Alison L. Ingraldi1,2, Darlene Lee, MD3, Aaron J. Tabor Ph.D., CTBS, CWCA1,2,3,4*
1Carmell Corporation, Pittsburg PA, USA
2Axolotl Biologix, Department of Research and Development, Flagstaff, AZ, USA*
3Axolotl Biologix, Department of Clinical Operations, Flagstaff, AZ, USA*
4Northern Arizona University, Biological Sciences, Flagstaff, AZ, USA
*prior to merger with Carmell Corporation
*Corresponding Author: Aaron J. Tabor Ph.D., CTBS, CWCA, Carmell Corporation, Pittsburg PA, USA, Axolotl Biologix, Department of Research and Development, Flagstaff, AZ, USA, Axolotl Biologix, Department of Clinical Operations, Flagstaff, AZ, USA, Northern Arizona University, Biological Sciences, Flagstaff, AZ, USA.
Introduction
Mohs micrographic surgery (MMS) is the standard care for high-risk basal cell carcinoma, squamous cell carcinoma, and other primary or recurrent contiguous skin cancers, such as melanoma or, occasionally, Merkel cell carcinoma. [1,7,20] MMS was initially developed by Dr. Frederic E. Mohs at the University of Wisconsin in the 1930s, initially dubbed chemosurgery using a zinc chloride paste. Currently, the technique excises thin layers of cancer tissue with minimal removal of the surrounding healthy tissue. The procedure is generally executed while the patient is conscious, with the malignant tissue area numbed by anesthesia injections, and can last several hours. [1,11] A small margin of normal-appearing tissue is traced around the cancer, and during excision, the tumor and the narrow margin are removed, and the sample is sent to an onsite laboratory for thin sections to be histopathologically analyzed. If residual cancerous cells remain within the tissue section margins, additional excision will occur, and the new margins will be analyzed until the tissue margins are lesion-free. In general, this procedure is associated with superior cure rates, sparing normal healthy tissue, and is becoming widespread amongst the dermatological community worldwide. [1,20] Due to the rising epidemic of skin cancer and an increase in the number of trained dermatologists in MMS, the use of this treatment modality has expanded exponentially since the 1930s. [1,11] As the number of MMS procedures increases, establishing an optimal treatment for post-Mohs surgical defects to ensure the return of healthy native tissue while avoiding the recurrence of skin cancer is the next step toward a successful outcome in the MMS field.
Following the procedure, the patient and surgeon can discuss care for the post-Mohs wound. Ultimately, the result is to aid in the closure of the surgically created wound; however, no standardized post-MMS care exists. Most post-Mohs wounds are repaired at the end of the surgery.
If it is a large or complex wound, the surgeon may temporarily close it using sutures until another repair operation can be completed. Depending on the characteristics of the post-Mohs wound (including the size and location of the wound, the discomfort experienced by the patient, and the availability of materials), post-surgical treatment modalities may include further surgical intervention using autologous skin grafting, closing the wound with sutures, pulling skin from nearby to cover the wound, tissue reconstruction, secondary intention healing, or the currently explored method of applying a biological tissue product, such as amnion tissue. The mechanisms of healing depend on the tissue layers involved (partial vs. total thickness) and the mechanism of wound closure (primary, secondary, or tertiary intention). Critical factors within this repair process include cells that generate the tissue and establish a clean wound bed, the growth factors and cytokines that control cellular activity, and the extracellular matrix that provides the necessary wound-healing environment that promotes cell migration and contains several substances that regulate the migration and activity of the growth factors and cytokines present. For patients who are adverse to and decline further surgical intervention after initial MMS, the alternatives include second-intention healing with or without adjunctive therapy or the application of biological tissue membranes. The benefits of using biological skin substitutes include the construction of natural dermis due to the native extracellular matrix structure and a basement membrane, a collection of growth factors, and antimicrobial properties. These products have shown efficacy in protecting wounds as a structural barrier or covering, reducing the bacterial load, controlling inflammation, and encouraging angiogenesis. [15,24]
In this paper, we present a retrospective analysis of six patients who received biologic amniotic tissue grafts to post-Moh’s surgical defects to assist and support the healing of the surgical site. This data suggests that biological amnion may be a minimally invasive clinical option, allowing physicians to have a post-MMS standard of care. It is suggested that a further multi-site, controlled clinical study should be explored based on the data herein.
