Maria-Jose Sanchez-Gonzalez*, Jose Barbarroja-Escudero, Teodora Matas-Dominguez, Josefa Monjo-Paz, Ana Laiseca-Anton, Lucia Gonzalez-Bravo, Melchor Alvarez-Mon
Hospital Universitario Príncipe de Asturias, Departamento de Medicina y Especialidades Médicas, Facultad de Medicina y Ciencias de la Salud, Universidad de Alcalá, Alcalá de Henares, Madrid, Spain.
*Corresponding Author: María José Sánchez González, Hospital Universitario Príncipe de Asturias, Departamento de Medicina y Especialidades Médicas, Facultad de Medicina y Ciencias de la Salud, Universidad de Alcalá, Alcalá de Henares, Madrid, Spain.
Abstract
The placebo effect has been studied and described in several fields. It's used in clinical trials, other areas (pain, cardiovascular development…), and allergological studies. Patients with IgE-mediated allergic diseases are very susceptible to placebo effects. There is also a negative repercussion of the placebo effect, which is the nocebo effect. Some placebo-treated patients will notice subjective symptoms or adverse events because they expect an active medication to have side effects or to worsen their condition.
The target of this study was to clarify the capacity of the placebo effect as a diagnosis or treatment in our Allergy Department patients.
We studied 56 patients who underwent 63 studies in our Allergy Department after giving informed consent for 12 months. They reported different symptoms. In 23 cases, we used a placebo as a diagnostic tool, and in 40 points, we used a placebo to treat the reaction the patient was experiencing.
We consider placebo a diagnostic tool that is very useful to elucidate if the patient is somatising, and given as treatment, we can discriminate between real and unreal allergic reactions.
Keywords: placebo, nocebo, allergy, allergology, drug allergy.
Introduction
Placebo, or placer in Latin, means "I will please." The placebo effect includes the psychological and physiological benefits of receiving treatment for a medical problem, independently of the pharmacological effects the prescribed treatment has.
On the other hand, we have the negative repercussion of the placebo effect; this is the nocebo effect ("I will harm"). This is the onset of troublesome reactions after administering an inert substance. Some placebo-treated patients will notice subjective symptoms or adverse events because they expect an active medication to have side effects or to worsen their condition [1,2].
The placebo effect has been studied and described in several fields. Its use is well-known in clinical trials. However, there are reports of its development in pain control and its application to discern the actual effect in cardiovascular, gastrointestinal, and respiratory diseases, psychiatric and neurological disorders, and surgical and other invasive procedures [3,4,5].
Patients with IgE-mediated allergic diseases are very susceptible to placebo effects. The European Academy of Allergy and Clinical Immunology's Task Force has reported a Position Paper reporting several essential topics related to the placebo effect in allergen immunotherapy (AIT) from different perspectives with concern to regulatory aspects of the placebo effect, the underlying neuroimmunological and psychological mechanisms [6].
The responsiveness of allergic reactions to psychological factors is also shown by high placebo response rates in clinical studies with allergic patients, in which placebos are usually used to test the effectiveness of a drug or a treatment [7,8].
In regular allergological studies such as drug and food challenges, a placebo is frequently used to discriminate among subjective symptoms the patient can experience. Drug provocation placebo control test (DPPCT) is commonly used for this purpose in studying drug allergy.
The purpose of our study is to elucidate the capacity of the placebo effect as a diagnosis or treatment in a series of patients referred to our outpatient Allergy Department.
Materials and Methods
We studied 56 patients who underwent 63 studies in our Allergy Department, after giving informed consent, during a period of 12 months, 5 men and 51 women, with a median age of 45,02 (17-74) years.
These patients had previous reactions to different substances: 14 with a beta-lactam antibiotic, 13 NSAID, 6 quinolones, 2 clarithromycin, 2 antiparasitic metronidazole, 2 cotrimoxazole (sulfamethoxazole and trimethoprim), 2 morphic, 2 polyethylene glycol and 2 rifaximin.
Other substances were (11) dexchlorpheniramine, methylprednisolone methylprednisolone, simvastatin, acenocoumarol, methotrexate, iron, diazepam, ranitidine, omeprazole, AIT and air freshener. They reported symptoms such as dyspnoea, pharyngeal discomfort and occupation, abdominal pain, dizziness, nausea, and objective signs such as pruritus, rash, erythema, exanthema, hives, and inflammation.
Results
We used an oral placebo consisting of empty oral capsules and a saline solution as a subcutaneous placebo. We used it to achieve a diagnosis and to treat the patient's symptoms, depending on the case, including the issues of nocebo effects.
