Parameters
Cardiovascular diseases included anamnestic or diagnosed CHD (coronary heart disease), stroke, peripheral artery disease (PAD), and atrial fibrillation (AF).
HT was defined using ESH/ESC criteria [29]: systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg, previous history of HT, and current anti-HT therapy. Blood pressure was measured in the office, while at rest (sitting). Two measurements were performed at 2-3 minutes intervals and the lesser value was retained. Controlled HT was diagnosed when the subject was taking anti-HT medication and had a measured SBP < 140 mmHg and DPB < 90 mmHg. Awareness was defined as the percentage of patients with a previous diagnosis of HT, from the total number of hypertensives.
Weight and height were measured and body mass index (BMI; kg/m2) was calculated. Subjects with a BMI of 25-29 were considered overweight, those with a BMI of ≥ 30, obese while those with BMI < 18.5, underweight. Abdominal circumference was measured to identify abdominal obesity, which was defined as ≥ 102 cm for men and ≥ 88 cm for women [30].
Smoking status was defined as a currently active smoker (if one smoked at least 1 cigarette per day) or former smoker (if he had ceased smoking for more than one year).
Diabetes mellitus (DM) was defined by fasting plasma glucose ≥ 126mg/dL, glycated hemoglobin (HbA1c) ≥ 6.5%, or a previous diagnosis made by a specialist, regardless of glucose or HbA1c values.
Lipid disorders were defined on the basis of NCEP ATPIII recommendations [31]: Hypertriglyceridemia was defined by a triglycerides (TG) serum level ≥ 150mg/dL, hypercholesterolemia was defined by a total serum cholesterol (Chol) level ≥ 200mg/dL. Elevated levels of both TG and Chol were considered mixed dyslipidemia.
The general CVD risk was estimated with the Framingham 10–year CV event risk score. The CVD risk was stratified into low (< 10 %), moderate (10 to 20 %), high (20 to 30 %), and very high (≥ 30 %). The 10-year risk of developing a fatal CV event was estimated using the SCORE (Systematic Coronary Risk Evaluation) risk chart. The formulas provided in the original articles were used for calculating the risk scores. All the limitations were considered when the scores were computed (i.e., age 40-65 for SCORE), which meant reducing the size of the analyzed cohort to those that fitted into the model [32,33]. The high-risk chart was used for SCORE according to the recommendations for this country/region.
Statistical analysis
Descriptive analysis (mean, median, the standard deviation for continuous data, and frequency analysis for categorical data) was performed on all target variables. The numerical variables that had a normal distribution were reported as mean and standard deviation. Kolmogorov-Smirnov and Shapiro-Wilk tests were used to evaluate the distribution of continuous data, according to which appropriate tests were used for comparison between groups: independent samples t-test or Mann-Whitney U test for differences between 2 independent groups, ANOVA or Kruskal-Wallis test for differences between ≥ 3 independent groups. The Chi-square test was used to analyze differences between categorical data. Binominal logistic regression was used to estimate the risk factors. An alpha level of P < 0.05 was used to test the statistical significance.
Results
The characteristics of the investigated cohort are summarized in Table I. The mean age was 55 (±16) years, 31 % were aged over 65 and 70 % were female.
