Dasaad Mulijono1,2,3*, Albert M Hutapea2,4, I Nyoman E Lister2,5, Mondastri K Sudaryo6, and Helwiah Umniyati7
1Department of Cardiology, Bethsaida Hospital, Tangerang-Indonesia
2Indonesia College of Lifestyle Medicine, Indonesia
3Department of Cardiology, Faculty of Medicine, Prima University, Medan-Indonesia
4Department of Pharmacy, Faculty of Life Sciences, Advent University, Bandung-Indonesia
5Department of Biomolecular and Physiology, Faculty of Medicine, Prima University, Medan-Indonesia
6Department of Epidemiology, Faculty of Public Health, University of Indonesia, Jakarta-Indonesia
7Department of Community Nutrition, Faculty of Dentistry, Yarsi University, Jakarta-Indonesia
*Corresponding Author: Prof. Dasaad Mulijono, Department of Cardiology, Bethsaida Hospital, Tangerang-Indonesia, Indonesia College of Lifestyle Medicine, Indonesia, Department of Cardiology, Faculty of Medicine, Prima University, Medan-Indonesia.
Abstract
The potential benefits of plant-based interventions in reducing the incidence and lessening the morbidity and mortality of COVID-19 have yet to be widely recognized. However, our groundbreaking research suggests that these interventions could be effective. In a recent publication, we utilized plant-based diets (PBDs) and supplements for 3470 high-risk elderly COVID-19 patients. The results were nothing short of remarkable, as we achieved a zero mortality rate, and none of our patients experienced worsening conditions or required hospitalization. This review article presents the mechanism of how PBD and supplement interventions can mitigate COVID-19 disease severity and avoid mortality. Our findings suggest that PBD and supplement interventions are not only effective but could revolutionize the management of coronavirus patients.
1. Introduction
Several studies have demonstrated the immense potential of a PBD in effectively treating chronic inflammatory diseases, such as obesity, atherosclerosis, hypertension, hyperlipidemia, and hypercholesterolemia. Dietary interventions not only manage these diseases but can potentially reverse them [1-2]. By reducing the consumption of foods that promote inflammation and contribute to chronic inflammatory diseases, one can further enhance the positive impact of a healthy diet. In our cardiology practice, we have witnessed the power of these interventions in patients suffering from chronic inflammation-related illnesses. These patients achieved remission with limited medications. Patients with hyperlipidemia could also meet their lipid targets according to International guidelines. We also noticed improved kidney function and successful management of glucose for non-insulin-dependent diabetes mellitus (NIDDM) or glucose intolerance patients without the need for excessive medication or insulin. Furthermore, we witnessed a regression of coronary obstruction in numerous cardiac patients, and there was a low occurrence of in-stent restenosis (ISR) and stent thrombosis (ST).
Based on our previous successful experiences in treating our patients with chronic inflammatory diseases using a PBD and dietary supplements, we hypothesized that PBD could decrease the incidence and severity of SARS-CoV-2 infection and may save lives. Our clinical trial was initiated in April 2020 and concluded in July 2023, enrolling a total of 3,470 participants, the majority of whom were elderly COVID-19 patients with multiple comorbidities, including cardiology patients. Notably, none of our patients experienced severe illness, hospitalization, or fatality at the end of the study. The details of our study on COVID-19 have been published in a separate article, which can be accessed through the title "Plant-Based Diet and Supplements in Mitigating COVID-19: Part 1. The Research Report" To the best of our knowledge, we were the first to implement the PBD intervention and provide dietary supplements to COVID-19 patients at the outset of the pandemic.
It is important to note that during the initial eighteen months of our investigation, no studies or recommendations emerged demonstrating the benefits of PBD and dietary supplementation in reducing the virus's incidence, severity, and mortality. Fortunately, we observed the promising outcomes at the onset of our study. So, despite significant skepticism and opposition from our Indonesian peers towards PBD intervention, we continued our research. Later on, despite the publication of positive findings in a prestigious journal indicating the crucial role of PBD in managing the coronavirus [3], our research still gained minimal traction. Undertaking interventional research with close contact with COVID-19 patients when no medications or vaccines were available was also a high-risk endeavor and very challenging. Our steadfast dedication to advancing our research is underpinned by our unshakable belief in PBD and supplementations, reinforced by our extensive expertise and hands-on experience with PBD and supplementations. We have successfully employed these interventions to manage persistent inflammatory disorders in our cardiology patients and are convinced of their efficacy. And most importantly, our ultimate goal is to save as many lives as possible, including ours.
We put forward the notion that PBD could potentially enhance nitric oxide (NO) availability, alter the gut microbiota, improve endothelial function, reduce inflammation, combat oxidative stress, boost mitochondrial function, extend telomeres, and facilitate caloric restriction (CR) as a means to combat COVID-19. These mechanisms can potentially decrease the incidence, morbidity, and mortality rate of COVID-19 patients. Severe inflammation and blood clotting are significant contributing factors to the high mortality rate of COVID-19 patients [4,5], and PBD may help to prevent these complications. Field experts have recently validated our hypotheses, inspiring us to share our findings and publish our study.
Our research sets itself apart from other PBD studies in several ways, including utilizing raw PBD, carefully selecting foods to maximize their potential, and paying close attention to food preparation techniques, such as avoiding high-temperature cooking of vegetables, which can neg-atively impact the NO content. Furthermore, we supplement our dietary intervention with vitamins, minerals, and nutraceuticals to maximize anti-inflammatory and antioxidant effects, minimize oxidative stress, improve NO availability, and repair the endothelium. These supplements are selected based on our usual supplements used for cardiology patients to fight their chronic inflammatory diseases [6,7]. However, only re-cently has the significance of vitamins, minerals, and nutraceuticals in relation to COVID-19 been recognized [8,9]. We propose that by com-bining our dietary intervention with strategic supplementation, we can decrease the severity and fatality rates of the coronavirus to the greatest extent possible.
2. Mechanism of Plant-Based in decreasing incidence, severity, hospitalization, and mortality of COVID-19
2.1. Mechanism of NO in fighting coronavirus
NO is a naturally occurring molecule that is present in various cell types and organ systems, and it serves a critical role in cardiovascular function. Its functions include regulating basal vascular tone, preventing platelet activation, and limiting leukocyte adhesion to the endothelium. Furthermore, it significantly contributes to regulating myocardial contractility [10]. Unfortunately, conditions like obesity, hypertension, hy-percholesterolemia, and NIDDM, which are commonly linked to risk factors for atherosclerosis, can lead to a decrease in NO release into the arterial wall due to synthesis dysfunction or enormous oxidative degradation [11]. Studies on humans indicate that natural NO production re-duces with age, which is relevant to the number of diseases that affect elderly people [12]. It is believed that the elderly's NO deficiency may contribute to the development of comorbidities, causing the severity and mortality of coronavirus [13].
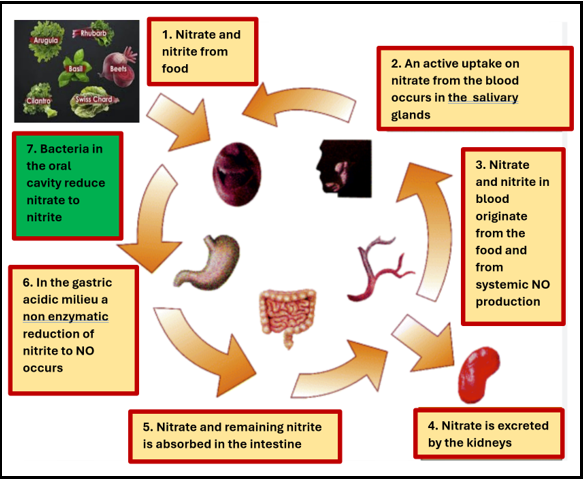
On the other hand, children and young adults (below 19 years old) have low COVID-19 mortality rates, which may be due to their high NO content [14]. To measure our study population's NO, we used a salivary strip that has a 96% accuracy rate [15]. We can improve our patients' NO readings by modifying their diet and lifestyle [16]. Figure 1 illustrates the conversion of nitrate-rich foods to nitric oxide [17].
Figure 1: Illustration of nitrate-rich diet metabolized into nitric oxide
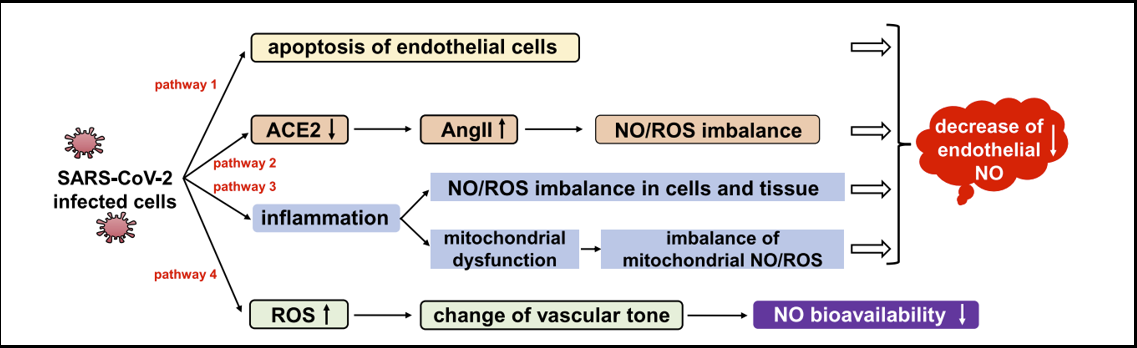
It has been observed that coronavirus has the potential to reduce the availability of NO in the endothelium [18], as shown in Figure 2.
Figure 2: Endothelial NO decreased by coronavirus-infected cells.
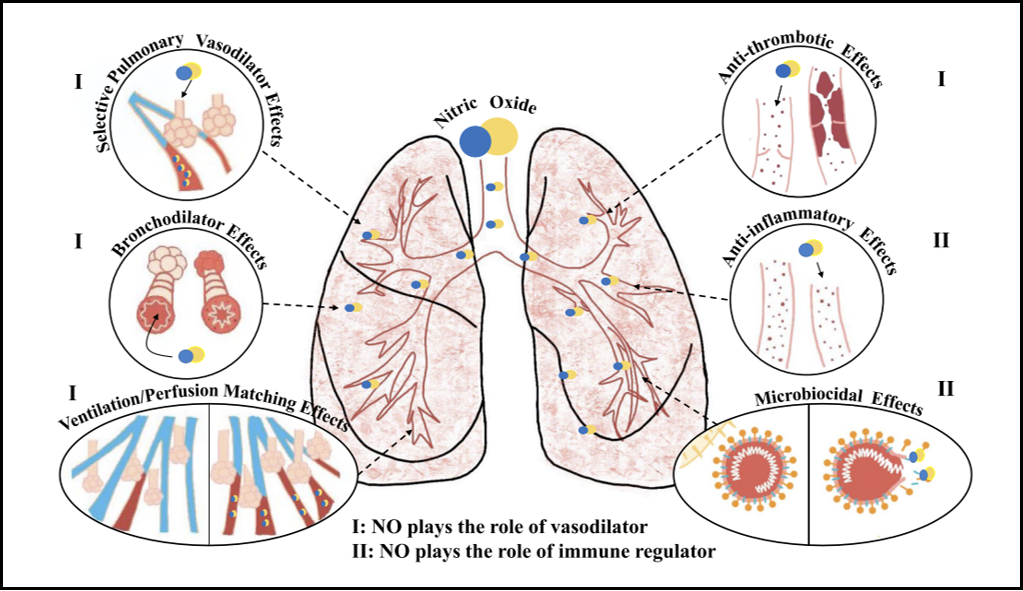
It is suggested that the virus could affect the cardiovascular system, resulting in conditions like acute coronary syndrome, pulmonary embolism, and heightened COVID-19 mortality. NO plays a vital role in the lungs and serves as a vasodilator, bronchodilator, anticoagulant, anti-inflammatory, and antiviral agent [18], as demonstrated in Figure 3.