Material & Methods
Process of Manufacturing Amnion-Amnion Membrane:
For many years, the human amniotic membrane has been utilized in many clinical applications. This biological intervention is derived from the inner and outer layers of donated human placental tissue that consists of two essential tissue layers known as the amnion and chorion. The amnion and chorion membranes maintain the necessary environment and protect the fetus during gestational development. [12] To date, various methods have been utilized to prepare, preserve, and sterilize amnion-based membranes, resulting in a unique allograft product with different morphological, mechanical, and biological properties. [24,25,26
The allograft membrane elected for application in this clinical study was Axolotl DualGraft TM, a bi-layered dehydrated human amnion membrane allograft derived from the amniotic lining of the placenta. This specific allograft source material is donated placental tissue collected from consenting mothers and is prepared accordingly. Upon receipt, the tissue is rinsed with sterile saline to remove residual blood and tissue debris, and then the amnion and chorion layers are separated via blunt dissection. The amnion layer is inspected for tears or damage and then further washed before folding the spongy intermediate layers together, with the epithelial surface facing outwards. Then, the membrane is smoothed to remove air pockets and ensure complete contact before being transferred to a drying rack and air-dried for a period. After dehydrating the membrane, it is cut to specific membrane sizes, packaged, and terminally sterilized using gamma irradiation. The final product results in a dual-layered membrane with the amnion epithelial surface exposed on each side for an omni-directional implantation method. Axolotl DualGraft TM is regulated solely under section 361 of the Public Health Service (PHS) Act, classified as minimally manipulated tissue, and follows FDA 21 CFR Part 1271 code of federal regulations (CFR). Axolotl DualGraft TM is indicated for full or partial-thickness, chronic and acute wounds, and maintains the ability of the structural tissue to act as a barrier.
Study Setting & Patient Selection:
The study occurred at Heartland Plastic & Reconstructive Surgery P.C. in Des Moines, Iowa. To ensure accurate and transparent reporting prior to the preparation of this retrospective case series, written consent was obtained from the patients, allowing for the use of de-identified personal health information and photographs, along with publication rights. Patients were included if the diagnosis was comprehensive, treatment was completed with Axolotl DualGraft TM, and the representative photos of different healing time intervals were clear and discernible. The demographic age group of the patients treated within this study ranged from 67 to 91 years old, with all patients having a different location of surgical intervention, see Table 1. Complications and self-reported issues after receiving MMS treatment are common in elderly patients, and the delay to beneficial and more intensive treatments may be a risk factor. Age and previous surgical or medical conditions can alter tissue characteristics and complicate or delay healing. Further investigation in difficult-to-heal tissue locations may demonstrate further intention of healing with biological tissue grafts. In this study, the patient defects range from full-thickness scalp wounds to neck, cheek, and hand post-MMS defects.
Table 1: Patient Demographics
|
|
Patient #1 |
Patient #2 |
Patient #3 |
Patient #4 |
Patient #5 |
Patient #6 |
|
Gender |
Female |
Male |
Male |
Male |
Male |
Male |
|
Age |
91 |
67 |
85 |
76 |
80 |
67 |
|
Surgical Indication |
SCC Scalp Mohs |
BCC Left forehead Mohs |
Right neck Mohs |
SCC Left Cheek Mohs |
BCC Right forehead & Nose Mohs |
SCC left hand Mohs |
|
Comorbidities |
Hypertension Heart Disease |
Hypertension M.S. |
Hypertension Kidney Disease Diabetes |
Hypertension Diabetes II Heart Disease |
Hypertension Heart Disease Arthritis |
Hypertension Heart Disease Arthritis Skin Cancer |
|
Average Healing Time (in weeks) |
>16 weeks |
5 weeks |
5 weeks |
6 weeks |
10 weeks |
4 weeks |
The patients reviewed in this case series were referred to a plastic and reconstructive surgeon for post-Mohs wound treatment. The option for Surgical closure of patients’ wounds was not feasible due to comorbidities such as age, medical history, and wound chronicity. However, each patient had both options of either surgery or biological membrane treatment explained, and it was ultimately the patient’s choice. All reconstructions were managed with the same postoperative protocol.