Table I. Characteristics of the investigated cohort
|
All |
Male |
Female |
p |
||||
|
N |
Mean or % (95% CI) |
N |
Mean or % (95% CI) |
N |
Mean or % (95% CI) |
||
|
Age |
2987 |
||||||
|
Years (mean ±SD) >65 years (%) |
920 |
54.9 (±16.3) 30.8 % |
909 306 |
56.6 (±16) 33.7 % (30.3-36.6) |
2078 614 |
54.2 (±16.3) 29.5 % (27.5-31.6) |
< 0.01 |
|
Sex; percent men |
2987 |
909 |
30.4 % |
2078 |
69.6 % |
||
|
Smoking |
2407 |
< 0.01 |
|||||
|
Non-smoker |
1778 |
73.9 % (72.1-75.6) |
374 |
51.60 % (48-54.9) |
1404 |
83.5 % (81.8-85.4) |
|
|
Smoker |
391 |
16.2 % (14.8-17.8) |
186 |
25.7 % (22.5-29) |
205 |
12.2 % (10.6-13.7) |
|
|
Former smoker |
238 |
9.9 % (8.7-11.1) |
165 |
22.8 % (19.7-25.9) |
73 |
4.3 % (3.3-5.3) |
|
|
Weight status |
2380 |
< 0.01 |
|||||
|
Underweight |
37 |
1.6 % (1.1-2.1) |
9 |
1.3 % (0.6-2.1) |
28 |
1.7 % (1.1-2.3) |
|
|
Normal weight |
778 |
32.7 % (30.8-34.5) |
259 |
36.1 % (32.5-40) |
519 |
31.2 % (29-33.3) |
|
|
Overweight |
819 |
34.4 % (32.5-36.3) |
287 |
40 % (36.5-44.1) |
532 |
32.0 % (29.9-34.3) |
|
|
Obese |
746 |
31.3 % (29.4-33.2) |
162 |
22.6 % (19.4-25.8) |
584 |
35.1 % (32.7-37.3) |
|
|
Abdominal obesity |
1397 |
59.2 % (57.1-61.2) |
277 |
39.5 % (36.2-43.2) |
1120 |
67.6 % (65.2-69.9) |
< 0.01 |
|
BMI (mean± SD) |
2380 |
27.9 (±5.9) |
717 |
26.7 (±4.8) |
1663 |
28.3 (±6.2) |
< 0.01 |
|
Diabetes mellitus Diabetes mellitus (all) |
2388 301 |
12.6 % (11.3-13.9) |
118 |
16.3 % (13.7-19.3) |
183 |
10.9 % (9.4-12.5) |
< 0.01 |
|
Awareness |
146 |
48.5 % (42.9-53.8) |
58 |
49.2 % (39.8-58.2) |
88 |
48.1 % (40.7-55.9) |
|
|
Newly diagnosed |
155 |
51.5 % (46.2-57.1) |
60 |
50.8 % (41.8-60.2) |
95 |
51.9 % (44.1-59.3) |
|
|
Dyslipidemia Dyslipidemia (all) |
2351 1523 |
64.7 % (62.7-66.6) |
434 |
61.3 % (57.6-64.7) |
1089 |
66.2 % (63.8-68.4) |
0.02 |
|
Hypercholesterolemia |
1008 |
42.9 % (41-45.1) |
256 |
36.2 % (32.9-39.6) |
752 |
45.7 % (43.1-48.5) |
|
|
Hypertriglyceridemia |
103 |
4.4 % (3.6-5.2) |
43 |
6.1 % (4.3-7.9) |
60 |
3.6 % (2.8-4.6) |
|
|
Mixed dyslipidemia |
409 |
17.4 % (15.8-18.8) |
134 |
19 % (16-21.9) |
275 |
16.7 % (14.9-18.5) |
|
|
Hypertension HTA (all) Newly diagnosed HT Previous history of HT Treated Controlled HT |
2407 1752 584 1168 759 128 |
72.8 % (71-74.6) 33.3 % (31.1-35.4) 66.7 % (64.5-68.8) 65 % (62.2-67.7) 17.2 % (14.6-19.9) |
559 238 321 179 35 |
77.1 % (73.9-80.3) 42.6 % (38.6-46.7) 57.4 % (53.4-61.3) 55.8 % (50.2-61.4) 19.7 % (14.2-25.5) |
1193 346 847 580 93 |
70.9 % (69-73.1) 29% (26.4-31.7) 71 % (68.4-73.5) 68.5 % (65.4-71.8) 16.4 % (13.2-1.7) |
< 0.01 < 0.01 < 0.01 < 0.01 < 0.01 0.05 |
|
SBP; (mean ±SD) DBP; (mean ±SD)) |
2407 2407 |
145 (±25.5) 87.8 (±13.9) |
724 742 |
147.1 (±23.1) 88.9 (±13.6) |
1665 1665 |