Figure 3: NO as a vasodilator, bronchodilator, anticoagulation, anti-inflammatory, and anti-viral.
Research has indicated that NO can provide therapeutic advantages, including reducing pulmonary hypertension and increasing blood flow in ventilated lung units [13]. Furthermore, a higher baseline level of NO has been linked to fewer cold symptoms, indicating that it may enhance mucosal immunocompetence and aid in preventing airway infections [19]. Recent studies have also found that inhaled nitric oxide (iNO) may be beneficial in treating COVID-19 patients by improving arterial oxygenation. Given the high risk of refractory hypoxemia in these patients, clinical guidelines need to consider the potential advantages of iNO in managing ARDS, particularly for critically ill individuals [20].
To increase NO levels in vital organs such as the hearts, brains, and lungs of coronavirus patients, we recommend incorporating dietary nitrates from sources such as raw green leafy vegetables, beets, cabbage, pomegranate, watermelon, garlic, lemongrass, ginger, turmeric into their daily diet [21]. Our study suggests that improving NO may lower morbidity and mortality rates in coronavirus patients, as previously explained.
2.2. The role of microbiota in coronavirus
The human body harbors roughly 38 trillion microorganisms, with the gut being the most biologically diverse and densely populated organ in the body. The gut microbiota is crucial for maintaining immune homeostasis. The gut-associated lymphoid tissue (GALT) and bronchial-associated lymphoid tissue, part of the mucosal immune system, serve as the first line of defense against infections. The GALT includes the Peyer's patches, appendix, and isolated lymphoid follicles of the intestinal mucosa. Interaction between the GALT and the gut microbiota is extremely important to regulate the immune system. Since most (70-80%) of immune cells are found in the gut, maintaining a healthy gut microbiota is essential [22]. The understanding of gut microbiota has been extensively studied compared to lung microbiota.
The relationship between the gut and lung systems, known as the "gut-lung axis," has been extensively researched, and evidence supports the connection between gut microbiota and lung immunity. Dysfunction of gut microbiota has been linked to impaired alveolar macrophage function and reduced bacteria-killing capacity. Antibiotics can disrupt the gut microbiota, leading to the survival and growth of pathogenic microorganisms in the lungs. The gut-lung axis is bidirectional; through the bloodstream, microbial components from the gut can impact the lungs, and lung inflammation may also impact the gut microbiota. Studies have shown that exposure to the influenza virus can result in the migration of CD4 T cells from the lungs to the intestine, leading to dysbiosis of the gut microbiota and an abnormal Th17 response, intestinal damage, and gastroenteritis [23]. Chronic inflammatory diseases have also been linked to the gut microbiota composition [24].
Many simple therapeutic techniques have been proposed to modify microbiota and combat chronic illnesses. These include changes to one's diet and the use of pre-and probiotics. These modifications have the potential to mitigate the severity of COVID-19 through a multitude of mechanisms while also impacting the course of chronic illnesses like hypertension, NIDDM, obesity, and coronary heart disease. Addressing these comorbidities can significantly reduce the likelihood of developing severe coronavirus disease and lower mortality rates.
Disrupting beneficial microorganisms in the gut's microbiota can lead to gut dysbiosis and may increase the risk of respiratory illnesses, sepsis, and ARDS. The intestinal barrier serves as a shield against harmful microbes and their byproducts from entering the bloodstream. However, when gut dysbiosis occurs, the gut barrier may become more permeable, resulting in a leaky gut. This condition has been linked to decreased short-chain fatty acids (SCFAs) produced by gut bacteria. This increase in gut permeability allows microbiota-derived lipopolysaccharides (LPS) and inflammatory components to enter the bloodstream, resulting in inflammation and immune activation. Toll-like receptor 4 (TLR4) has a significant role in immune activation, and its activation by LPS can exacerbate a range of clinical problems. Research has indicated that TLR4 activation by LPS can worsen mortality rates in cases of influenza infections [25].
SCFAs are essential for regulating immune and inflammatory responses. By promoting mucin production and maintaining an acidic pH in the gut environment, harmful microbes are discouraged from growing. Furthermore, SCFAs maintain the integrity of the gut epithelium. So, epithelium leakage or translocation can be avoided. SCFAs are also powerful histone deacetylase (HDAC) inhibitors that can reduce inflammation by boosting the numbers and functions of regulatory T-, T helper-, and Th17 effector- cells. SCFAs can activate G protein-coupled receptors (GPCRs) like GPR43 and inhibit the Nf-kB pathway, which has an anti-inflammatory effect. Recent research indicates a potential gut-lung axis, as small amounts of SCFAs are present in the lungs [26]. The studies have also shown that SCFAs assist in creating macrophage and dendritic cell progenitors in the bone marrow. Additionally, SCFAs protect against airway inflammation and respiratory tract infections by enhancing the function of T cells [27].
Younger people may experience less severe symptoms and less risk of developing cytokine storms due to their less inflammatory response toward the coronavirus [28]. On the other hand, the elderly are more likely to have an imbalance in their gut microbiota, so the chances of having severe inflammation and the risk of developing cytokine storms will be higher. Also, beneficial strains of microbiota like Bifidobacteria and Lactobacillus and bacteria that produce SCFAs, which help maintain intestinal barrier integrity, will be altered in the elderly. Many shreds of evidence support that gut dysbiosis is important in chronic aging-related diseases. Therefore, the morbidity and mortality rates of coronavirus in elderly patients over 65 with comorbidities such as NIDDM, obesity, hyperlipidemia, hypertension, and cardiovascular disorders are high [29].
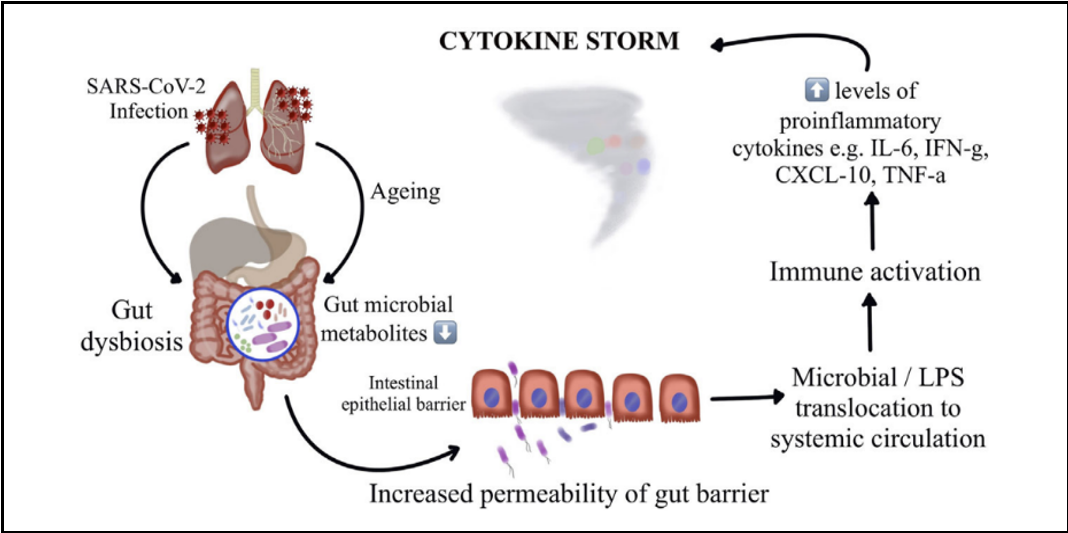
Numerous Studies reveal that immunological aging is connected with "inflammaging," a subclinical inflammatory state that relies heavily on Th1 immune responses. Children, on the other hand, exhibit more Th2 response, which leads to less production of pro-inflammatory molecules. Recent research has extensively studied and found that gut microbiota links to various chronic inflammatory diseases such as chronic respiratory diseases, inflammatory bowel disease, NIDDM, cardiovascular disease, depression, and hypertension [30]. Demonstrating a strong association between the disruption of gut microbiota and the severity and clinical outcomes of COVID-19 in elderly individuals with chronic inflammatory conditions. [31]. Multiple studies have suggested that increased cytokine and chemokine production, leading to viral hyperinflammation (cytokine storm), is mainly responsible for coronavirus mortality, as illustrated in Figure 4.
Figure 4: Gut microbiota perturbation leading to cytokine storm causing severe COVID-19 and death.
Maintaining an optimized and balanced immune response is essential in preventing severe inflammatory reactions that could potentially be life-threatening. This can be achieved by cultivating a healthy gut microbiota. A well-regulated immune response is critical for determining clinical outcomes and consequences, and it must be neither overly reactive nor under-reactive. Consuming a nutritious diet and taking supplements can help achieve a harmonious immune response balance.
Research has shown that dietary carnitine, primarily found in animal protein, can negatively impact human vascular health. Gut flora converts carnitine into trimethylamine (TMA), which is then metabolized into trimethylamine N-oxide (TMAO) in the liver. Increased TMAO in the bloodstream is linked to major adverse cardiovascular events, such as stroke, myocardial infarction, congestive heart failure, and mortality. In COVID-19 patients, a correlation between elevated serum TMAO and inflammation and thrombosis has been observed. Gut dysbiosis will also produce TMAO. Various molecular mechanisms, such as the nuclear factor kappa (NF-kB) and the expression of scavenger receptors (SRs) on the surface of macrophages, will be upregulated by TMAO and lead to inflammation. TMAO can also induce the expression of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-𝛼) and interleukin 1ß(IL-1ß), which increase the inflammatory response.
Moreover, TMAO will reduce the expression of anti-inflammatory cytokines such as interleukin-10 (IL-10). TMAO can also enhance platelet aggregation and adhesion to collagen, which may increase the risk of thrombosis. Recent studies have shown that high TMAO levels may lead to coronavirus severity [32].
Previous studies revealed that those who adhere to a vegan or vegetarian diet tend to have decreased levels of TMAO. Instead, consuming red meat, processed meat, egg yolks, fish, and full-fat dairy products results in elevated TMAO levels.
The microorganisms that reside in our gastrointestinal (GI) tract, from the mouth to the anus, are an essential part of our overall health. Our well-being is maintained by the gut, which is connected to various organs in our body through the gut-organ axis. Moreover, the gut-brain axis (GBA) significantly influences our emotions and behavior. The gut-brain-immune system axis operates bi-directionally and can impact the progression of COVID-19, particularly when stress is involved. Several communication systems exist between the gut and brain, including the autonomic nervous system (ANS), the enteric nervous system (ENS), the immune system, and neuroendocrine signaling systems, which can all impact the gut microbiota. As a result, having a healthy balance of gut microbiota may help alleviate stress levels during the pandemic [33].
In light of the coronavirus pandemic, it's crucial to maintain a healthy mood and behavior to prevent cytokine storms. Several risk factors for cardiovascular disease can lead to dysbiosis, weakening the gut barrier and causing inflammation. Recent research has shown that this can increase the severity of coronavirus symptoms. Furthermore, studies have shown a close association between gut microbiota, dietary lipid intake, and atherosclerosis development, involving metabolic and inflammatory factors. A novel pathway has been identified that connects these components, with the production of TMAO being linked to reduced bile acid synthesis and inhibited reverse cholesterol transport, ultimately contributing to the development of atherosclerosis. Microbial dysbiosis and abnormal metabolite production have been associated with exacerbation of acute heart failure [34], which can worsen the prognosis of COVID-19 patients who already suffer from both atherosclerosis and heart failure (gut-heart axis).