Method of Application:
Following an explanation of treatment and patient consent, an initial assessment of the post-Mohs wound occurred. A small amount of damage debridement was done with blunt dissection using tissue forceps around the wound margins to remove necrotic and scab or eschar tissue formation that would prevent further healing. Sterile normal saline (0.9 % sodium chloride) was utilized to moisten the clean membrane sample before transferring with tissue forceps into the flat section of the wound. It is important to note that the membrane was not packed into the damage, and there is no directional issue of the application using Axolotl DualGraftTM, it being an omni- directional membrane. After application, the membrane was not affixed to the tissue, as it naturally adhered to the wound bed due to moisture content. A topical dressing was then placed to cover the wound – this varied upon wound size – utilizing non-adherent dressing as a protective barrier and for patient comfort. No harmful pressure therapies were applied. The patient follow-ups occurred every week or based on patient availability, with representative photos of healing taken at each visit. The reapplication of Axolotl DualGraftTM was completed based on the physician’s discretion.
Results
A total of 6 patients participated in this retrospective clinical case study, each treated with Axolotl DualGraftTM. It remains unclear as to the optimal frequency of membrane application as this was based solely on the physician’s discretion, which included wound history, type, comorbid conditions, and visual inspections of the wounds. The average healing time for the post-Mohs defects varied based on location and wound size, ranging between 4 to 16 weeks.
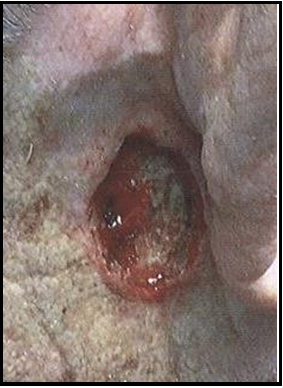
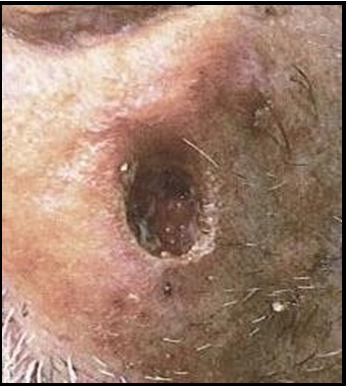
Case 1: A 91-year-old female came for a consult following MMS of the scalp due to squamous cell carcinoma (SCC). Social history includes retired lifestyle with no nicotine use, negative for non- recreational drug use, or caffeine, and social alcohol practice. Due to her comorbidities (myocardial infarct, chronic hypertension, and cardiac disease with post-oral anticoagulant therapy) and family concern for the delayed closure and developing chronicity of her wound, the surgical option was denied and permission for application of biological allograft was granted. Initial examination of wound measured to be 6.8x7.6x1.0 cm area of a full-thickness, non-healing scalp wound with exposed bone. Wound margins are exposed and identifiably healthy, without evidence of undermining or tunneling. Acute infection was not present, and the wound was moist with a non- purulent, sanguineous exudate. The coloration was normal, based on wound presentation. Using aseptic technique, the amniotic membrane product was initially pre-soaked with a 0.9% sterile normal saline solution. The moistened amniotic membrane was then aseptically placed within the wound margins, without fixation. Following clinical application of the membrane product, the wound was observed for a two-week period, with reapplication performed, as needed. By week 5 of treatment, tissue granulation commenced, starting at the wound edges, with full granulation tissue coverage complete by week 9, at which time, no exposed bone was observed.
Initial Wound Exam: Full-thickness non-healing post-Mohs surgical scalp wound, initial area measured to be 6.8 x 7.6 x 1.0cm. Moderate amount of bloody exudate (sanguineous) with exposed bone and defined wound margins.
