Diané kouao Maxime1,2*, N'dri Kouamé Mathias1,3, Fernique Konan1,4, Dosso Mireille1, Guessennd-Kouadio Nathalie1,4, Tiembre Issiaka5
1Pasteur Institute de Côte d’Ivoires
2Biological Resource Center
3Epidemiology and Clinical Research Unit
4Antibiotics and Natural Substances Unit
5Department of Public Health UFR Medical Science -UFHB
*Corresponding Author: Diané kouao Maxime, Pasteur Institute de Côte d’Ivoire, Biological Resource Center.
Abstract
Context: With the birth of modern laboratory science, especially microbiology and serology, disease prevention and control activities have developed a more rational scientific basis and, therefore, more effective results. These laboratories have played a crucial role in the prevention and fight against diseases such as tuberculosis, HIV/AIDS, avian influenza, the Ebola virus, and, more recently, the COVID-19 pandemic. A National Reference Laboratory (NRL) is the highest level of laboratory in a country responsible for ensuring high-quality standards for laboratory testing. Effective evaluations should provide recommendations to improve a surveillance system's quality, efficiency, and usefulness. This work aims to study the methods of assessing national reference laboratories for the surveillance of infectious diseases in the literature.
Methods and analysis: This are a protocol for a systematic review of published studies on Methods for Assessing National Reference Laboratories in Infectious Disease Surveillance in Africa. Relevant information will be accessible from the following databases: PubMed, Google Scholar, Google, EBSCO Host, and Web of Science. The studies will be listed in two stages: stage 1 will consist of recording the studies descriptively according to purpose and method; Stage 2 will include additional inclusion criteria, quality assessment, and data extraction performed by two reviewers in parallel. Data will be synthesized using relevant systematic research tools.
Conclusion: Evaluating public health surveillance systems ensures that crucial public health issues are monitored effectively and efficiently. However, studies evaluating NRLs in African countries are few. Therefore, with the increase in emerging and re-emerging communicable diseases in the African population, it is essential to study NRLs globally and in Africa.
Systematic review registration: International prospective Register of Systematic Reviews (PROSPERO) number: CRD42023437489
Keywords: Method, infectious disease surveillance, reference laboratory, evaluation, Côte d'Ivoire
1. Introduction
Disease surveillance, involving collecting and interpreting health-related data to identify appropriate actions, developed entirely during the 19th century as public health methods improved.[1] Public health laboratories are among the areas where public health practice has improved in preventing communicable diseases. This was possible thanks to the birth of modern laboratory science, especially microbiology and serology. Subsequently, disease prevention and control activities have developed a more rational scientific basis allowing for more effective results.[2]
The first public health laboratory was established in 1893 at the Institute Pasteur in Paris, where scientists began to study infectious diseases and develop vaccines to prevent them.[3], [4]
Over time, many other communicable disease surveillance laboratories have been established worldwide, including in the United States, Great Britain, and Australia. These laboratories have played a crucial role in the prevention and fight against diseases such as tuberculosis, HIV/AIDS, avian influenza, the Ebola virus, and, more recently, the COVID-19 pandemic.[5],[7]
The National Reference Laboratory (NRL) is the highest laboratory level in a country. He is responsible for ensuring high-quality standards for laboratory testing. It plays a crucial role in validating test methods, training laboratory personnel, standardizing test protocols, and managing the quality of results. National reference laboratories often specialize in specific fields, such as microbiology, virology, parasitology, or toxicology. They are also involved in coordinating the activities of clinical laboratories and public health laboratories to ensure an effective response to health emergencies.[8], [9]
The global public health community has long recognized that communicable disease surveillance NRLs are essential to pandemic preparedness.[10]
In Africa, the national reference laboratory actively participates in the surveillance of infectious diseases. This is possible because it is equipped to perform specialized and precise tests to detect and diagnose infectious diseases. The support of reference laboratories is, therefore, of the utmost importance.[10] The emergence of SARS- CoV-2 in 2019 is the perfect illustration of this.