Numerous health benefits have been associated with healthy microbial metabolites, such as anti-inflammatory, antioxidant, anti-lipid, antiproliferative, anti-obesity, antihypertensive, and immunomodulatory. Recent research has shown that the gut microbiota can adapt rapidly following a change in diet, whether PBD or omnivorous, with noticeable alteration appearing in three days [35]. This information suggests that even those with unhealthy eating habits can improve their microbiota through dietary changes, which is especially relevant for new COVID-19 patients in acute settings. A shift in gut microbiota composition occurs when transitioning from PBD to an omnivorous diet, with increased bile acid-metabolizing species, which can induce an inflammatory process. On the other hand, PBD is effective in combating inflammation, as it has been demonstrated to decrease chronic inflammation markers such as Fibrinogen, IL-6, and CRP [36]. Interestingly, individuals who primarily follow PBD do not experience increased TMAO levels when occasionally consuming animal-based foods [37]. This is postulated due to the microbiota environment established over time.
Many experts suggest that the current COVID-19 pandemic presents a rare chance to assess how nutritional interventions may help fight infectious diseases. With this in mind, conducting tests during the pandemic can yield valuable information [38]. Studies have revealed a significant link between consuming foods that include fresh, nutrient-rich foods like vegetables, legumes, whole grains, healthy fats (seeds, nuts, and avocado), and fruits and limiting one's intake of sugary products, high-calorie empty nutrients, and high-salt foods, leading to a decrease in COVID-19 severity and mortality [3,38,39-41].
2.3. Mechanism of inflammation and endothelial dysfunction in coronavirus
The role of chronic inflammation in developing vascular lesions cannot be overstated. This process causes endothelial dysfunction and triggers several other processes that contribute to the worsening of atherosclerosis. These processes include platelet aggregation, leucocyte adhesion, cytokine production, and increased endothelial permeability [42]. Unfortunately, atherosclerosis is often associated with acute coronary events, and COVID-19 can exacerbate this situation by inducing a severe inflammatory state that may trigger similar events. It's important to note that coronary artery disease resulting from atherosclerosis, as well as heart failure, hypertension, and atrial fibrillation, are all considered comorbidities for COVID-19, and they are also caused by chronic inflammation [43]. Patients with heart conditions who contract coronavirus are at an increased risk of developing arrhythmia, acute coronary syndrome, and acute heart failure, which can lead to higher mortality rates.
Eating unhealthy foods such as sugary drinks, snacks, cakes, pastries, sweets, added salt, saturated fat, trans-fat, cholesterol, dairy products, processed meat, red meats, poultry, and eggs can contribute to chronic inflammation [44]. Coronavirus is classified as an acute inflammatory disorder, and it can increase inflammatory markers such as ferritin, procalcitonin, LDH, D-Dimer, and acute phase response proteins. This virus can also trigger a cytokine storm, a severe inflammatory reaction that can be fatal [45]. Coronavirus patients with chronic inflammation are at risk for severe inflammation regardless of their comorbidities. In that case, their body will have to work extra hard to combat the inflammation and its complications. Their chances of experiencing a cytokine storm and facing a higher mortality risk are understandably higher [46,47]. Conversely, eating foods that decrease dietary inflammatory index (DII) is recommended to combat the inflammatory response in our coronavirus patients (Table 1).
Table 1: List of foods that increase and decrease inflammation.
|
Foods that increase DII |
Foods that decrease DII |
|
Red meat (steak and hamburgers) Animal products (including eggs and dairy products) Processed meat Commercial baked goods White flour (bread and noodles), white rice Deep-fried foods Sugary products Products with trans-fats Saturated fats (especially animal fats) Cholesterol (red meats, processed meats, eggs, fried foods and dairy products) |
Plant-based proteins (beans, lentils, chickpeas, edamame, hemp seeds, tofu, tempeh, and nuts) Whole grains (oatmeal, buckwheat, quinoa, pigmented rice) Starchy vegetables (sweet potatoes and beets) Seeds (flaxseeds and chia seeds) Green leafy vegetables (raw) Colorful vegetables (raw) Fruits (berries, apples, grapes, oranges, peaches, figs, bananas, and kiwi) Spices and herbs (turmeric, ginger, cumin, peppermint, cinnamon, chili, parsley, bay leaf, and basil) |
Rich polyphenols are found in vegetables and fruits and can inhibit the binding of coronavirus spike protein to the ACE2 receptor. Thus, viral entry into host cells can be prevented, and viral RNA replication and protein processing can be suppressed [48]. Consuming red meat, refined sugar, high cholesterol, saturated and trans fats foods will promote inflammation and can expedite the binding of coronavirus to host cells [49].
Adopting a healthy lifestyle for an extended period can significantly improve chronic disease conditions. Therefore, an individual with multiple comorbidities who embraces a healthy PBD and lifestyle may observe a normalization of most of their chronic inflammatory markers. These can decrease the risk of coronavirus severity and mortality. Is PBD intervention a viable option for individuals with chronic comorbidities who contract COVID-19 in the acute setting? While research has indicated that PBD intervention can effectively reduce acute arthritic pain [50,51], our experience with COVID-19 patients has demonstrated the same efficacy. Even though inflammatory markers may not improve, patients reported symptom relief. Thus, our study supports this hypothesis.
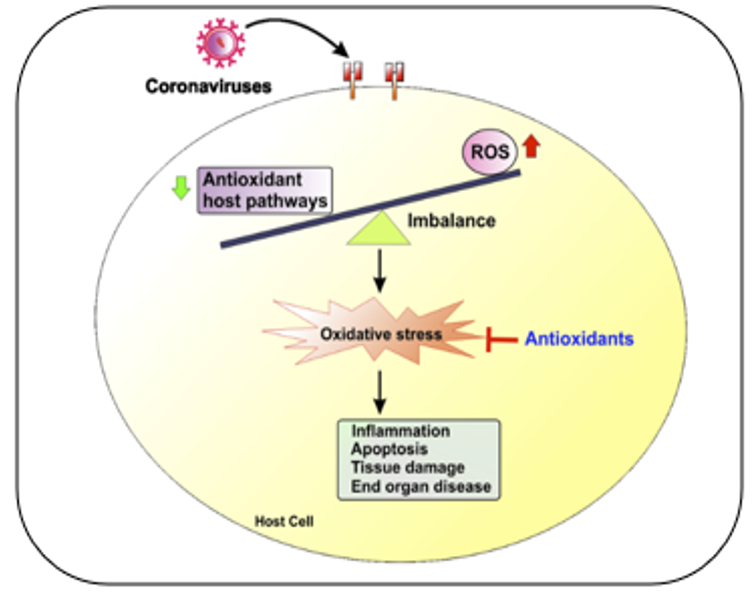
2.4. Role of oxidative stress in coronavirus
One mechanism through which the coronavirus causes imbalances within the body is by generating reactive oxygen species (ROS) and diminishing the body's capacity to produce antioxidants that can combat the virus. Automatically, this will induce redox stress, decreasing the body's ability to fight off the virus and increasing inflammation and cell damage. Ultimately, this will damage tissues and organs in the body. A diagram illustrating this process can be found in Figure 5.
Figure 5: Redox imbalance in coronavirus infections.
ROS is a natural byproduct of metabolic processes in different organelles, including endoplasmic reticulum (ER), plasma and nuclear membranes, mitochondria, and peroxisomes. These ROS play an essential role in various cellular processes. ROS can cause oxidative damage to mitochondria, dysregulated gene expression, aberrant cellular signaling, and impaired host defense. Notably, the primary source of ROS is from mitochondria named mito-ROS, which are formed during energy production. ROS levels can increase during viral infections, and while they have harmful effects on tissues and cells, they are also essential as an antiviral [52].
Too much ROS in the body can harm essential parts of cells like proteins, lipids, and DNA. ROS can also affect immune functions, cause Inflammation, and damage organs and tissues. Research has found that oxidative stress is crucial in viral respiratory infections like influenza and RSV. In severe cases of COVID-19, elevated oxidative stress can induce inflammation, harm endothelial cells, plus form blood clots. Eventually, it will damage multiple organs [53,54].
Research suggests that elderly patients are more susceptible to oxidative stress. There is a reciprocal link between ROS and chronic Inflammation, in which chronic Inflammation leads to the buildup of ROS, and ROS also contributes to chronic Inflammation [55]. Consequently, older individuals who have chronic inflammatory conditions are at greater risk of experiencing serious illness in the event of contracting SARS-CoV-2 infections [56].
Research has shown that consuming meat products, refined sugars, and fats can increase ROS levels, resulting in Inflammation. However, studies indicate that incorporating PBD can potentially reduce Inflammation and oxidative stress, which play a significant role in coronavirus infection [57-60]. Exploring dietary changes for COVID-19 patients may be prudent as the current omnivorous or similar diets can increase oxidative stress and Inflammation. Adopting PBD can help manage chronic inflammatory conditions and enhance the likelihood of surviving the COVID-19 pandemic.
2.5. The link between Mitochondria health and COVID-19 severity and mortality
As we grow older, the powerhouses of our cells, known as mitochondria, undergo changes that cause a decline in their function. This decline is caused by ROS-inducing accumulation of oxidative damage and mutations. As a result, the volume, integrity, and functionality of mitochondrial DNA (mtDNA) decrease. Moreover, the mitochondria of older adults are characterized by significant increases in ROS and decreased antioxidant defense, which lead to impaired functions. These include decreased ATP production, lowered oxidative capacity, and reduced oxidative phosphorylation. In addition, as individuals age, mitochondrial biogenesis will gradually decrease. This decline is caused by the inhibition of mitophagy and alterations in mitochondrial dynamics, which are active processes requiring numerous specialized proteins, including mechanical enzymes that physically modify mitochondrial membranes and adaptor proteins that regulate the interaction of these enzymes with organelles through processes called fission and fusion. Furthermore, the autophagy process that removes defective mitochondria will also deteriorate with age.
Acute and chronic inflammatory diseases are characterized by an excessive generation of ROS. This may cause damage to mtDNA, mitochondrial proteins, and lipids. This, in turn, negatively affects normal mitochondrial function and dynamics. Inflammation is generated by various mitochondrial products called damage-associated molecular patterns (DAMPs) and is released into the cytosol or extracellular environment. Protective measures are in place to prevent mitochondria from triggering harmful inflammatory responses, such as disposing of damaged mitochondria through autophagy. However, if these mechanisms are overwhelmed or not functioning correctly, inflammatory reactions instigated by mitochondria can become problematic and contribute to developing disorders associated with autoimmunity. Furthermore, inefficient inflammatory pathways can exacerbate infectious diseases and impede healing.
Atherosclerosis occurs due to the dysfunction of endothelial cells and the infiltration of lipids. Mitochondrial dysfunction can negatively impact various cells within the arterial wall, including endothelial cells, smooth muscle cells, macrophages, and lymphocytes, leading to heightened ROS levels. This can cause chronic Inflammation, oxidative stress, and intracellular lipid deposition. Moreover, mitochondrial dysfunction plays a significant role in chronic inflammatory diseases like hypertension, obesity, and asthma [61,62].
As we learn more about COVID-19, it becomes increasingly clear that certain factors may contribute to its severity and mortality. These include age, age-related conditions, and underlying disorders like CVD, metabolic syndrome (including NIDDM), obesity, and hypertension. In coronavirus disease development, a potential role for mitochondria has been discovered. Mitochondria and its mtDAMPs control the immune system and impact coronavirus infection. It is suggested that mitochondria hijacked by the coronavirus could significantly affect COVID-19 pathogenesis [63], potentially causing massive Inflammation and damage to multiple organs. A new study in the journal Nature sheds light on why some people with COVID-19 develop these severe symptoms, revealing that the virus may be able to infect and cause fatalities in vital immune cells within the bloodstream and lungs. As we continue to investigate this virus, it has been proven that mitochondrial dysfunction significantly eliminates these immune cells [64].