144.1 (±26.5) 87.2 (±14.1) |
<0.01 <0.01 |
|
Cardiovascular disease CVD (all) |
2407 338 |
14.0 % (12.8-15.5) |
104 |
14.3 % (11.9-17) |
234 |
13.9 % (12.2-15.6) |
0.77 |
|
CHD |
216 |
9 % (7.9-10.1) |
64 |
8.8 % (6.8-10.9) |
152 |
9 % (7.7-10.5) |
0.86 |
|
Stroke |
71 |
2.9 % (2.3-3.7) |
27 |
3.7 % (2.3-5.2) |
44 |
2.6 % (1.9-3.5) |
0.14 |
|
PAD |
32 |
1.3 % (0.9-1.8) |
9 |
1.2 % (0.6-2.1) |
23 |
1.4 % (0.9-1.9) |
0.8 |
|
AF |
76 |
3.2 % (2.5-3.9) |
27 |
3.7 % (2.5-5.1) |
49 |
2.9 % (2.1-3.7) |
0.29 |
SD= standard deviation
Cardiovascular risk factors Smoking
The smoking frequency was relatively low, 16.2 % were smokers and 9.9 % were former smokers. The majority of smokers and former smokers were male (25.7 % vs 12.2 %, respectively,22.8 % vs. 4.3 %; p=0.001) [Table I].
Overweight and obesity
About 66 % of the cohort had a BMI above 25kg/m2, and 31.3 % were obese. Obesity and abdominal obesity were more frequent in females (35.1 % vs 22.6 %, p < 0.001) and (67.6 % vs 39.5 %; p < 0.001) [Table I].
Diabetes mellitus
The prevalence of diabetes mellitus was 12.6 % and was higher in men (16.3 % vs 11 %, p < 0.001). Half of the diabetics (51.5 %) were newly diagnosed [Table I].
Dyslipidemia
In this cohort, 64.7 % had dyslipidemia (42.9 % hypercholesterolemia, 4.4 % hypertriglyceridemia and 17.4 % mixed dyslipidemia). Dyslipidemia was more frequent in females (66.2 % vs 61.3 %, p = 0.02). [Table I].
Hypertension
The mean SBP/ DBP was 145/87.8 mmHg and was higher in males than in females (147.1/88.9 vs 144.1/87.2 mmHg) [Table I].
The HT prevalence (known and newly diagnosed) in the whole cohort was 72.8%. HT prevalence was related to age and gender: it was more frequent in males, and significantly increased with age in both sexes, reaching a plateau around 90% at ages above 60 years [Figure 2].
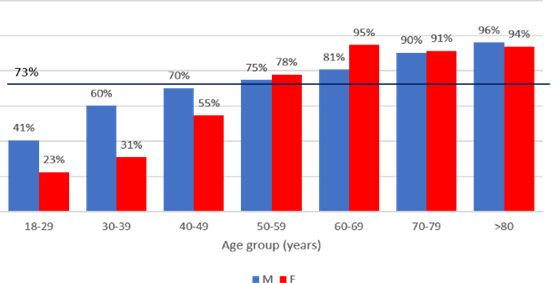
Figure 2. Prevalence of HT according to gender and age group
HT was newly diagnosed in 584 participants (33 % of hypertensives). Accordingly, 66 % of hypertensives were aware that they had HT. About two-thirds (65 %) of those with known HT were treated, but only 17.2 % were on the target. More important, when counting all hypertensive subjects, i.e., known and newly diagnosed, HT was controlled in only 8 % [Figure 3].

Figure 3. Total, known, treated, and controlled HT.
In a model of binary logistic regression, sex, age, obesity, and diabetes mellitus, but not smoking were retained as factors independently associated with HT. Males were 1.3 times more likely to have HT than females, increasing age was associated with an increased likelihood of HT (1 % per year), the odds of participants with obesity and diabetes to have HT were 2.9 and, respectively, 2.5 times higher (Table II).