Week 2 Follow-Up: Reapplication of membrane – seen as textured pattern on wound bed: wound exudate is moderate serosanguineous with a slightly edematous pink wound border showing signs of re-epithelialization. Wound coloration was Red, Pink, Black lacking yellow discoloration, indicating a lack of acute infection.
Week 4 Follow-Up: A moist exudate appears sanguineous with advancing/migrating pink edges of wound. Wound moisture increased with a reduction in black coloration indicating elimination of necrotic tissue.
Week 5 Follow-Up: Granulating tissue (islands) are present advancing inward from the wound edges, indicating the beginning of the remodeling phase.
Week 7 Follow-Up: Increase in red moist granulating tissue, beginning to cover exposed bone in middle of wound bed.
Week 9 Follow-Up: Full Granulation tissue coverage of wound bed; exposed bone is no longer visible.
Week 11 Follow-Up: The granulation tissue continues to fill in the defect and has a healthy viable appearance.
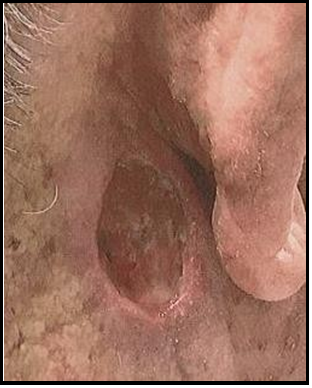
Week 15 Follow-Up: The wound appears smaller and there is hair regrowth up to the edges of the defect. The granulation tissue remains healthy.
Week 16 Follow-Up: 6cm x 5cm circumference of scalp wound.
The granulation tissue surface is smooth, and the wound edges have re-epithelialized and exhibit hair regrowth.
Case 2: A 67-year-old male, with a past medical history of hypertension and multiple sclerosis (MS), presented for treatment of a left forehead MMS defect, status post removal of a basal cell carcinoma (BCC). The retired patient reported use of caffeine, and no practice of nicotine, recreational drug use, alcohol, or exercise. Upon description of treatment options between further surgical intervention or biological tissue graft, the patient preferred avoidance of surgery and requested the use of the biological tissue graft. Initial examination of the forehead wound demonstrated a 1.9x2.1x0.75 cm area of full thickness, skin and soft tissue forehead wound, with defined healthy borders with slightly rolled, dusky edges, indicating wound stagnation.
Initial Wound Exam: Front left forehead, post-Mohs full thickness surgical wound with 1.9x2.1x0.75cm area. Exposed wound margins are healthy with no undermining, slight rolled edges indicating wound stagnation with dusky inflammatory edges. Red & pink wound bed, focal black tissue around edges indicates post-surgical eschar. Ther is no indication of purulence. Light bloody exudate.
Week 2 Follow-Up: One-week follow-up after initial treatment with reapplication of graft: wound exudate is minimal, and clear. Slightly edematous wound border with beginnings of tissue granulation. The full wound coloration is red border with pink tissue center, without any indication of infection.
Week 3 Follow-Up: Moist exudate with deep red wound center and undefined granulated wound pink wound border, the overall size of the wound has decreased and now measures 1.3x1.3cm. The wound appears moist, with a reduction in exudate. The increased red coloration implies angiogenesis.
Week 5 Follow-Up: Light pink re-epithelization; no exúdate was present and complete wound closure was noted.
Case 3: A 85-year-old male with type II diabetes was referred for treatment of an MMS defect located on the neck behind right ear. At the initial presentation, the wound area measured 2.3x2.0cm. The patient’s medical history includes type II diabetes, kidney disease, hypertension, and a previous stroke. He is a caffeine and social drinker but denies the use of tobacco or recreational drugs. The neck wound appeared to be full thickness wound, penetrating into fatty tissue of neck. The wound has a clearly defined surgical wound border, without undermining or tunneling of wound margins. Initial inspection revealed moderate wet and bloody exudate with red wound edges and small foci of black coloration within wound bed.
Initial Wound Exam: A 2.3x2.0 cm surgical wound, with dark red defined wound border, moderate wet red, and tan exudate.