Studies have proposed that people living in "blue zones" lead long and healthy lives by adhering to healthy habits such as exercising regularly, eating a balanced diet, mainly being PBD, abstaining from harmful substances, managing stress effectively, having strong social support, and getting enough rest. These lifestyle choices contribute to maintaining healthy mitochondria. Mitochondrial function can be improved by consuming PBD foods, specific natural products, caloric restriction, intermittent fasting, and exercise [65-67]. Our elderly coronavirus cardiology patients have been living a Blue Zone lifestyle for many years, leading us to hypothesize that this has resulted in less severe symptoms and zero mortality due to their healthy mitochondria.
2.6. Potential benefits of telomere manipulation in coronavirus treatment
As the Centers for Disease Control and Prevention (CDC) reported, the most significant factor in determining poor outcomes and severe illness in individuals with COVID-19 is aging. Data from the National Vital Statistics System (NVSS) at the CDC reveals that people aged 50-64 with COVID-19 are 25 times more likely to die than those under 30 years old. For individuals aged 65-74, the risk of death increases to 60 times, while for those over 85, it jumps to 340 times. This data includes all deaths in America from February 2020 to July 1, 2022, regardless of vaccination status [68,69].
Short telomeres are affiliated with a higher probability of all-cause mortality and disease-specific mortality in the general population [70]. Studies suggest that COVID-19 severity in older individuals may be influenced by the same molecular pathways that cause aging. One of these pathways involves gradual telomeres shortening. Telomeres are protective structures at the ends of chromosomes. When telomeres become excessively short, they can hinder tissue regeneration and disrupt tissue homeostasis, leading to disease. Since the coronavirus infects various cell types, it triggers cell turnover and regeneration to support homeostasis. Research has shown that people with shorter telomeres are at an increased risk of experiencing severe coronavirus symptoms. The risk of developing severe coronavirus pathologies has been associated with individuals with higher percentiles of short telomeres and lower percentiles of telomere length [71-73]. Myeloid and lymphoid cells contribute to innate and adaptive immunity, which is essential in defending against coronavirus [74]. To resist the infection, the production of myeloid cells is rapidly activated when the virus enters the body.
The length of telomeres will shorten with age; this will affect the production of T and B cells, which are crucial to clearing the virus. Understandably, older people will have a higher risk of a T-cell shortfall when contracting coronavirus compared to younger people. People with inherently short T-cell telomeres will experience poor adaptive immune response caused by a T-cell deficit, which might contribute to the severity of COVID-19. These individuals might also generate inadequate T-cell response to anti-SARS-Cov-2 vaccination, which is vital to know [75]. Furthermore, ACE2 (Angiotensin Converting Enzyme 2), the receptor for SARS-CoV-2, is upregulated by shortened telomeres. So, telomere shortening in elderly individuals increases their susceptibility to coronavirus infection and increases their risk for coronavirus severity and mortality [76].
Chronic Inflammation can worsen the shortening of telomeres, altering the genes related to telomeres, and decreasing telomerase enzymes, which control the release of cytokines that cause Inflammation. All of these mechanisms will cause severe COVID-19 disease and mortality. There is a two-way relationship between telomere shortening and Inflammation; telomere attrition and dysfunction can cause low-grade Inflammation. On the other hand, Inflammation can speed up telomere wear and telomere dysfunction. [77].
Chronic Inflammation and oxidative stress can accelerate telomere shortening. However, consuming healthy PBD, which is full of anti-inflammatory and antioxidant properties, counteracts this process. Observational studies have indicated that adopting a healthy PBD, consuming seeds and their derivatives, and incorporating carotenoids will slow telomere shortening. This can lead to improved overall health and longevity [78,79].
Elizabeth Blackburn, a Nobel Prize winner, discovered that switching to a vegan diet can change over 500 genes in just three months. This diet can activate genes that help prevent diseases and deactivate genes that cause chronic inflammatory diseases [80]. There are various ways to preserve our telomeres, including exercising regularly, avoiding smoking, and consuming a diet rich in plant-based foods that protect telomeres. Dean Ornish and Elizabeth Blackburn conducted a study demonstrating how PBD can increase telomerase activity, the enzyme responsible for maintaining telomere length. The ability to prevent telomere from shortening is crucial for longevity. While we cannot reverse chronological age, we can reverse biological age, which can help us reverse chronic illnesses in our patients and protect them from severe morbidity and mortality from coronavirus. Additionally, reducing our patients' biological age by one or two decades will automatically decrease their risk of developing severe coronavirus. We hypothesized that one of the mechanisms implies that the elderly coronavirus patients in our study have milder symptoms, avoid hospitalization, and experience no mortality, which is linked to their telomere superiority.
2.7. Caloric Restriction is emerging as an essential factor in the fight against Inflammation
Reducing caloric intake, commonly known as Caloric Restriction or CR, has been shown to have consistent anti-aging effects across various organisms. Age-related diseases, including chronic inflammatory disorders such as stroke, NIDDM, cardiovascular disease, hypertension, and cancer, have been shown to improve with the practice of CR. CR can reduce oxidative stress and Inflammation while boosting the production and activity of antioxidant enzymes and anti-inflammatory mediators. Studies have also revealed that CR can improve overall health and well-being, reduce ROS and Inflammation, enhance cellular protection, optimize energy metabolism, improve insulin sensitivity and glucose regulation, induce functional changes in the neuroendocrine systems, and even shape the gut microbiota [81].
DNA and RNA viruses, including coronavirus, use the Mammalian Target of Rapamycin (mTOR) as their signaling system to replicate and persist in host cells. CR has been found to inhibit the mTOR pathway, similar to the effect of Rapamycin in laboratory research. One of the mechanisms CR benefits in coronavirus is mTOR inhibition and autophagy promotion. Moreover, CR could help fight the virus by interrupting the viral cycle (protein synthesis) [82].
Increased glucose variability and poor glycemic control have been associated with coronavirus severity [83,84]. Metabolic syndrome and insulin resistance, as shown by elevated triglycerides and glucose (TyG) index, are associated with coronavirus severity and morbidity [85,86]. Eating healthy PBD with CR will help the patients have proper glycemic control, achieve good metabolism, and avoid insulin resistance.
In our modern society, unhealthy habits like consuming excessive calories and living a sedentary lifestyle are significant determinants of health issues and inflammatory disorders. The overconsumption of food, especially unhealthy food, can pose big problems. Therefore, COVID-19 patients who consume excessive calories with poor nutrients will struggle to fight Inflammation, especially if their bodies are already inflamed [87]. We hypothesized that the whole food PBD emphasizing CR may have contributed to the mild illness and zero mortality observed in our COVID-19 elderly patients with multiple comorbidities study.
3. Mechanism of supplements in decreasing incidence, severity, hospitalization, and mortality of COVID-19
In the preliminary phase of our research, we did not come across any academic literature demonstrating the role of dietary supplements in mitigating the frequency, severity, and mortality of coronavirus infection. Therefore, the literature we used to determine the types of vitamins, minerals, and nutraceuticals in our study was taken from the literature we used in previous studies, where we combatted chronic inflammatory diseases with PBD and dietary supplements. Those dietary supplements have been known to increase NO levels, reduce Inflammation, serve as immunomodulatory, decrease the likelihood of cytokine storms, fight oxidative stress, and exhibit antiviral properties. We also studied extensively literature about dietary supplements that have shown their benefits in previous viral pandemics.
Most adults consume a poor-quality diet and lack essential nutrients such as magnesium, calcium, potassium, iron, vitamins A, C, D, E, fiber, and choline [88]. Therefore, we provide our patients with multivitamins based on the literature and blood levels. It is worth noting that all of our cardiology patients who follow PBD have been advised to take B12, dietary multivitamins, and supplements with antioxidant, anti-inflammatory, endothelial-supporting, and other properties relevant to cardiovascular health.
3.1. Vitamin C
Due to its anti-infective properties and safety, many experts have proposed vitamin C as a potential treatment for coronavirus. Studies have shown that the severity and duration of colds can be reduced by taking vitamin C [89]. Numerous studies have revealed that vitamin C may support cellular health and defense against coronavirus infection. This is believed because vitamin C has anti-oxidative stress and anti-inflammatory properties [90]. In severe coronavirus cases, high doses of vitamin C help fight oxidative stress and relieve the severity of cytokine storms. A high dose of intravenous vitamin C shortens patients' time in the ICU, and if combined with corticosteroids and thiamin, it also reduces mortality [91].
In treating patients who require mechanical ventilation caused by an acute inflammatory lung injury, vitamin C has positive effects due to its oxidative stress properties. A study from China suggested that high-dose intravenous vitamin C can effectively treat coronavirus patients. Those patients who received intravenous bolus vitamin C had their oxygenation improved, and no mortality was observed [89].
Recent meta-analyses of randomized controlled trials have shown that vitamin C may improve mortality rates in patients with severe coronavirus infections [92,93]. In light of the information provided, it is highly recommended that vitamin C be utilized to mitigate the severity of COVID-19, prevent hospitalization, and reduce mortality.
3.2. Vitamin D
Our previous experience with cardiology patients has shown that most of them, regardless of their dietary status, had a deficiency of vitamin D. Therefore, we recommend that our patients with PBD take vitamin D supplements and adjust the dosage based on their blood vitamin D levels. Vitamin D is crucial in combating chronic inflammatory diseases, such as coronary heart disease, and it may also play a role in combating COVID-19, which is characterized by Inflammation.
T regulatory lymphocytes (Tregs) are essential in fighting against uncontrolled Inflammation and viral infections. However, many COVID-19 patients have low Tregs levels. One way to increase Tregs levels is through supplementation with vitamin D. Decreased vitamin D has been linked to increased inflammatory cytokines, which significantly increase the risk of infection in the upper respiratory tract, including viral and pneumonia [94]. The recent paper has acknowledged the role of vitamin D in reducing the risk of COVID-19. The proposed mechanism is the induction of cathelicidin and defensins, which decreases the risk of viral and bacterial infections and diseases, including pneumonia. Additionally, vitamin D may reduce the binding of the virus to the ACE2 receptor, which could potentially lessen the risk of cytokine storms in coronavirus infection [95].
The likelihood of thrombotic events, commonly observed in coronavirus patients, has been associated with vitamin D deficiency [96-97]. Many coronavirus patients developed severe diseases during the pandemic or died due to vitamin D deficiency [98]. A recent meta-analysis has suggested that vitamin D supplementation could positively impact the incidence, severity, hospitalization, and mortality of SARS-CoV-2 illness, especially in patients with vitamin D deficiency [99-101].
3.3. Vitamin B3, precursor of NAD+
There are three primary types of vitamin B3, i.e., Nicotinic acid (NA), nicotinamide riboside (NR), and nicotinamide (NAM). These vitamins are not only helpful in preventing pellagra but may also prevent some of the physiological changes that occur with age-related diseases and promote healthy aging, particularly NR [102]. It has been suggested that increasing NAD+ levels might enhance the body's ability to fight off viruses and reduce Inflammation. NA, NR, and NAM play essential roles as precursors of NAD+ and provide the production of NAD+ through different metabolic pathways.
Studies indicate that different types of B3 vitamins will support NAD+ levels differently. To increase NAD+ concentrations, one can give NMN, which is derived from NAM and in the body will be converted into NAD+. NMN only requires one enzymatic step to be transformed into NAD+ and is currently the most effective method to raise NAD+ levels [103,104]. Research on NAD+ in vascular aging and chronic inflammatory diseases like coronary artery disease has been promising. NAD+ has been shown to reduce chronic Inflammation, induce reactivate autophagy and assist mitochondrial biogenesis. These findings suggest that supplementing with precursor NAD+ may be a useful therapeutic approach for treating these conditions [105], as we have done for our elderly cardiology patients long before the coronavirus pandemic. Further, more sophisticated research is required before recommending precursor NAD+ to reduce the morbidity and mortality of coronavirus patients.