Table II. Predictors of HT
|
B |
S.E. |
Exp(B) |
95% CI for EXP(B) |
Sig. |
||
|
Sex (male) |
0.3 |
0.1 |
1.3 |
1.02 |
1.76 |
0.04 |
|
Age (years) |
0.1 |
0 |
1.075 |
1.066 |
1.085 |
<0.001 |
|
Smoking (yes) |
-0.2 |
0.1 |
0.8 |
0.6 |
1.1 |
0.17 |
|
Obesity (yes) |
1 |
0.1 |
2.8 |
2.13 |
3.8 |
<0.001 |
|
Diabetes mellitus (yes) |
0.9 |
0.3 |
2.5 |
1.49 |
4.19 |
<0.001 |
|
Constant |
-3.4 |
0.2 |
0 |
- |
- |
<0.001 |
|
Binary logistic regression. Dependent variable HT Yes/No Independent variable entered in the first step: Sex, Age, Smoking, Obesity, Diabetes mellitus |
||||||
|
Chi2 504.7; p < 0.001; Cox & Snell R2=0.24 Hosmer & Lemeshow Chi2 6.5; p=0.59 |
||||||
HT was closely related to cardiovascular disease: 94 % of patients with cardiovascular disease had HT.
Cardiovascular disease Prevalence
The prevalence of CVD was 14 %: CHD (8.1 %), stroke (2.7 %), PAD (1.2 %), and AF (2.7 %). The distribution of CVDs was even between sexes but increased with age, reaching a prevalence of over 20 % in those older than 60 years (Table III).
Table III. Prevalence of CVD according to age group and sex
|
Age group (years) |
||||||||
|
18-29 |
30-39 |
40-49 |
50-59 |
60-69 |
70-79 |
>80 |
||
|
CVD |
M |
3.1 % |
0 % |
3.5 % |
11 % |
19.1 % |
22.1 % |
39.6 % |
|
F |
0.9 % |
1 % |
3.5 % |
10 % |
21 % |
30 % |
31.8 % |
|
|
Total |
1.4 % |
0.8 % |
3.5 % |
10.3 % |
20.4 % |
27.7 % |
35.3 % |
|
Risk factors
The CV risk factors were evaluated by logistic regression. Although increasing age was independently associated with an increased likelihood of CVD, obesity, smoking, and diabetes increased the likelihood of CVDs by 1.7 times; HT was the most important risk factor as it increased the risk by 2.7-fold (Table IV).
Table IV. Predictors of CVD
|
B |
S.E. |
Exp(B) |
95% CI for EXP(B) |
Sig. |
||
|
Sex (male) |
0.06 |
0.15 |
1 |
0.7 |
1.4 |
0.68 |
|
Age (years) |
0.06 |
0 |
1.06 |
1.05 |
1.07 |
< 0.001 |
|
Obesity (yes) |
0.55 |
0.15 |
1.7 |
1.2 |
2.3 |
< 0.001 |
|
Smoking (yes) |
0.58 |
0.28 |
1.7 |
1 |
3.1 |
0.04 |
|
Hypertension (yes) |
1 |
0.28 |
2.7 |
1.5 |
4.7 |
< 0.001 |
|
Diabetes mellitus (yes) |
0.53 |
0.17 |
1.7 |
1.2 |
2.3 |
0.02 |
|
Constant |
-7.3 |
0.52 |
0 |
- |
- |
< 0.001 |
|
Binary logistic regression. Dependent variable CV disease (Yes/No). Independent variables entered in the first step: Sex, Age, Obesity, Smoking, Hypertension, Diabetes mellitus |
||||||
|
Chi2 247.35; p<0.01; Cox & Snell R2=0.24 Hosmer & Lemeshow Chi2 6.9; p=0.53 |
||||||
Framingham cardiovascular risk
A high risk (>20 %) of developing a CV in the next 10 years was noted in 33 % of participants (N=1740) [Figure 4].
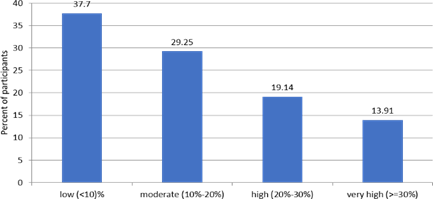
Figure 4. The 10-yeat risk of a CV event (Framingham)
SCORE
The mean global 10-year risk of fatal CV events of the entire cohort was 3.4 %; 22 % of participants had risk over 5 % (N = 945) [Figure 5].