Week 1 Follow-Up: One-week follow-up treatment with reapplication of graft. The wound exhibits slight tissue granulation on attached wound edges. There is a small amount of clear exudate, without purulence or necrosis.
Week 3 Follow-Up: Two-week follow-up; the wound defect is completely filled with reddish-pink granulated tissue. No exudate present and wound margins are clean and clear.
Week 5 Follow-Up: The wound has completely closed, with re-epithelialization of the surface.
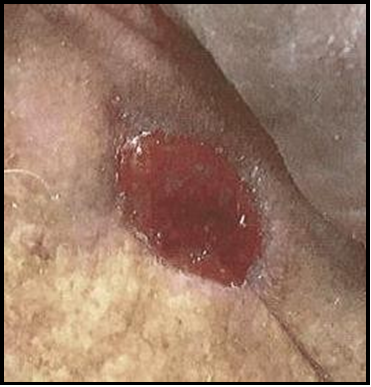
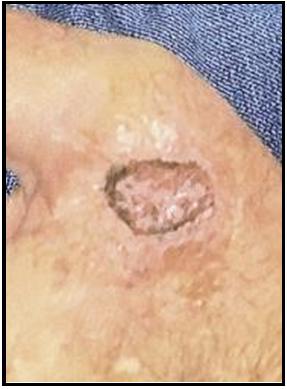
Case 4: A retired 76-year-old male with heart disease, type II diabetes and hypertension came in for plastic surgery consult following MMS of the left cheek due to squamous cell carcinoma (SCC). Social history includes social alcohol practice and no use of caffeine, nicotine, exercise, or recreational drugs. Following the explanation of procedure options, the patient preferred a nonsurgical route and chose biological graft treatment. Examination of the left cheek wound noted a 1.4x1.0 cm defect, with exposed deep tissue. The surrounding tissue was edematous but viable, with slight epidermal sloughing, moderate serosanguinous exudate, and defined wound borders.
Initial Wound Exam: Full-thickness non-healing post-Mohs surgical defect of the left cheek, measuring 1.4x1.0 cm, excised to the depth of the deep soft tissue. A mild amount of blood-tinged exudate covers the surgical bed and respects the defined surgical wound margins.
Week 1 Follow-Up: The wound bed exhibits even discoloration with a scant surface exudate. The wound margins are well defined, with signs of decreasing inflammation and granulation. The edema and erythema of the has decreased.
Week 2 Follow-Up: The wound size has noticeably decreased, the wound bed is a deep red coloration, consistent with angiogenesis.
Week 6 Follow-Up: Wound healing is complete, and the erythema and edema have resolved.
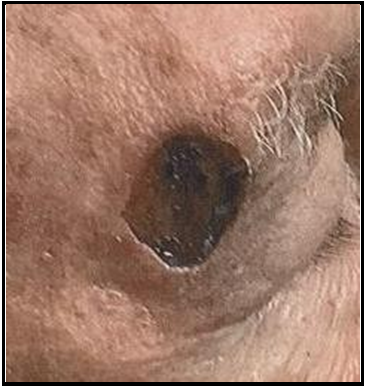
Case 5: An 80-year-old male with a history of heart disease, hypertension and arthritis was referred for treatment of a BCC post-Mohs surgical defect of the lateral right eyebrow, which measured 2x1.5x0.5 cm. There is no social history reported, but the patient granted permission to be treated with a biological graft.
Initial Wound Exam: The wound margins are sharply defined, and there are no features of undermining or tunneling. The wound bed is covered by a layer of yellow and black crust, which is an indication of a non-healing wound bed. The surrounding tissue does not appear to be inflamed or infected.
Week 2 Follow-Up: Re-epithelialization of the wound margins has occurred, and the wound bed is filled in by with healthy granulation tissue. There is no exudate present.
Week 10 Follow-Up: At the conclusion of the treatment protocol. The wound has completely healed and is surrounded by healthy tissue.
Initial Wound Exam: The wound margin and wound bed is covered by pink and yellow scale with early dark eschar formation. A small amount of thin clear exudate is present in the wound bed. The wound margins are regular and identifiable with no undermining or rolling present. The surrounding tissue appears healthy and viable.