3.4. Zinc
Oral zinc (Zn) supplementation can reduce the 30-day death rate, shorten the duration of symptoms, and decrease the ICU admission rate, as shown in a recent randomized, double-blind controlled trial [106]. Trace element zinc is crucial in stimulating innate and acquired immunity. A recent meta-analysis has found that zinc supplementation can reduce coronavirus mortality in hospitals. Due to the scientific evidence of zinc's role in coronavirus patients, it should be considered an adjunct therapy [107].
3.5. Copper
Both copper (Cu) and zinc play an essential role in the production of an important antioxidant enzyme known as superoxide dismutase (SOD). This enzyme aids in combating free radicals and decreasing oxidative stress. Maintaining the appropriate ratio between the two is essential to ensure the optimal functioning of this enzyme. It is important to know that zinc can lead to copper deficiency. Therefore, seeking guidance from a qualified healthcare professional is crucial to prevent adverse effects. Cu plays a critical role in assisting the functions of macrophages, neutrophils, and immune cells such as B cells, T helper cells, and natural killer (NK) cells. These blood cells kill infectious microbes, produce specific antibodies against pathogens, and mediate cell-mediated immunity [108,109]. In humans with Cu deficiency, there is an increased risk for infections due to these blood cells' decreased number and function. We routinely incorporate zinc and copper into our treatment regimen, as the presence of zinc can result in copper deficiency, and copper may aid patients' immune systems in combating COVID-19.
3.6. Selenium
Several studies have suggested that lacking selenium may negatively affect viral disorders. The correlation between selenium and coronavirus disease severity research has yielded mixed results [110]. According to the report, a shortage of selenium is linked to a higher risk of chronic diseases with inflammatory pathogenesis [111]. The use of selenium has demonstrated encouraging results in treating viral disorders. However, it is important to consider the host's nutritional status, as this can lead to mutations in the viral genome from a benign or mildly pathogenic virus to a highly virulent one under oxidative stress. These mutations can further spread in hosts even with adequate selenium intake [112]. This is assumed due to selenium's immune-boosting, anti-inflammatory, and antithrombotic effects [113]. Many of our cardiology patients have been taking selenium supplements before contracting the coronavirus, which may explain why many experienced mild symptoms upon presentation.
3.7. Coenzyme Q10 (CoQ10)
CoQ10 benefits cardiovascular health by helping reduce total cholesterol, improving endothelial function, and fighting Inflammation and oxidative stress. This also makes it a suitable option for treating COVID-19 [114,115]. A recent meta-analysis found that CoQ10 supplements taken for 10 weeks may lower malondialdehyde (MDA), IL-6, and TNF-α levels, which are all involved in COVID-19 inflammation [116].
In the course of the resurgence of the coronavirus pandemic in China in 2023, there has been a growing demand for Ubiquinol - the active form of CoQ10. Supplementing with Ubiquinol can significantly increase the level of CoQ10 in whole blood, platelets, and plasma. CoQ10 can also accelerate the regeneration of mitochondrial function [117,118]. Recent research has shown that patients recovered from coronavirus have reduced levels of platelet mitochondrial function and decreased endogenous CoQ10. Mitochondria are the energy powerhouses of our cells and are vital for cellular metabolism and immune responses. Further research has proposed that mitochondria have a role in antiviral defense [109,120]. Our practice has consistently utilized CoQ10 for our cardiology patients, and it may also provide benefits during their battle against coronavirus infections.
3.8. Astaxanthin
Studies have shown that astaxanthin significantly reduces oxidative stress biomarkers, such as blood MDA levels. This effect is particularly notable in NIDDM patients, especially those who are overweight, where astaxanthin has been found to improve superoxide dismutase activity and reduce serum isoprostane, which has an antioxidant effect and can neutralize free radicals [121].
Natural astaxanthin can potentially alleviate cytokine release syndrome by regulating inflammatory cytokines. It does this by inhibiting the activities of key players such as JAK/STAT-3, NF-kB, and NLRP3 while regulating the expression of pro-inflammatory factors such as IL-6, IL-8, TNF-α, and IL-1ß. Additionally, natural astaxanthin can prevent oxidative damage. Given these benefits, it is reasonable to consider supplementation with astaxanthin since it has a potential therapeutic effect against inflammation and cytokine storms in coronavirus patients [122,123].
3.9. Quercetin
Quercetin may reduce the likelihood of coronavirus patients being admitted to ICU, as has been shown in recent systematic reviews and meta-analyses. It also can lower hospitalization rates and potentially decrease mortality [124,125]. Quercetin, as an antioxidant, has also been known to possess antiviral and anti-inflammatory properties. According to studies, Quercetin has been found to interfere with 85% of coronavirus proteins in human cells [126]. A study recently highlighted Quercetin's capability to block ACE2 receptors, thereby interfering with viral replication. Moreover, protease enzymes that play a crucial role in virus replication may be inhibited by Quercetin [127].
Flavonoid quercetin in PBD has been found to block the active site of 6LU7, the major protease in coronavirus. This will inhibit virus replication [128,129]. In addition to its anti-inflammatory and antioxidant effects, Quercetin exhibits a potent iron-chelating property, which helps decrease the severity of coronavirus symptoms. Due to these properties, we believe Quercetin can be a valuable therapeutic option for treating COVID-19 [130].
3.10. Curcumin
Curcumin is a natural polyphenolic compound with various benefits, such as anti-inflammatory, antiviral [131], cytoprotective, anticoagulant, and antiplatelet properties. It has been proven to help reduce the progression of several inflammatory diseases [132,133]. These effects have made Curcumin a potential treatment option for coronavirus patients. The pathophysiology of COVID-19 involves severe inflammatory reactions, coagulopathy, and the development of cytokine storms. The benefits of Curcumin can be due to its anti-inflammatory effects, such as inhibiting inflammasome formation. By binding to the primary protease (Mpro) enzyme of coronavirus, which is necessary for viral replication, Curcumin has been found to have antiviral effects.
Additionally, Curcumin can effectively block viral attachment to entry into human cells [134]. According to modeling studies, ACE2 receptor and spike proteins are both inhibited by Curcumin, thus preventing virus-receptor interaction [135]. In a randomized trial, it was suggested that Curcumin could speed up the recovery of acute inflammatory immune response.
In coronavirus patients who received nano-curcumin, the mRNA expression of interleukin-6 and interleukin- 1ß was significantly decreased, which may reduce the systemic inflammatory reaction. Moreover, further trials showed a significant reduction in gene expression, the number of Th17 cells, and serum levels of Th17-mediated cytokines upon administering Curcumin. These mechanisms may benefit coronavirus patients [136-138]. Systematic reviews and meta-analyses of randomized trials recently concluded that oral administration of Curcumin as a supplement in coronavirus patients could significantly decrease their death risk [139]. Thus, our cardiac center employs curcumin supplementation as a regular measure to combat chronic inflammatory diseases, including coronavirus infection.
3.11. Taurine
Various studies suggest Taurine can act as an antioxidant, anti-inflammatory, and antiviral substance. Taurine may also help regulate vascular function. Its potential effects are wide-ranging, from stopping viral invasion through the AT1R-mediated coronavirus/ACE2 endocytosis pathway to reducing vascular injury and inflammation by affecting both inflammatory and coagulation pathways. Like other infections, COVID-19 decreases Taurine levels, which limits its protective properties under normal circumstances. It is hypothesized that early Taurine administration during COVID-19 onset can halt cytokine storms, reducing morbidity and mortality. An increasing number of studies have demonstrated that Taurine supplementation is effective in guarding against pathologies linked to mitochondrial defects, including aging, mitochondrial diseases, and metabolic syndrome.
Furthermore, this pathway may also play a role in the beneficial effects of Taurine in reducing the severity of COVID-19 [140-142]. Taurine seems to be a promising, supplementary option for coronavirus patients.
4. Healthy lifestyles their significant role in managing the COVID-19 pandemic
As we demonstrated, the significance of PBD and supplementations in managing coronavirus patients has been emphasized. In addition to following a balanced diet, everyone must adopt certain lifestyle practices to mitigate chronic inflammatory conditions and prevent severe coronavirus and its associated mortality. These practices include maintaining a healthy weight, engaging in regular physical activity, reducing stress, abstaining from harmful substances such as smoking and alcohol, ensuring adequate sleep, and fostering positive personal relationships.
To enhance the immune system, consuming 2-3 cups of coffee daily [143] and cabbage and fermented vegetables in one's diet is recommended. Cabbage is rich in sulforaphane, a natural and potent Nrf2 activator. Fermented vegetables are abundant in lactobacilli, another potent Nrf2 activator with antioxidant properties, helping alleviate the severity of coronavirus infections [144].
During the COVID-19 pandemic, we recommended that our patients receive sun exposure. Research has demonstrated that sunlight exposure is associated with decreased mortality rates for COVID-19 patients, independent of its role in increasing vitamin D levels. It is believed that ultraviolet A and B radiation may promote the release of NO from the skin, which can benefit cardiovascular health and reduce the risk of coronavirus infection [145-147].
5. Discussion
PBD has been associated with reduced COVID-19 incidence, severity, hospitalization, and mortality rates over time, as demonstrated by various studies [39-41,148-152]. However, in early 2024, certain experts in the field, including Rayman M, Stewart G, Mellor D, and McCoway K, expressed strong reactions to a recent study published in BMJ Nutrition, Prevention & Health by Acosta-Navarro et al. that investigated the relationship between various dietary patterns and COVID-19 infection. The experts specifically highlighted the deficiency of essential nutrients, such as vitamins B12 and D, and minerals like iodine, iron, zinc, selenium, and calcium, in PBD interventions, which can be detrimental, particularly for pregnant individuals. The experts also drew attention to the ambiguous definition of PBD in the study, which did not consider factors such as the quality, quantity, and food preparation methods, as well as the loose definition of PBD encompassing ovo- and pesco-vegetarians. Since most of those studies used questionnaires, further research with a clear definition of PBD and adequate supplementation is needed to provide valuable insights and shed additional light on the subject, per the expert commentary.
Our study is a novel and unique contribution to the field of COVID-19 research, as it is the only one to address the issue of PBD specifically. We have given careful consideration to the opinions of experts and have included micronutrients in the dietary supplements of our coronavirus participants. These micronutrients include vitamin C, vitamin D, vitamin B3/NAD+, zinc, copper, selenium, natural anti-inflammatory products such as astaxanthin, curcumin, quercetin, as well as CoQ10, Taurine, and multivitamins (which contain a variety of small doses of minerals and vitamins as recommended for daily RDA). To the best of our knowledge, as of the writing of this paper, no previous PBD studies on COVID-19 have included the use of supplements. Our study aims not only to ensure the adequacy of supplementation but also to validate our hypothesis that dietary supplements can enhance the anti-inflammatory, immune regulatory, and antiviral properties of PBD in combating coronavirus. In recent years, experts have emphasized the significance of utilizing supplements in managing the virus. However, it is important to note that the aforementioned experts are not affiliated with PBD.
Our research distinguishes itself from others through a meticulous accounting of variations in food quality, quantity, and preparation methods and closely monitoring participants' dietary intake throughout their illness. These factors are of paramount importance and warrant special consideration, especially given the disparities between our findings and those of other studies. Our study's unique characteristics, such as the mild symptoms exhibited by our participants and the absence of disease progression, hospitalization, and mortality, deserve special attention. A detailed account of our study can be accessed separately in our article “Plant-Based Diet and Supplements in Mitigating COVID-19: Part 1. The Research Report”
Our work has resulted in improved outcomes for individuals diagnosed with COVID-19, including an enhanced quality of life and a longer lifespan. Moreover, our findings may protect against future pandemics, such as disease X, as the World Health Organization (WHO) predicted. It is evident from historical records that adopting a healthier lifestyle and emphasizing the principles of plant-based diets (PBD) has protected numerous individuals from various pandemics since the beginning of the 20th century [153,154]. Furthermore, our extensive knowledge of supplementation will enrich our understanding of how to combat COVID-19 and may motivate us to prepare for future pandemics.