Figure 5. The 10-year risk of a fatal CV event (SCORE)
Discussions
This is the first study targeting the Romanian rural population, a population exposed at risk of poverty and social exclusion, with poor access to health services. The participants’ profile highlights the main characteristics of this population: old age and a higher proportion of females. An impressive high prevalence of hypertension was found. Moreover, the proportion of subjects unaware of HT was remarkably high, as was the low proportion of controlled HT.
This study revealed an extremely high prevalence of hypertension (72.8 %) in the rural adult population, much higher than reported in the Romanian general adult population (45 %). Importantly, 33.3 % of hypertensives were newly diagnosed, and HT was controlled in only 17.8 % as compared to 19 % and 31 % in the Romanian general population. The biggest study on HT prevalence, the SEPHAR study reports lately interesting results with lower prevalence of HT in the rural population: 46 %, as well as lower awareness rates (41 %) and with a higher percentage of treated and controlled population (74 % and 29 %) [34]. The proportion of treated hypertensives and of those having controlled HT were lower (65 % vs. 74 %, and 11 % vs. 29 %), which underlines limits in providing medical care in the investigated Romanian rural areas.
However, other studies have also reported high HT prevalence in the rural population. For instance, the CARLA study conducted on rural residents from East Germany, reported HT prevalence of 74 % and 26 % had unknown HT, a proportion much closer to our findings [35].
The other CV risk factors analyzed were consistent with reported prevalence in the general population: obesity (31.3 % vs 31.9 %), dyslipidemia (67.1 % vs 64.6 %), diabetes (12.6 % vs 11.6 %). Notably, more than a half of the investigated subjects with diabetes were newly diagnosed [8,9,10].
Interestingly, there are fewer smokers among the rural residents than the national average. The latest WHO data report a smoking prevalence of 27 % in Romania, however, our responders declared that only 16 % were regular smokers (at least 1 cigarette/day) [36]. There is an economic aspect that needs to be considered: rural residents may have financial constraints that prohibit them from engaging in “expensive” habits.
CV risk scores were calculated to highlight the CV risk of the examined population. Two risk scores were chosen because each one has advantages and limitations [37]. SCORE was computed from European cohorts and is specially designed to work well on the European population. However, the score is limited for the population aged 40-65 and estimates the chance of a fatal cardiovascular event in 10 years.38 Alternatively, the Framingham score, even though it was extrapolated from American studies, applies to a much wider range of population (30+) and estimates the 10-year risk of developing any cardiovascular event [32].
Strengths and limitations
The high number of patients included in the study makes it highly relevant. Although the study areas were located in South-East Romania, the results can be extrapolated to the entire rural population. There are missing variables (for example from patients who performed blood tests but did not subsequently undergo a physical examination or vice versa) and CV risk estimates were calculated only for subjects with complete data. The data collected were intended for clinical and not research purposes, therefore some relevant variables may not have been consistently documented. The difference in study design, which in our case, was not initially intended as an epidemiologic study and did not focus on acquiring specific data (i.e., history of CVD) can partially explain the low CVD prevalence.
Another limitation concerns BP measurements which were done on a single visit, leaving room for “white coat hypertension” bias.
Conclusion
This is the first study that focused on the health of the rural population in Romania. The data was collected from mobile health caravans, a concept that is in continuous growth as a reaction to the reduced access to healthcare of the rural inhabitants. The results showed an unexpectedly high prevalence of HT, as well as a high risk of developing cardiovascular disease, pointing to the need for strategies to improve medical care.
The results revealed an extremely high prevalence of hypertension in the rural adult population (72.8 %) and showed a high percentage of undiagnosed patients (33.3 %) as well as a small number of treated hypertensives that are on target therapy.