Week 1 Follow-Up: The wound bed is now red with a small amount of serosanguinous exudate. The wound margins retain black eschar but are well defined.
Week 2 Follow-Up: The wound margins are pink, and the adjacent wound bed is dark red, with granulation tissue islands evident along the wound periphery. Small amounts of bloody exudate are present.
Week 4 Follow-Up: The wound is completely closed, with re-epithelialization occurring over the majority of the area.
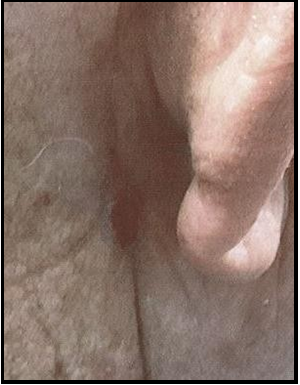
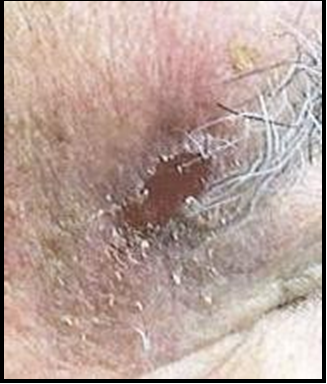
Case 6: A 67-year-old male with a history of skin cancer, hypertension and heart disease was referred for treatment of a post- MMS surgical defect after removal of a SCC on the radical dorsal aspect of the left hand. The surgical wound measured 2.0x1.0cm. After procedures and options were explained, the patient granted consent for the use of biological tissue graft for treatment.
Discussion
The goal of skin cancer treatment is the complete removal of the lesion, minimize functional disability, and achieve a positive aesthetic result. For geriatric patients, it is essential to consider how to improve or maintain a patient's active lifestyle, increase disease-free survival, and limit the impact of disability or morbidity while avoiding complications or outcomes that would result in exacerbation of underlying medical conditions that would increase health-related costs or the possibility of mortality. With the progressive aging of populations coinciding with the rising risk of skin cancer, geriatric care is a significant issue for health authorities. This preliminary retrospective case series has demonstrated the beneficial implications of the once-geriatric approach in the clinical management of post-MMS and surgical skin cancer treatment. A regenerative medicine approach to skin cancer treatment and post-surgical care may provide physicians with more treatment options and deliver faster and improved care for difficult-to-heal elderly patients.
Treatment with a biological tissue graft is less invasive, resulting in faster wound closure, fewer postoperative complications, decreased requirements for additional surgical procedures, and less patient discomfort. All of these are noteworthy beneficial effects, resulting in improved patients' outcomes and an overall reduction in healthcare costs. For a future geriatric-centered study, the authors recommend a multi-disciplinary interpretation of patient's results following a Comprehensive Geriatric Assessment (CGA) outline or a similar system to understand better and summarize patient conditions throughout the entire process.
As demonstrated in this study, the Axolotl DualGraft TM integrates into the wound bed and encourages tissue granulation rapidly without the need for meticulous wound care protocols. Table two details that re-epithelialization occurred in all six patients while complete epidermal covering closure was visible present in five of the six patients.
Table 2: Patient Healing Time
|
|
Patient #1 |
Patient #2 |
Patient #3 |
Patient #4 |
Patient #5 |
Patient #6 |
|
Surgical MMS location |
SCC Scalp Mohs |
BCC Left forehead Mohs |
Right neck Mohs |
SCC Left Cheek Mohs |
BCC Right forehead & Nose Mohs |
SCC left hand Mohs |
|
Re-epithelization |
☒ |
☒ |
☒ |
☒ |
☒ |
☒ |
|
Complete Epidermal covering |
☐ |
☒ |
☒ |
☒ |
☒ |
☒ |
|
Average Healing Time (in weeks) |
>16 weeks |
5 weeks |
5 weeks |
6 weeks |
10 weeks |
4 weeks |
Preparations of amniotic-tissue-derived allograft have been shown to contain cytokines, growth factors, protease inhibitors, angiogenin, and extracellular matrix components that, when exposed to the wound bed, promote cell proliferation, migration in vitro and overall assistance in wound healing. [4,15]
Figure one: Left, represents the dehydrated allograft amniotic product, pre-application. Right, represents the amniotic ECM proteins.