Several studies have emphasized the significance of PBD in managing COVID-19, and our research has provided substantial evidence to reinforce this notion. Despite this, the medical community remains cautious and skeptical about the application of PBD in treating the virus. The World Health Organization has not endorsed PBD or supplementation specifically in their guidelines for managing coronavirus. We hope that future research will further elucidate the benefits of PBD and supplementation in treating viral illnesses, leading to greater acceptance and integration within the medical community.
6. Conclusion
This review article complements our earlier research, which demonstrated that PBDs and supplements were successful in helping our 3,470 elderly COVID-19 cardiac patients. Compared to the general COVID-19 Indonesian population (GCIP), our patients exhibited quicker recovery rates (12±1.4 vs. 21±7 days), lower severity levels (2% vs. 10-20%), fewer hospitalizations (0 vs. 5-10%), and no fatalities (0 vs. 15-17%).
The method employed in our study differs significantly from those employed in other studies, as we pay close attention to the quality, quantity, and food processing of PBDs. Moreover, we supplement our PBD regimen with various supplements to address deficiencies commonly observed in PBD followers. These supplements possess anti-viral, anti-inflammatory, antioxidant, antithrombotic, and immunomodulatory properties, which serve to enhance the effectiveness of PBDs in combating COVID-19. Therefore, our interventional research is both compelling and innovative, offering a unique contribution to the research community, particularly in the realm of COVID-19 management.
Author Contributions: Conceptualization, D.M.; Writing-original draft, D.M.; Writing-review and editing, D.M., A.M.H., I.N.E.L., M.K.S., and H.U. All authors have read and agreed to the published version of the manuscript.
Funding: This research was supported by the National Institute of Health (1UØ1 CKØØØ577-Ø1).
Conflicts of Interest: The authors declare no conflict of interest.
References
- Wang P, Song M, Eliassen AH, Wang M, Fung TT, et al. (2023) Optimal dietary patterns for prevention of chronic disease. Nat Med. 29(3): 719-728.
- Peña-Jorquera H, Cid-Jofré V, Landaeta-Díaz L, Petermann- Rocha F, Martorell M, et al. (2023) Plant-Based Nutrition: Exploring Health Benefits for Atherosclerosis, Chronic Diseases, and Metabolic Syndrome- A Comprehensive Review. Nutrients. 15(14): 3244.
- Kim H, Rebholz CM, Hedge S, LaFiura C, Raghavan M, et al. (2021) Plant-based diets, pescatarian diets and COVID-19 severity: a population-based case-control study in six countries. BMJ Nutrition, Prevention & Health. 4: e000272.
- Oboza P, Ogarek N, Olszanecka-Glinianowicz M, Kocelak P (2023) The main causes of death in patients with COVID-19. Eur Rev Med Pharmacol Sci. 27(5): 2165-2172.
- Dessie ZG, Zewotir T (2021) Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 21(1): 855.
- Gupta SC, Prasad S, Aggarwal BB (2016) Anti-inflammatory Nutraceuticals and Chronic Diseases. Advances in Experimental Medicine and Biology. 928.
- Chojnacka K, Skrzypczak D, Izydorczyk G, Mikula K, Szopa D, et al. (2021) Antiviral Properties of Polyphenols from Plants. Foods. 10(10): 2277.
- Alesci A, Aragona M, Cicero N, Lauriano ER (2021) Can nutraceuticals assist treatment and improve covid-19 symptoms? Natural Product Research.
- Alam S, Sarker MMR, Afrin S, Richi FT, Zhao C, et al. (2021) Traditional Herbal Medicines, Bioactive Metabolites, and Plant Products Against COVID-19: Update on Clinical Trials and Mechanism of Actions. Frontiers in Pharmacology. 12: 671498.
- Loscalzo J, Welch G (1995) Nitric Oxide and its role in cardiovascular system. Prog Cardiovasc Dis. 38(2): 87-104.
- Cannon RO (1998) Role of nitric oxide in cardiovascular disease: focus on the endothelium. Clin Chem. 44(8Pt2): 1809-19.
- Torregrossa AC, Aranke M, Bryan NS (2011) Nitric Oxide and geriatrics: Implications in diagnostics and treatment of the elderly. J Geriatr Cardiol. 8(4): 230-242.
- Nikolaidis A, Kramer R, Ostojic S (2022) Nitric Oxide: The Missing Factor in COVID-19 Severity? Med Sci (Basel). 10(1): 3.
- Flaxman S, Whittaker C, Semenova E, Rashid T, Parks RM, et al. (2023) Assessment of COVID-19 as the Underlying Cause of Death Among Children and Young People Aged 0 to 19 Years in the US. 6(1): e2253590.
- Babateen A, Shannon O, Mathers JC, Siervo M (2019) Validity and reliability of test strips for the measurement of salivary nitrite concentration with and without the use of mouthwash in healthy adults. Nitric Oxide. 91: 15-22.
- Kobayashi J, Ohtake K, Uchida H (2015) NO-Rich Diet for Lifestyle-Related Diseases. Nutrients. 7(6): 4911-4937.
- Rajendran S, Shen X, Glawe JD, Kolluru GK, Kevil CG (2019) Nitric Oxide and Hydrogen Sulfide Regulation of Ischemic Vascular Growth and Remodeling. Chapter in Comprehensive Physiology. 9(3): 1213-1247.
- Xiao S, Yuan Z, Huang Y (2023) The Potential Role of Nitric Oxide as a Therapeutic Agent against SARS-CoV-2 Infection. Int J Mol Sci. 24(24): 17162.
- Ritz T, Trueba AF, Vogel PD, Auchus RJ, Rosenfield D (2018) Exhaled nitric oxide and vascular endothelial growth factor as predictors of cold symptoms after stress. Biol Psychol. 132: 116- 124.
- Alqahtani JS, Aldhahir AM, Al Ghamdi SS, AlBahrani S, AlDraiwiesh IA, et al. (2022) Inhaled Nitric Oxide for Clinical Management of COVID-19: A Systematic Review and Meta- Analysis. Int J Environ Public Health. 19(19): 12803.
- Mir JM, Maurya RC (2021) Nitric oxide as a therapeutic option for COVID-19 treatment: a concise perspective. New J Chem. 45(4): 1774.
- Wiertsema SP, van Bergenhenegouwen J, Garssen J, Knippels LMJ (2021) The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients. 13(3): 886.
- Dumas A, Bernard L, Poquet Y, Lugo-Villarino G, Neyrolles O (2018) The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell Microbiol. 20(12): e12966.
- Vijay A, Valdes AM (2022) Role of the gut microbiome in chronic diseases: a narrative review. European Journal of Clinical Nutrition. 76(4): 489-501.
- Vignesh R, Swathirajan CR, Tun ZH, Rameshkumar MR, Solomon SS, et al. (2021) Could Perturbation of Gut Microbiota Possibly Exacerbate the Severity of COVID-19 via Cytokine Storm? Frontiers in Immunology. 11: 607734.
- Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, et al. (2017) Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 15(1): 55-63.
- Dang At, Marsland BJ (2019) Microbes, metabolites, and gut- lung axis. Mucosal Immunol. 12(4): 843-850.
- Ramatillah DL, Gan SH, Pratiwy I, Syed Sulaiman SA, et al. (2022) Impact of cytokine storm on severity of COVID-19 disease in a private hospital in West Jakarta prior to vaccination. PloS One. 17(1): e0262438.
- Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, et al. (2020) Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 55(5): 2000524.
- Groves HT, Higham SL, Moffatt MF, Cox MJ, Tregoning JS (2020) Respiratory Viral Infection Alters the Gut Microbiota by Inducing Inappetence. mBio. 11(1): e03236-19.
- Gou W, Fu Y, Yue L, Chen GD, Cai X, et al. (2020) Gut microbiota may underlie the predisposition of healthy individuals to COVID-19. MedRxiv. 22: 20076091.
- Abadi MSS, Kodashahi R, Aliakbarian M, Beiraghdar F, Arjmand MH (2024) The Association Between the Gut Microbiome and COVID-19 Severity: The Potential Role of TMAO Produced by the Gut Microbiome. Archives of Clinical Infectious Diseases. 18(6): e140346.
- Foster JA, Baker GB, Dursun SM (2021) The Relationship Between the Gut Microbiome- Immune System-Brain Axis and Major Depressive Disorder. Front Neurol. 12: 721126.
- Kamo T, Akazawa H, Suda W, Saga-Kamo A, Shimizu Y, et al. (2017) Dysbiosis and compositional alterations with aging in the gut microbiota of patients with heart failure. PloS One. 12(3): e0174099.
- Leeming ER, Johnson AJ, Spector TD, Le Roy CI (2019) Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients. 11(12): 2862.
- Craddock JC, Neale EP, Peoples GE, Probst YC (2019) Vegetarian-Based Dietary Patterns and their Relation with Inflammatory and Immune Biomarkers: A Systematic Review and Meta-Analysis. Advances in Nutrition. 10(3): 433-451.
- Sidhu SRK, Kok CW, Kunasegaran T, Ramadas A (2023) Effect of Plant-Based Diets on Gut Microbiota: A Systematic Review of Interventional Studies. Nutrients. 15(6): 1510.
- Hibino S, Hayashida K (2022) Modifiable Host Factors for the Prevention and Treatment of COVID-19: Diet and Lifestyle/ Diet and Lifestyle Factors in the Prevention of COVID-19. Nutrients. 14(9): 1876.
- Soltanieh S, Salavatizadeh M, Ghazanfari T, Jahromi SR, Yari Z, et al. (2023) Plant-based diet and COVID-19 severity: results from a cross-sectional study. BMJ Nutrition, Prevention & Health. 6(2): 182-187.
- Rust P, Ekmekcioglu C (2023) The Role of Diet and Specific Nutrients during the COVID-19 Pandemic: What Have We Learned over the Last Three Years? Int J Environ Res Public Health. 20(7): 5400.
- Acosta-Navarro JC, Dias LF, de Gouveia LAG, Ferreira EP, de Oliveira MVPF, et al. (2024) Vegetarian and plant-based diets associated with lower incidence of COVID-19. BMJ Nutrition, Prevention & Health. 2024: e000629.
- Dovignoan J, Ganz P (2004) Role of Endothelial Dysfunction in Atherosclerosis. Circulation. 109(23 Suppl 1): III-27-III-32.
- Akinrinmade AO, Obitulata-Ugwu VO, Obijiofor NB, Victor F, Chive M, et al. (2022) COVID-19 and Acute Coronary Syndrome: A Literature Review. Cureus. 14(9): e29747.
- Margină D, Ungurianu A, Purdel C, Tsoukalas D, Sarandi E, et al. (2020) Chronic Inflammation in the Context of Everyday Life: Dietary Changes as Mitigating Factors. Int J Environ Res Public Health. 17(11): 4135.
- Hojyo S, Uchida M, Tanaka K, Hasebe R, Tanaka Y, et al. (2020) How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 40: 37.
- Moludi J, Qaisar SA, Alizadeh M, Jafari Vayghan H, Naemi M, et al. (2021) The relationship between Dietary Inflammatory Index and disease severity and inflammatory status: a case-control study of COVID-19 patients. British Journal of Nutrition. 2021: 1-9.
- Zhao L, Wirth MD, Petermann-Rocha F, Parra-Soto S, Mathers JC, et al. (2023) Diet-Related Inflammation Is Associated with Worse COVID-19 Outcomes in the UK Biobank Cohort. Nutrients. 15(4): 884.