Other CV risk factors were similar to the nationally reported prevalence: obesity (31.3 %), dyslipidemia (64.7 %), diabetes (12.6 %). Obesity, smoking, and diabetes increased the likelihood of participants having CVD by 1.7 times with HT being the leading risk factor by 2.7-fold. The Framingham and SCORE cardiovascular risk scores revealed that an alarming one-third of the population (33 %) has a high risk (>20 %) of developing a CVD, while almost a quarter of the population (22 %) has a risk of above 5 % of undergoing a fatal CV event in the following decade.
While this analysis of the health status of Romania’s rural population needs to be further expanded to include areas from all regions of the country, the large number of participants makes this study highly relevant. The results show that there is a segment of the population – the rural population – that has an underestimated prevalence of HT and has a high risk of developing CVDs; a population with a deficit of healthcare, education, and infrastructure that requires better access to medical services.
Acknowledgments
We thank the “Doctors’ Caravan Association” for their efforts in collecting data and providing medical care to the most distant rural settlements of Romania.
Ethics: The study received ethics approval from the Ethics Review Board of Fundeni Clinical Institute and is filed under ID number 53986.
Disclosure of interest: The authors report no conflict of interest.
Funding: This research did not receive grants from any funding agency in the public, commercial or not-for-profit sectors.
Data availability: Berbecar, Vlad (2021), “Romania rural health dataset 2015-2017”, Mendeley Data, V1, DOI: 10.17632/fs4kwd29gf.1
References
- Abubakar II, Tillmann T, Banerjee A, Wang H, Lozano R, et al . (2015) Global, regional, and national age-sex specific all- cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 385(9963): 117-171.
- Forouzanfar MH, Afshin A, Alexander LT, Anderson HR, Bhutta ZA, et al . (2016). Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 388(10053): 1659-1724.
- Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, et al. (2016) Cardiovascular disease in Europe: epidemiological update 2016. Eur heart j. 37(42): 3232-3245.
- Yusuf S, Reddy S, Ôunpuu S, Anand S (2001) Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 104(22): 2746-2753.
- Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, et al. (2015) Global disparities of hypertension prevalence and control: A systematic analysis of population-based studies from 90 countries. Circulation. 134(6): 441-50.
- Wilkins E, Wilson L, Wickramasinghe K, Bhatnagar P, Leal J, et al . (2017) European cardiovascular disease statistics 2017. European Heart Network: Brussels, Belgium.
- Dorobantu M, Tautu OF, Dimulescu D, Sinescu C, Gusbeth- Tatomir P, et al . (2018) Perspectives on hypertension's prevalence, treatment and control in a high cardiovascular risk East European country: data from the SEPHAR III survey. J hypertens. 36(3): 690-700.
- Popa S, Moţa M, Popa A, Moţa E, Serafinceanu C, et al . (2016) Prevalence of overweight/obesity, abdominal obesityand metabolic syndrome and atypical cardiometabolic phenotypes in the adult Romanian population: PREDATORR study. J endocrinol invest. 39(9): 1045-1053.
- Mota M, Popa SG, Mota E, Mitrea A, Catrinoiu D, et al. (2016) Prevalence of diabetes mellitus and prediabetes in the adult Romanian population: PREDATORR study. J Diabetes. 8(3):336-344.
- Popa S, Mota M, Popa A, Mota E, Timar R, et al. (2019) Prevalence of dyslipidemia and its association with cardiometabolic factors and kidney function in the adult Romanian population: The PREDATORR study. Diabetes Metab Syndr. 13(1), 596-602.
- Kannel WB (2000) Risk stratification in hypertension: new insights from the Framingham Study. Am j hypertens. 13(S1), 3S-10S.
- Poulter N (2003) Global risk of cardiovascular disease. Heart. 89(suppl 2): ii2-ii5.
- Trialists’Collaboration B P L T, Turnbull F, Neal B, Ninomiya T, Algert C, et al. (2008) Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger people: meta-analysis of randomised trials. Bmj. 336 (7653): 1121-1123.
- Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, et al. (2005) Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 366(9493): 1267-1278.
- Collaboration ERF, Sarwar N, Gao P, Seshasai SRK, Gobi R. (2010) Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 375(9733): 2215-2222.