The images represent the amniotic allograft product applied and the amniotic layers with the extracellular matrix proteins found in placental tissue. It is this ECM and its constituents that aid in the process of wound closure as seen with patients in this study (images created by authorship group).
The use of amniotic membrane (AM) in this retrospective clinical trial setting was successful in complete would healing and closure, cosmetically pleasing outcomes, and overall patient satisfaction.
Conclusion
There is a lack of published data and articles analyzing comparisons between the use of AM vs standard of care for post-Mohs wounds healing by secondary intention. Future randomized, multi-center, controlled studies should focus on analyzing potential quantitative differences in initial defect area, the relationship between initial defect size and time to closure in relation to tissue type. Further investigation into the efficacy of dehydrated human amniotic membrane allografts (Axolotl DualGraft TM) as a treatment modality for a variety of post-Mohs secondary intention wounds and how it impacts elderly patients is warranted.
Limitations
Due to limitations of sample size, the variances in wound location, dimensions and patient demographics, the wound closure rates and percentages were not statistically obtained. Although rate was not calculated to show statistical significance, the qualitative outcomes demonstrated positive results i.e. granulation tissue formation, and it is believed that time of wound closure improved in patients treated with AM. Future studies with the goal of further evaluating amniotic membrane and the rate of wound closure post-Mohs micrographic surgery should effectively control the aforementioned limitations to determine an accurate rate of wound closure using biologic amniotic interventions.
Acknowledgements
The authors would like to express their appreciation to the Heartland Plastic & Reconstructive Surgery P.C group in Des Moine, Iowa. Additionally, the authors would like to express gratitude to the patients represented in this publication for enhancing the field of medicine.
Conflicts of Interest
The authors have the following relevant disclosures: Alison Ingraldi and Aaron J. Tabor were employed by Axolotl Biologix (Flagstaff, AZ, USA) which has merged with Carmell Corporation (Pittsburg, PA, USA), which offers membrane-based wound care products. Darlene Lee (Flagstaff, AZ, USA) serves as an acting clinical pathologist and medical director for tissue banks that offer wound care membrane-based commercial products.
Author Contributions: All authors contributed equally to this project. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
References
- Bittner GC, Cerci FB, Kubo EM, Tolkachjov SN (2021) Mohs micrographic surgery: a review of indications, technique, outcomes, and considerations. An Bras Dermatol. 96(3): 263-77.
- Castellanos G, Bernabé-García Á, Moraleda JM, Nicolás FJ (2017) Amniotic membrane application for the healing of chronic wounds and ulcers. Placenta. 59: 146-153.
- Cerrati EW, Thomas JR (2017) Scar Revision and Recontouring Post-Mohs Surgery. Facial Plast Surg Clin North Am. 25(3): 463- 471.
- Diller RB, Tabor AJ (2022) The Role of the Extracellular Matrix (ECM) in Wound Healing: A Review. Biomimetics (Basel). 7(3): 87.
- Garcovich S, Colloca G, Sollena P, Andrea B, Balducci L, et al. (2017) Skin Cancer Epidemics in the Elderly as An Emerging Issue in Geriatric Oncology. Aging and Disease. 8(5): 643-661.
- Ilic D, Vicovac L, Nikolic M, Lazic Ilic E (2016) Human amniotic membrane grafts in therapy of chronic non-healing wounds. Br Med Bull. 117(1): 59-67.
- van Kester MS, Goeman JJ, Genders RE (2019) Tissue-sparing properties of Mohs micrographic surgery for infiltrative basal cell carcinoma. J Am Acad Dermatol. 80(6): 1700-1703.
- Liu KY, Silvestri B, Marquez J, Huston TL (2020) Secondary Intention Healing After Mohs Surgical Excision as an Alternative to Surgical Repair: Evaluation of Wound Characteristics and Esthetic Outcomes. Ann Plast Surg. 85(S1 Suppl 1): S28-S32..