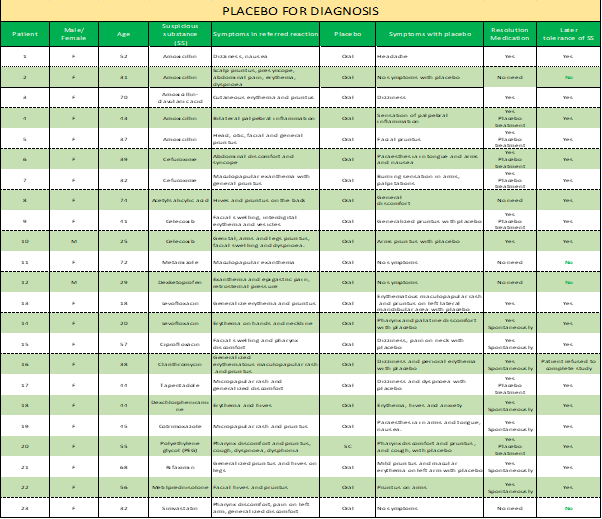
In 23 cases of reactions to drugs, we used a placebo as a diagnostic tool; (Table 1) this is, we gave it to the patients pretending to be the culprit drug of the initial reaction to prove the suspicious drug was not implicated. In 19 cases, we induced a nocebo effect, and the patients experienced different symptoms such as pruritus (7), dizziness (4), nausea (2), paraesthesia (2), pharynx discomfort (2), maculopapular rash (2), erythema (1), general pain (1), urticaria (1), cough (1), palpebral swelling (1), palpitations (1), dyspnoea (1), burning sensation in arms (1), pain (1) and headache (1). In the other 4 cases, the patients did not have any reaction. The referred nocebo effects ceased with no treatment in 12 and placebo treatment in 7 cases. (Table 1)
Table 1: Placebo for diagnosis.
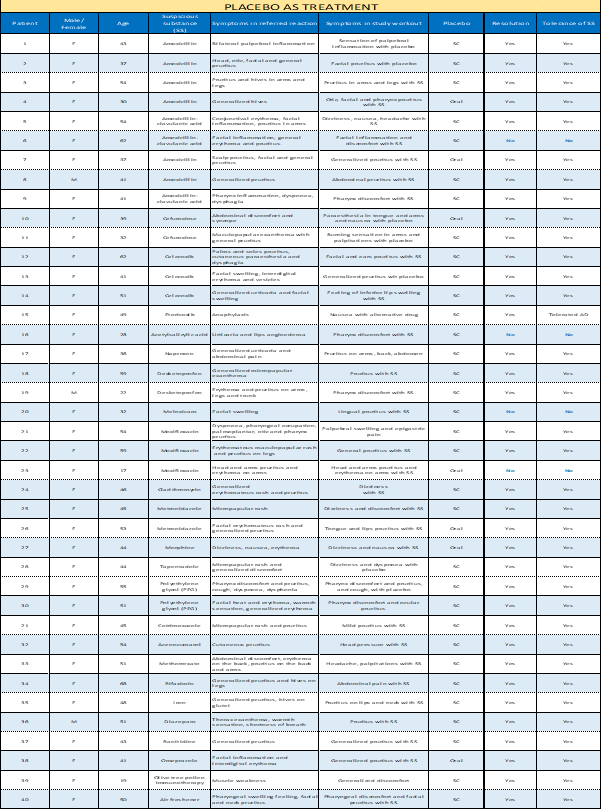
In 40 cases, we used a placebo to treat the reaction the patient was experiencing after giving the suspicious drug in 33 points and a placebo as a diagnosis in 7 cases (Table 2). We shared an oral placebo 6 times and a subcutaneous placebo in 34. We treated different symptoms the patient reported with the substance we had already given: pruritus (22), pharynx discomfort (6), dizziness (5), inflammation (4), nausea (3), palpitations (2), abdominal pain (2), headache (2), head pressure (1), paraesthesia (1), burning sensation (1), general discomfort (1), cough (1), dyspnoea (1) and erythema (1). (Table 2)
The reported symptoms stopped in 36 cases. The four patients (6, 16, 20 and 23 of table 2) who didn't respond were treated with methylprednisolone and dexchlorpheniramine, solving the reaction. We could diagnose these patients as allergic to the given drug.
Table 2: Placebo as treatment.
Discussion
Placebo as a diagnostic tool is well known, and there are many reports of the nocebo effect related to drug allergy studies. The DPPCT is a common practice in the allergological study of drug reactions. Different articles concerning the nocebo effect during challenge or provocation drug tests have been reported [9,10,1]. They studied the factors that lead to a nocebo effect in patients undergoing drug provocation tests. Baybek et al. observed a majority of subjective influences, and many patients had abnormal results on the hospital anxiety depression questionnaire [9].
The nocebo effect occurs frequently in clinical practice, and subjects with high education, non-atopy, and older drug hypersensitivity reaction history seem more likely to experience the nocebo effect during oral drug provocation tests [10,11]. These risk factors should be considered and managed accordingly to successfully complete the drug provocation procedure. Our patients with nocebo effect due to the placebo given as a diagnostic tool had similar characteristics.
Medical treatments have specific effects (pharmacodynamics) and nonspecific effects related to the psychocognitive impacts, patients' perceptions and expectations, and variations of the symptom's severity and psychosomatic [12].
In managing adverse drug reactions through oral challenge tests, the nocebo effect is mandatory to recognize false positive responses [10,11].
Placebo and nocebo effects are linked to the patient's expectations and beliefs about the drug they think they are receiving or taking.