- Paraiso IL, Revel JS, Stevens JF (2020) Potential use of polyphenols in the battle against COVID-19. Current Opinion in Food Science. 32: 149-155.
- Wang Y, Uffelman C, Hill E, Anderson N, Reed J, et al. (2022) The Effects of Red Meat Intake on Inflammation Biomarkers in Humans: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr Dev Nutr. 6(Suppl 1): 994.
- Hofseth LJ, Hébert JR (2022) Diet and acute and chronic, systemic, low-grade inflammation. Diet, Inflammation, and Health. 2022: 85-111.
- Buck AN, Vincent HK, Newman CB, Batsis JA, Abbate LM, et al. (2023) Evidence-Based Dietary Practices to Improve Osteoarthritis Symptoms: An Umbrella Review. Nutrients. 15(13): 3050.
- Gain C, Song S, Angtuaco T, Satta S, Kelesidis T (2023) The role of oxidative stress in the pathogenesis of infections with coronavirus. Frontiers in Microbiology. 13: 1111930.
- Li M, Zhu D, Yang J, Yan L, Xiong Z, et al. (2021) Clinical treatment experience in severe and critical COVID-19. Mediat Inflamm. 2021: 9924542.
- Alam MS, Czajkowsky DM (2022) SARS-CoV-2 infection and oxidative stress: pathophysiology insight into thrombosis and therapeutic opportunities. Cytokine Growth Factor Rev. 63: 44- 57.
- Gorni D, Finco A (2020) Oxidative stress in elderly population: A prevention screening study. Aging medicine. 3(3): 205-213.
- Al-Zahrani J (2021) SARS-CoV-2 associated COVID-19 in geriatric population: A brief narrative review. Saudi Journal of Biological Sciences. 28(1): 738-743.
- Macho-González A, Garcimartín A, López-Oliva ME, Bastida S, Benedí J, et al. (2020) Can Meat and Meat-Products Induce Oxidative Stress? Antioxidants. 9(7): 638.
- Macho-González A, Bastida S, Garcimartín A, López-Oliva ME, González P, et al. (2021) Functional Meat Products as Oxidative Stress Modulators: A Review. Advances in Nutrition. 12(4): 1514-1539.
- Aleksandrova K, Koelman L, Rodrigues CE (2021) Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biology. 42: 101869.
- Akbari B, Baghaei-Yazdi N, Bahmaie M, Mahdavi Abhari F (2022) The Role of plant-derived natural antioxidants in reduction of oxidative stress. BioFactors. 48(3): 611-633.
- Marchi S, Guilbaud E, Galluzzi L (2023) Mitochondrial control of inflammation. Nature Reviews Immunology. 23: 159-173.
- Ciccarelli G, Conte S, Cimmino G, Maiorano P, Morrione A, et al. (2023) Mitochondrial Dysfunction: The Hidden Player in the Pathogenesis of Atherosclerosis? Int J Mol Sci. 24(2): 1086.
- Shoraka S, Samarasinghe AE, Ghaemi A, Mohebbi SR (2023) Host mitochondria: more than an organelle in SARS-CoV-2 infection. Frontiers in Cellular and Infection Microbiology. 13: 1228275.
- Junqueira C, Crespo Â, Ranjbar S, de Lacerda LB, Lewandrowski M, et al. (2022) FcyR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature. 606(7914): 576-584.
- Khalil M, Shanmugam H, Abdallah H, John Britto JS, Galerati I, et al. (2022) The Potential of the Mediterranean Diet to Improve Mitochondrial Function in Experimental Models of Obesity and Metabolic Syndrome. Nutrients. 14(15): 3112.
- Pollicino F, Veronese N, Dominguez LJ, Barbagallo M (2023) Mediterranean diet and mitochondria: New findings. Experimental Gerontology. 176: 112165.
- Kyriazis ID, Vassi E, Alvanou M, Angelakis C, Skaperda Z, et al. (2022) The impact of diet upon mitochondrial physiology (Review). International Journal of Molecular Medicine. 50(5): 135.
- Ahmad FB, Cisewski JA, Miniño A, Anderson RN (2021) Provisional Mortality Data- United States, 2020. MMWR Morb Mortal Wkly Rep. 70(14): 519-522.
- Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, et al. (2020) Coronavirus Disease 2019 Case Surveillance – United States, January 22-May 30,2020. MMWR Morb Mortal Wkly Rep. 69(24): 759-765.
- Wang Q, Zhan Y, Pedersen NL, Fang F, Hägg S (2018) Telomere length and all-cause mortality: a meta-analysis. Ageing Res Rev. 48: 11-20.
- Sanchez-Vazquez R, Guío-Carrión A, Zapatero-Gaviria A, Martínez P, Blasco MA (2021) Shorter telomere lengths in patients with severe COVID-19 disease. Aging (Albany NY). 13(1): 1-15.
- Mahmoodpoor A, Sanaie S, Eskandari M, Behrouzi N, Taghizadeh M, et al. (2023) Association between leucocyte telomere length and COVID-19 severity. Egypt J Med Hum Genet. 24(1): 37.
- Retuerto M, Lledó A, Fernandez-Varas B, Guerrero-López R, Usategui A, et al. (2022) Shorter telomere length is associated with COVID-19 hospitalization and with persistence of radiographic lung abnormalities. Immunity & Ageing. 19(1): 38.
- Dos Santos GA, Pimenta R, Viana NI, Guimarães VR, Romão P, et al. (2021) Shorter leukocyte telomere length is associated with severity of COVID-19 infection. Biochemistry and Biophysics Reports. 27: 101056.
- Aviv A (2022) The bullwhip effect, T-cell telomeres, and SARS- CoV-2. Lancet. 3(10): E715-E721.
- Sepe S, Rossiello F, Cancila V, Iannelli F, Matti V, et al. (2022) DNA damage response at telomeres boosts the transcription of SARS-CoV-2 receptor ACE2 during aging. EMBO rep. 23(2): e53658.
- Liu S, Nong W, Ji L, Zhuge X, Wei H, et al. (2023) The regulatory feedback of inflammatory signaling and telomere/telomerase complex dysfunction in chronic inflammatory disease. Experimental Gerontology. 174: 112132.
- Crous-Bou M, Molinuevo J-L, Sala-Vila A (2019) Plant-Rich Dietary Patterns, Plant Foods and Nutrients, and Telomere Length.Adv Nutr. 10(Suppl_4): S286-S303.
- D’Angelo S (2023) Diet and Aging: The Role of Polyphenol-Rich Diets in Slow Down the Shortening of Telomeres: A Review. 12(12): 2086.
- Blackburn E, Epel E (2017) The Telomere Effect: A Revolutionary Approach to Living Younger, Healthier, Longer. Grand Central Publishing.
- Kökten T, Hansmannel F, Ndiaye NC, Heba AC, Quilliot D, et al. (2021) Calorie Restriction as a New Treatment of Inflammatory Diseases. Adv Nutr. 12(4): 1558-1570.
- Gnoni M, Beas R, Vásques-Garagatti R (2021) Is there any role of intermittent fasting in the prevention and improving clinical outcomes of COVID-19?: intersection between inflammation, mTOR pathway, autophagy and caloric restriction. VirusDis. 32(4): 625-634.
- Martin Bagos JP, Erick M, Matawaran B (2023) Predictors of Poor Glycemic Control and Increased Glucose Variability Among Admitted Moderate to Critical COVID-19 Patients with Type 2 Diabetes Mellitus: A Single Center Cross-sectional Study. J ASEAN Fed Endocr Soc. 38(2): 57-64.
- Sultan K, Kal S, Issagholian L, Thind BS, Neeki SC, et al. (2023) The Effect of Glycemic Control on Morbidity and Mortality in Critically Ill COVID-19 Patients. Cureus 15(10): e47991.
- Garcia-Carretero R, Vazquez-Gomez O, Lopez-Lomba M, Gil- Prieto R, Gil-de-Miguel A (2023) Insulin Resistance and Metabolic Syndrome as Risk Factors for Hospitalization in Patients with COVID-19: Pilot Study on the Use of Machine Learning. Metab Syndr Relat Disord. 21(8): 443-452.
- Alvarado M, Campos-Campos L, Guerrero-Romero F, Simental- Mendía LE (2024) The Triglycerides and Glucose Index Is an Independent Risk Factor for Acute Respiratory Distress Syndrome in Patients with Covid-19. Metab Syndr Relat Disord. 2024.
- Horne BD, May HT, Muhlestein JB, Le VT, Bair TL, et al. (2022) Association of periodic fasting with lower severity of COVID-19 outcomes in the SARS-CoV-2 prevaccine era: an observational cohort from the INSPIRE registry. BMJ Nutrition, Prevention & Health. 5(2): 145-153.
- U.S. Department of Agriculture, Agriculture Research Service, Scientific Report of the 2020 Dietary Guideline Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services, Published 2020.
- Cheng RZ (2020) Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID_19)? Med Drug Discov. 5: 100028.
- Chen L, Hu C, Hood M, Zhang X, Zhang L, et al. (2020) A novel combination of vitamin C, curcumin and glycyrrhizin acid potentially regulates immune and inflammatory response associated with coronavirus infections: a perspective from system biology analysis. Nutrients. 12(4): 1193.
- Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J (2017) Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. 151(6): 1229-1238.
- Kow S-K, Hasan SS, Ramachandram DS (2023) The effect of vitamin C on the risk of mortality in patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials. Inflammopharmacology. 31(6): 3357-3362.
- Olczak-Pruc M, Swieczkowski D, Ladny JR, Pruc M, Juarez-Vela R, et al. (2022) Vitamin C Supplementation for the Treatment of COVID-19: A Systematic Review and Meta-Analysis. Nutrients. 14(19): 4217.
- Fisher SA, Rahimzadeh M, Brierley C, Gration B, Doree C, et al. (2019) The role of vitamin D in increasing circulating T regulatory cell numbers and modulating T regulatory cell phenotypes in patients with inflammatory disease or in healthy volunteers: A systematic review. PloS One. 14(9): e0222313.
- Gönen MS, Alaylıoğlu M, Durcan E, Özdemir Y, Şahin S, et al. (2021) Rapid and Effective Vitamin D Supplementation May Present Better Clinical Outcomes in COVID-19 (SARS-CoV-2) Patients by Altering Serum INOS1, IL1B, IFNg, Cathelicidin- LL37, and ICAM1. Nutrients. 13: 4047.
- Giannis D, Ziogas IA, Gianni P (2020) Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS- CoV and lessons from the past. J Clin Virol. 127: 104362.
- Mohammad S, Mishra A, Ashraf MZ (2019) Emerging role of Vitamin D and its associated molecules in pathways related to pathogenesis of thrombosis. Biomolecules. 9(11): 649.
- Weir EK, Thenappan T, Bhargava M, Chen Y (2020) Does vitamin D deficiency increase the severity of COVID-19?. Clin Med (Lond). 20(4): e107-e108.
- D'Ecclesiis O, Gavioli C, Martinoli C, Raimondi S, Chiocca S, et al. (2022) Vitamin D and SARS Cov-2 infection, severity and mortality: A systematic review and meta-analysis. PLoS One. 17(7): e0268396.
- Meng J, Li X, Liu W, Xiao Y, Tang H, et al. (2023) The role of vitamin D in the prevention and treatment of SARS-CoV-2 infection: A meta-analysis of randomized controlled trials. Clinical Nutrition. 42(11): 2198-2206.
- Sartini M, Del Puente F, Oliva M, Carbone A, Bobbio N, et al. (2024) Preventive Vitamin D Supplementation and Risk for COVID-19 Infection: A Systematic Review and Meta- Analysis. Nutrients. 16(5): 679.
- Bogan KL, Brenner C (2008) Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr. 28: 115- 130.
- Zheng M, Schultz MB, Sinclair DA (2022) NAD+ in COVID- 19 and viral infections. Trends in Immunology. 43(4): 283-295.