- Lyons AB, Chipps LK, Moy RL, Herrmann JL (2018) Dehydrated human amnion/chorion membrane allograft as an aid for wound healing in patients with full-thickness scalp defects after Mohs micrographic surgery. JAAD Case Reports. 4(7): 688-691.
- Mamede AC, Botelho MF (2015) Biochemical Properties of Amniotic Membrane. Amniotic Membrane. (Chapter 2), 19–40.
- Mansouri B, Bicknell LM, Hill D, Walker GD, Fiala K, et al. (2017) Mohs Micrographic Surgery for the Management of Cutaneous Malignancies. Facial Plastic Surgery Clinics of North America. 25(3): 291–301.
- Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, et al. (2008) Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 15: 88-99.
- Olson MD, Hamilton GS (2017) Scalp and Forehead Defects in the Post-Mohs Surgery Patient. Facial Plast Surg Clin North Am. 25(3): 365-375.
- Park KK, Hagstrom E, Berrios R, Tung RC (2016) A Novel Purse- String Suture and Dehydrated Human Amnion/Chorion Membrane Allograft Closure Technique for the Repair of Defects Following Mohs Micrographic and Excisional Surgery. J Clin Investigat Dermatol. 4(1): 3.
- Seaton K, Mullens D, Barr J, Hull E, Averitte R (2021) Use of amniotic tissue-derived allografts post-Mohs micrographic surgery: a preliminary study assessing wound closure rate. Wounds. 33(7): 185-191.
- Sheikh ES, Sheikh ES, Fetterolf DE (2014) Use of dehydrated human amniotic membrane allografts to promote healing in patients with refractory non healing wounds. Int Wound J. 11(6): 711–717
- Snow SN, Stiff MA, Bullen R, Mohs FE, Chao WH (1994) Second-intention healing of exposed facial-scalp bone after Mohs surgery for skin cancer: review of ninety-one cases. J Am Acad Dermatol. 31(3 Pt 1): 450-4.
- Swanson NA (1983) Mohs surgery: technique, indications, applications, and the future. Archives of dermatology. 119(9): 761-773.
- Thornton JF, Carboy JA. (2018) Approach to the Post Mohs Patient. Seminars in Plastic Surgery. 32(2): 057–059.
- Tolkachjov SN, Brodland DG, Coldiron BM, Fazio MJ, Hruza GJ, et al. (2017) Understanding Mohs Micrographic Surgery. Mayo Clinic Proceedings. 92(8): 1261–1271.
- Toman J, Michael GM, Wisco OJ, Adams JR, Hubbs BS (2022) Mohs Defect Repair with Dehydrated Human Amnion/Chorion Membrane. Facial Plast Surg Aesthet Med. 24(1): 48-53.
- Wilmas KM, Patel J, Silapunt S, Doan HQ, Migden MR (2023) The Use of a Dehydrated Complete Human Placental Membrane Allograft for Mohs Surgical Defects of the Nose. Dermatol Surg. 49(4): 343-347.
- Wisco OJ (2016) Case series: The use of a dehydrated human amnion/chorion membrane allograft to enhance healing in the repair of lower eyelid defects after Mohs micrographic surgery. JAAD Case Reports. 2(4): 294–297.
- Odet S, Louvrier A, Meyer C, Nicolas FJ, Hofman N, et al. (2021) Surgical Application of Human Amniotic Membrane and Amnion-Chorion Membrane in the Oral Cavity and Efficacy Evaluation: Corollary With Ophthalmological and Wound Healing Experiences. Front Bioeng Biotechnol. 9: 685128.
- Dadkhah Tehrani F, Firouzeh A, Shabani I, Shabani A (2021) A Review on Modifications of Amniotic Membrane for Biomedical Applications. Front Bioeng Biotechnol. 8: 606982.
- Gholipourmalekabadi M, Farhadihosseinabadi B, Faraji M, Nourani MR (2020) How preparation and preservation procedures affect the properties of amniotic membrane? How safe are the procedures? Burns. 46(6): 1254-1271.