Patient hopes about the treatment and conditionings are cognitive factors involved in the placebo response in different diseases and physiological systems. The psychological mechanisms activate intricate neurobiological phenomena as distinct brain areas activation and peripheral physiology, including the release of endogenous substrates [13,14].
When we give the placebo, pretending it is the drug that produced the patient's reaction, many patients have symptoms. In most of our cases, 19 out of 23 patients experienced symptoms similar to the ones they had taking the drug. This makes us be on the watch for a possible unreal reaction in these patients when giving them the natural medicine afterward. So, if they again report symptoms with the suspected drug as with the placebo, we can anticipate the referred symptoms to stop spontaneously without using placebo treatment. Many patients do not experience symptoms when given the actual drug after first having symptoms with the diagnostic placebo.
Allergic responses of type 1 hypersensitivity, including asthma, are affected by psychological factors such as stress and anxiety and can be controlled by interventions other than conventional drug therapy [15].
In our study, most of the patients experienced symptom relief after administering the placebo as treatment.
In our opinion, trained nurses are crucial in giving the placebo with confidence, particularly when it is used as treatment, speaking and soothing the patient, and providing them a feeling of security. They also watch the patients closely and have a close relationship with them and their companions to know how to approach them.
We should be pretty sure the patient is somatising, and it is not a natural reaction when deciding to give a placebo as treatment. When the patient's symptoms don't disappear with the placebo treatment, we use standard drugs such as antihistamines and corticosteroids, as we did in of the 40 patients, with good results.
Conclusion
We consider placebo treatment helps allergological studies discriminate between real or unreal reactions.
As a diagnosis given before the suspicious substance, a placebo helps identify symptoms from possible somatization.
Allergologists and qualified nurses form a team in these studies, and in Allergy Departments, they have a significant role in giving the placebo and managing it individually for each patient.
Contribution
All coauthors have contributed to the article according to the requirements approved by the International Committee of Medical Journal Editors (ICMJE).
Conflicts of interest: Any author has any conflict of interest.
Funding: The authors declare that no funding was received for the present study.
References
- Gupta A, Thompson D, Whitehouse A, Collier T, Dahlof B, Poulter N et al. (2017) Adverse events associated with unblinded, but not with blinded, statin therapy in. the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid-Lowering. Arm (ASCOT-LLA): a randomised double-blind placebo-controlled. trial and its non- randomised non-blind extension phase. Lancet. 389(10088): 2473-2481.
- Tobert JA, Newman CB (2016) The nocebo effect in the context of statin. intolerance. J Clin Lipidol. 10(4): 739-747.
- Hróbjartsson A, Gøtzsche PC (2010) Placebo interventions for all clinical conditions. Cochrane Database Syst Rev. 2010(1): CD003974.
- Meissner K, Kohls N, Colloca L (2011) Introduction to placebo effects in medicine: mechanisms and clinical implications. Philos Trans R Soc Lond B Biol Sci. 366(1572): 1783-1789.
- Jonas WB, Crawford C, Colloca L, Kaptchuk T, Moseley B, et al. (2015) To what extent are surgery and invasive procedures effective beyond a placebo response? A systematic review with meta-analysis of randomised, sham controlled trials. BMJ Open. 5(12): e009655.
- Pfaar O, Agache I, Bergmann KC, Bindslev-Jensen C, Bousquet J, et al. (2021) Placebo effects in allergen immunotherapy-An EAACI Task Force Position Paper. Allergy. 76(3): 629–647.
- Eccles R (2007) The power of the placebo. Curr Allergy Asthma Rep. 7(2): 100-4.
- Mansfield LE, Hampel F, Haeusler JM, Georges G (2010) Study of levocetirizine in seasonal allergic rhinitis. Curr Med Res Opin. 26(6): 1269-75.
- Bavbek S, Aydın Ö, Sözener ZÇ, Yüksel S (2015) Determinants of nocebo effect during oral drug provocation tests. Allergol Immunopathol (Madr). 43(4): 339-45.
- Liccardi G, Senna G, Russo M, Bonadonna P, Crivellaro M, et al. (2004) Evaluation of the nocebo effect during oral challenge in patients with adverse drug reactions. J Investig Allergol Clin Immunol. 14(2): 104-7.
- Lombardi C, Gargioni S, Canonica GW, Passalacqua G (2008) The nocebo effect during oral challenge in subjects with adverse drug reactions. Eur Ann Allergy Clin Immunol. 40(4): 138-41.
- Schedlowski M, Enck P, Rief W, Bingel U (2015) Neuro-bio-behavioral mechanisms of placebo and Nocebo responses: implications for clinical trials and clinical practice. Pharmacol Rev. 67(3): 697-730.
- Benedetti F, Carlino E, Pollo A (2011) How placebos change the patient's brain. Neuropsychopharmacology. 36(1): 339-354.
- Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, et al. (2011) The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. 3(70): 70ra14.
- Langewitz W, Izakovic J, Wyler J, Schindler C, Kiss A, et al. (2005) Effect of selfhypnosis on hay fever symptoms - a randomised controlled intervention study. Psychother Psychosom. 74(3): 165-72.