Since the role of the NRL in the surveillance of infectious diseases is essential, its periodic evaluation is necessary to ensure that they are fit for purpose.[11], [12] The purpose of evaluating all public health surveillance systems is to ensure that critical public health issues are monitored effectively and efficiently.[13] Several approaches are used to evaluate surveillance systems.[14]
This research protocol aims to systematically review the methods for evaluating national reference laboratories for infectious diseases.
2. Objectives
The general objective of the literature review was to obtain an overview of the methods for evaluating national infectious disease surveillance reference laboratories, as well as the determinants and other factors that promote or reduce the evolution of NRLs, both in Côte d'Ivoire and globally.
Specifically, these are:
1. Describe the factors and determinants that promote or reduce the activity of NRLs for communicable disease surveillance
2. Describe the characteristics of NRLs
3. Describe lessons learned from NRL evaluations
3. Identification of the research question
1. How are national infectious disease surveillance reference laboratories assessed? In other words, what methodological approaches are used for the evaluation?
2. What are the characteristics of the national surveillance reference laboratories that have been assessed?
3. What lessons have been learned from the evaluations?
4. Methods
According to Cronin, Ryan, and Coughlan, the systematic review is the identification and the most extensive assessment of the documentation concerning a precise theme in a specific field, covering a well-determined period.[15]
The protocol development for this systematic review will follow the recommendations outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.[16],[18] and the Cochrane Handbook for Intervention Reviews.[19] This protocol has been submitted for registration on an appropriate website, the International Prospective Register of Systematic Reviews (PROSPERO) database.
4.1 Identification of the research question
How are national surveillance reference laboratories assessed? In other words, what methodological approaches are used to evaluate NRLs?
4.2. Eligibility criteria
This review will include studies evaluating national reference laboratories for infectious diseases regardless of the implementation period.
Population: All studies relating to the evaluation of national reference laboratories will be included in the study.
Types of studies: All observational studies reporting on the assessment of national reference laboratories for infectious diseases in humans will be included.
The Laboratories must be clearly defined as an NRL surveillance system and must include virological, bacteriological, and parasitic tests on human beings.
The inclusion of studies will be limited to those published in or after 2006.
Studies relating to national reference laboratories for non-communicable diseases will be excluded. Also, will be excluded:
-studies evaluate innovations not part of the active system, such as testing new algorithms.
-Studies evaluating only laboratory methods, techniques, or tests.
-Studies are piloting a new system or technique for use in a surveillance system.
-Studies that only report the results of surveillance.
-Articles from systematic reviews, narrative reviews, meta-analyses on clinical laboratories or public health, and gray literature.
4.3 Research Strategy and Sources of Information
The research technique will make it possible to use rigorous and explicit criteria to identify, critically evaluate and synthesize the documentation available on the theme of interest to help determine what is known and what is not. This is not in evaluating studies of NRLs against transmissible diseases.
The studies included in this search will be collected based on previously defined search strategies.
We will conduct systematic searches in the following databases: Science Direct, Embase, PubMed/Medline, Africa Wide, and Africa journal online (AJOL). Additionally, we will search electronic academic databases, health agency websites, electronic gray literature, and Internet search engines (Google Scholar).
The search will be based on the keywords and the Medical Subject Heading (MeSH) in Table 1. The search strategy will combine the key concepts and their synonyms: "assessment," "National reference laboratories," "health laboratory public," "national reference centers," "infectious diseases," "transmissible diseases," and "transmissible disease surveillance."
Search strategies will be developed from these keywords indexed in Medical Subject Headings (MeSH) and Descriptors of Health Sciences (DeCS). The Boolean operators will be combined with descriptors related to the interest and the context of the 'PICO' (population, intervention, control, and outcomes in French population, intervention, management, and results)[20] defined for the research to ensure that the product of the study takes into account the theme of the NRL in its evaluation method section. The search will be carried out according to the same procedure after having translated these keywords into English to increase the possibility of collecting the most significant number of relevant documents (Table 1).