- Bogan-Brown K, Nkrumah-Elie Y, Ishtiaq Y, Redpath P, Shao A (2022) Potential Efficacy of Nutrient Supplements for Treatment of Prevention of COVID-19. Journal of Dietary Supplements. 19(3): 336-365.
- Abdellatif M, Bugger H, Kroemer G, Sedej S (2022) NAD+ and Vascular Dysfunction: From Mechanisms to Therapeutic Opportunities. J Lipid Atheroscler. 11(2): 111-132.
- Ben Abdallah S, Mhalla Y, Trabelsi I, Sekma A, Youssef R, et al. (2023) Twice-Daily Oral Zinc in the treatment of Patients With Coronavirus Disease 2019: A Randomized Doble- Blind Controlled Trial. Clin Infect Dis. 76(2): 185-191.
- Olczak-Pruc M, Szarpak L, Navolokina A, Chmielewski J, Panasiuk L, et al. (2022) The effect of zinc supplementation on the course of COVID-19- A systematic review and meta-analysis. Ann Agric Environ Med. 29(4): 568-574.
- Francis Z, Book G, Litvin C, Kalivas B (2022) The COVID- 19 Pandemic and Zinc-Induced Copper Deficiency: An Important Link. Am J Med. 135(8): e290-e291.
- Rani I, Goyal A, Bhatnagar M, Manhas S, Goel P, et al. (2021) Potential molecular mechanisms of zinc- and copper-mediated antiviral activity on COVID-19. Nutr Res. 92: 109-128.
- Fakhrolmobasheri M, Mazaheri-Tehrani S, Kieliszek M, Zeinalian M, Abbasi M, et al. (2022) COVID-19 and Selenium Deficiency: a Systematic Review. Biol Trace Elem Res. 200(9): 3945-3956.
- Gröber U, Holick MF (2021) The coronavirus disease (COVID-19)- a supportive approach with selected micronutrients. Int J Vitam Nutr Res Int Z Vitam. Ernahrungsforschung J Int Vitaminol Nutr. 92(1): 13-34.
- Guillin OM, Vindry C, Ohlmann T, Chavatte L (2019) Selenium, selenoproteins and viral infection. Nutrients. 11(9): 2101.
- Kieliszek M, Lipinski B (2020) Selenium supplementation in the prevention of coronavirus infection (COVID-19). Med Hypotheses. 143: 109878.
- Dludla PV, Nyambuya TM, Orlando P, Silvestri S, Mxinwa V, et al. (2020) The impact of coenzyme Q10 on metabolic and cardiovascular disease profiles in diabetic patients: A systematic review and meta-analysis of randomized controlled trials. Endocrinol Diabetes Metab. 3(2): e00118.
- Alarcón-Vieco E, Martínez-García I, Sequí-Domínguez I, Rodríguez-Gutiérrez E, Moreno-Herráiz N, et al. (2023) Effect of coenzyme Q10 on cardiac function and survival in heart failure: an overview of systematic reviews and meta-analyses. Food Funct. 14(14): 6302-6311.
- Jorat MV, Tabrizi R, Kolahdooz F, Akbari M, Salami M, et al. (2019) The effects of coenzyme Q10 supplementation on biomarkers of inflammation and oxidative stress in among coronary artery disease: a systematic review and meta-analysis of randomized controlled trials. Inflammopharmacology. 27(2): 233-248.
- Sue-Ling CB, Abel WM, Sue-Ling K (2022) Coenzyme Q10 as adjunctive Therapy for Cardiovascular Disease and Hypertension: A Systematic Review. The Journal of Nutrition. 152(7): 1666-1674.
- Hargreaves IR, Mantle D (2021) COVID-19, Coenzyme Q10, and Selenium. Adv Exp Med Biol. 1327: 161-168.
- Dabbaghi Varnousfaderani S, Musazadeh V, Ghalichi F, Kavyani Z, Razmjouei S, et al. (2023) Alleviating effects of coenzyme Q10 supplements on biomarkers of inflammation and oxidative stress: result from an umbrella meta-analysis. Frontiers in Pharmacology. 14: 1191290.
- Sumbalová Z, Kucharská J, Rausová Z, Palacka P, Kovalčíková E, et al. (2022) Reduced platelet mitochondrial respiration and oxidative phosphorylation in patients with pst COVID-19 syndrome are regenerated after spa rehabilitation and targeted ubiquinol therapy. Front Mol Biosci. 9: 1016352.
- Ma B, Lu J, Kang T, Zhu M, Xiong K, et al. (2022) Astaxanthin supplementation mildly reduced oxidative stress and inflammation biomarkers: a systematic review and meta-analysis of randomized controlled trials. Nutrition Research. 99: 40-50.
- Talukdar J, Bhadra B, Dattaroy T, Nagle V, Dasgupta S (2020) Potential of natural astaxanthin in alleviating the risk of cytokine storm in COVID-19. Biomed Pharmacother. 132: 110886.
- Ahmadi A-R, Ayazi-Nasrabadi R (2021) Astaxanthin protective barrier and its ability to improve the health in patients with COVID-19. Iran J Microbiol. 13(4): 434-441.
- Cheema HA, Sohail A, Fatima A, Shahid A, Shahzil M, et al. (2023) Quercetin for the treatment of COVID-19 patients: A systematic review and meta-analysis. Reviews in Medical Virology. 33(2): e2427.
- Ziaei S, Alimohammadi-Kamalabadi M, Hasani M, Malekahmadi M, Persad E, et al. (2023) The effect of quercetin supplementation on clinical outcomes in COVID-19 patients: A systematic review and meta-analysis. Food Science & Nutrition. 11(12): 7504-7514.
- Mrityunjaya M, Pavithra V, Neelam R, Janhavi P, Halami PM, et al. (2020) Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19. Front Immunol. 11(2337): 570122.
- Smith M, Smith JC (2020) Repurposing therapeutics for COVID-19: supercomputer-based docking to the SARS-CoV-2 viral spike protein and viral spike protein-human ACE2 interface. ChemRxiv.
- Khaerunnisa S, Kurniawan H, Awaluddin R, Suhartati S, Soetjipto S (2020) Potential inhibitor of COVID-19 main protease (Mpro) from several medicinal plant compounds by molecular docking study.
- Derosa G, Maffioli P, D’Angelo A, Di Pierro F (2021) A role for quercetin in coronavirus disease 2019 (COVID-19). Phytother Res. 35(3): 1230-1236.
- Lammi C, Arnoldi A (2021) Food-derived antioxidants and COVID-19. J Food Biochem. 45(1): e13557.
- Hassaniazad M, Eftekhar E, Inchehsablagh BR, Kamali H, Tousi A, et al. (2021) A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin- containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients. Phytotherapy Research. 35(11): 6417-6427.
- Sadeghi M, Dehnavi S, Asadirad A, Xu S, Majeed M, et al. (2023) Curcumin and chemokines: mechanism of action and therapeutic potential in inflammatory diseases. Inflammopharmacology. 31(3): 1069-1093.
- Rattis BAC, Ramos SG, Celles MRN (2021) Curcumin as a potential treatment for COVID-19. Frontiers in Pharmacology. 12: 675287.
- Dourado D, Freire DT, Pereira DT, Amaral-Machado L, N Alencar É, et al. (2021) Will curcumin nanosystems be the next promising antiviral alternatives in COVID-19 treatment trials? Biomedicine & Pharmacotherapy. 139: 111578.
- Manoharan Y, Haridas V, Vasanthakumar KC, Muthu S, Thavoorullah FF, et al. (2020) Curcumin: A wonder drug as a preventive measure for COVID-19 management. Indian Journal of Clinical Biochemistry. 35(3): 373-375.
- Valizadeh H, Abdolmohammadi-Vahid S, Danshina S, Ziya Gencer M, Ammari A, et al. (2020) Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. International Immunopharmacology. 89(pt B): 107088.
- Tahmasebi S, El-Esawi MA, Mahmoud ZH, Timoshin A, Valizadeh H, et al. (2021) Immunomodulatory effects of nanocurcumin on Th17 cell responses in mild and severe COVID- 19 patients. Journal of Cellular Physiology. 236(7): 5325-5338.
- Kow CS, Ramachandram DS, Hasan SS (2022) The effect of curcumin on the risk of mortality in patients with COVID-19: A systematic review and meta-analysis of randomized trials. Phytotherapy Research. 36(9): 3365-3368.
- Shafiee A, Athar MMT, Shahid A, Ghafoor MS, Ayyan M, et al. (2023) Curcumin for the treatment of COVID-19 patients: A meta-analysis of randomized controlled trials. Phytotherapy Research. 37(3): 1167-1175.
- Jong C.J, Sandal P, Schaffer S.W (2021) The Role of Taurine in Mitochondria Health: More Than Just an Antioxidant. Molecules 26(16): 4913.
- van Eijk LE, Offringa AK, Bernal ME, Bourgonje AR, van Goor H, et al. (2022) The Disease-Modifying Role of Taurine and Its Therapeutic Potential in Coronavirus Disease 2019 (COVID- 19). Adv Exp Med Biol. 1370: 3-21.
- Rubio-Casillas A, Gupta RC, Redwan EM, Uversky VN, Badierah R (2022) Early taurine administration as a mean for halting the cytokine storm progression in COVID-19 patients. Explor Med. 3: 234-48.
- Vu TT, Rydland KJ, Achenbach CJ, Van Horn L, Cornelis MC (2021) Dietary Behaviors and Incident COVID-19 in the UK Biobank. Nutrients. 13(6): 2114.
- Bousquet J, Anto JM, Czarlewski W, Haahtela T, Fonseca SC, et al. (2021) Cabbage and fermented vegetables: From death rate heterogeneity in countries to candidates for mitigation strategies of severe COVID-19. Allergy. 76(3): 735-750.
- Gorman S, Weller RB (2020) Investigating the Potential for Ultraviolet Light to Modulate Morbidity and Mortality From COVID-19: A Narrative Review and Update. Frontiers in Cardiovascular Medicine. 7: 616527.
- Cherrie M, Clemens T, Colandrea C, Feng Z, Webb DJ, et al. (2021) Ultraviolet A radiation and COVID-19 deaths in the USA with replication studies in England and Italy. Br J Dermatol. 185(2): 363-370.
- Pelegrino MT, Paganotti A, Seabra AB, Weller RB (2020) Photochemistry of nitric oxide and S-nitrosothiols in human skin. Histochem Cell Biol. 153(6): 431-441.
- Kendrick KN, Kim H, Rebholz CM, Selvin E, Steffen LM, et al. (2023) Plant-Base Diets and Risk of Hospitalization with Respiratory Infection: Results from the Atherosclerosis Risk in Communities (ARIC) Study. Nutrients. 15: 4265.
- Wang J, Sato T, Sakuraba A (2021) Worldwide association of lifestyle-related factors and COVID-19 mortality. Annals of Medicine. 53(1): 1528-1533.
- Storz MA (2021) Lifestyle Adjustments in Long-COVID Management: Potential Benefits of Plant-Based Diets. Current Nutrition Reports. 10(4): 352-363.
- Lange KW, Nakamura Y (2020) Lifestyle factors in the prevention of COVID-19. Global Health Journal. 4(4): 146-152.
- Moreb NA, Albandary A, Jaiswal S, Jaiswal A.K (2021) Fruits and Vegetables in the Management of Underlying Conditions for COVID-19 High-Risk Groups. Foods. 10(2): 389.
- Khaleova H, Barnard ND (2022) The Role of Nutrition in COVID-19: Taking a Lesson from the 1918 H1N1 Pandemic. Am J Lifestyle Med. 17(1): 161-163.
- Kahleova H, Barnard ND (2022) Can a plant-based diet help mitigate COVID-19?. European Journal of Clinical Nutrition. 76(7): 911-912.