- Chiuve SE, McCullough ML, Sacks FM, Rimm EB (2006) Healthy lifestyle factors in the primary prevention of coronary heart disease among men: benefits among users and nonusers of lipid-lowering and antihypertensive medications. Circulation. 114(2): 160-167.
- Chomistek AK, Chiuve SE, Eliassen AH, Mukamal KJ, Willett WC, et al. (2015) Healthy lifestyle in the primordial prevention of cardiovascular disease among young women. J Am Coll Cardiol. 65(1): 43-51.
- Ignat R, Stoian M, Roşca V (2014) Socio-economic Aspects of rural Romania. Procedia Economics and Finance. 15(1): 1331-1338.
- Mărginean, I. (2005) Condiţiile de viaţă din mediul rural, Centru de Informare şi Documentare Economică, Academia Română, Bucureşti.
- Sirodoev I, Schvab AC, Ianos IL, Ion F (2015). Rural towns in Romania: a reality asking for specific sustainable development policies. Carpathian Journal of Earth and Environmental Sciences. 10(3): 147-156.
- Arcury TA, Gesler WM, Preisser JS, Sherman J, Spencer J, et al. (2005) The effects of geography and spatial behavior on health care utilization among the residents of a rural region. Health ser res. 40(1): 135-156.
- McGrail MR, Humphreys JS (2009) The index of rural access: an innovative integrated approach for measuring primary care access. BMC Health Ser Res. 9(1): 124.
- Colwill JM, Cultice JM, Kruse RL (2008) Will generalist physician supply meet demands of an increasing and aging population? Health Aff. 27(3): w232-w241.
- Befort CA, Nazir N, Perri M G (2012) Prevalence of obesity among adults from rural and urban areas of the United States: findings from NHANES (2005‐2008). J Rural Health, 28(4), 392-397.
- Lindroth M, Lundqvist R, Lilja M, Eliasson M (2014) Cardiovascular risk factors differ between rural and urban Sweden: the 2009 Northern Sweden MONICA cohort. BMC Public Health. 14(1): 825.
- Rice N, Smith PC (2001) Ethics and geographical equity in health care. J Medical Ethics. 27(4), 256-261.
- Chan L, Hart LG, Goodman DC (2006) Geographic access to health care for rural Medicare beneficiaries. J Rural Health. 22(2): 140-146.
- Berbecar VT, Cocheci RM, Acasandre A, Ismail G, Mircescu G (2020) Quality of living assessment in rural romania. An analysis of settlements with low accessibility to medical services. Journal of Urban and regional studies.12(2): 165 – 180.
- Williams B, Mancia G, Spiering W, Rosei EA, Azizi M, et al. (2018) 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. 36(10): 1953-2041.
- Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, et al . (2009) Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 120(16): 1640-1645.
- Report of the National Cholesterol Education Program (NCEP) (2002) Detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III).
- D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, et al. (2008) General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 117(6): 743-753.
- Conroy R. M, Pyörälä K, Fitzgerald AE, Sans S, Menotti A, et al. (2003) Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur heart j. 24(11): 987-1003.
- DOROBANŢU M, BĂDILĂ E, Ghiorghe S, DARABONT RO, Olteanu M, et al. (2008) Total cardiovascular risk estimation in Romania. Data from the SEPHAR study. Rom J Intern Med. 46(1): 29-37.
- Lacruz ME, Kluttig A, Hartwig S, Löer M, Tiller D, et al. (2015) Prevalence and incidence of hypertension in the general adult population: results of the CARLA-cohort study. Medicine, 94(22): e952.
- World Health Organization (2019) WHO report on the global tobacco epidemic 2019: Offer help to quit tobacco use.
- Cooney MT, Dudina AL, Graham IM (2009) Value and limitations of existing scores for the assessment of cardiovascular risk: a review for clinicians. J Am Coll Cardiol. 54(14): 1209-1227.
- Mortensen M. B, Falk E (2017) Limitations of the SCORE- guided European guidelines on cardiovascular disease prevention. Eur heart j. 38(29): 2259-2263.