Table 1: Keywords and Medical Subject Heading (MeSH)
|
Synonym CISMEF |
Synonym Mesh |
Synonym in English |
|
Laboratory Laboratory.mc [TER_MSH] |
laboratory |
Facilities equipped to carry out investigative procedures. |
|
reference center - national reference centers - national reference center public health laboratory national reference centre.tr[TER_CIS] |
|
national health reference center public health laboratory |
|
infectious pathology - transmissible pathologies - transmissible diseases - infectious diseases - infectious diseases - Infectious diseases Communicable disease.mc[TER_MSH] |
infectious diseases - communicable disease - diseases, infectious - infectious disease - disease, communicable - diseases, communicable - disease, infectious |
communicable diseases An illness caused by an infectious agent or its toxins that occurs through the direct or indirect transmission of the infectious agent or its products from an infected individual or via an animal, vector or the inanimate environment to a susceptible animal or human host. |
|
Africa.mc [TER_MSH] |
|
africa The continent south of EUROPE, east of the ATLANTIC OCEAN and west of the INDIAN OCEAN. |
|
West Africa.mc[TER_MSH] |
|
african, western The geographical area of Africa comprising BENIN; BURKINA FASO; IVORY COAST; GAMBIA; GHANA; GUINEA; GUINEA-BISSAU; LIBERIA; MALI; MAURITANIA; NIGER; NIGERIA; SENEGAL; SIERRA LEONE; and TOGO. |
|
vaccine-preventable diseases |
Disease, Vaccine -Preventable - Diseases, Vaccine -Preventable - Vaccine-Preventable Disease - Vaccine Preventable Diseases - Diseases, Vaccine Preventable - Preventable Disease, Vaccine - Preventable Diseases, Vaccine - Vaccine Preventable Disease |
Vaccine -Preventable Diseases Diseases for which vaccines exist that can confer partial or complete protection. (World Health Organization vaccine-safety-training.org)
|
|
Laboratory monitoring bio-monitoring biological monitoring Biological monitoring.mc[TER_MSH] |
Bio-Monitoring - Bio Monitoring - Biomonitoring - Monitoring, Biological - Biologic Monitoring - Monitoring, Biologic |
Biological Monitoring Definition Monitoring of the level of toxins, chemical pollutants, microbial contaminants or other harmful substances in the bodies of living organisms, by DIAGNOSTIC IMAGING or by analysis of BLOOD, URINE, BREAST MILK or SALIVA, etc. |
In addition to this documentary search based on keywords, an additional documentary search based on the reference lists of documents previously deemed relevant for inclusion in the review will be conducted. This process will allow the identification of a more significant number of papers dealing with the theme.
Listed studies will be saved in the Zotero v5.0.81 document manager (Zotero.org; Virginia). This software will be used to remove duplicates. After excluding duplicates, the title and abstracts of the remaining articles will be assessed for eligibility based on the inclusion and exclusion criteria.
4.4 Study selection process
Retrieved articles will be imported from Zotero Document Manager into Rayyan Smart Systematic Review software (http://rayyan.qcri.org) for initial selection from titles and abstracts. Rayyan is a free web tool allowing reviewers to select titles, abstracts, and full text independently. [21]
The studies will be selected independently by two reviewers, considering the previously established eligibility criteria, and in the event of contradictory decisions, a third reviewer will be consulted. The study selection process will be indicated in the flowchart formulated based on the PRISMA recommendations.[18] The results of the study selection process will be reported in the review and a flowchart according to (Figure 1). [17], [22]
Also, at this stage, reviewers will independently read and assess the full text of potentially eligible studies retrieved. Any conflicting decisions between them on the eligibility of specific studies will be resolved by discussion with a third reviewer.
All studies excluded at each stage will be listed in a table with their reasons for exclusion. The two reviewers will check the final list of included studies.
Supplementary material will be identified by a citation search forward and backward of the included articles. These will be included if they meet the inclusion criteria.
Rayyan's web-based systematic review software will be used to store and manage the review process.
4.5. Ethics and dissemination:
This literature review will not require ethics approval, and the results will be published in peer-reviewed journals and presented at local and international conferences. The findings of this review will inform all stakeholders of current and future guidance on the assessment of NRLs, especially in African countries.
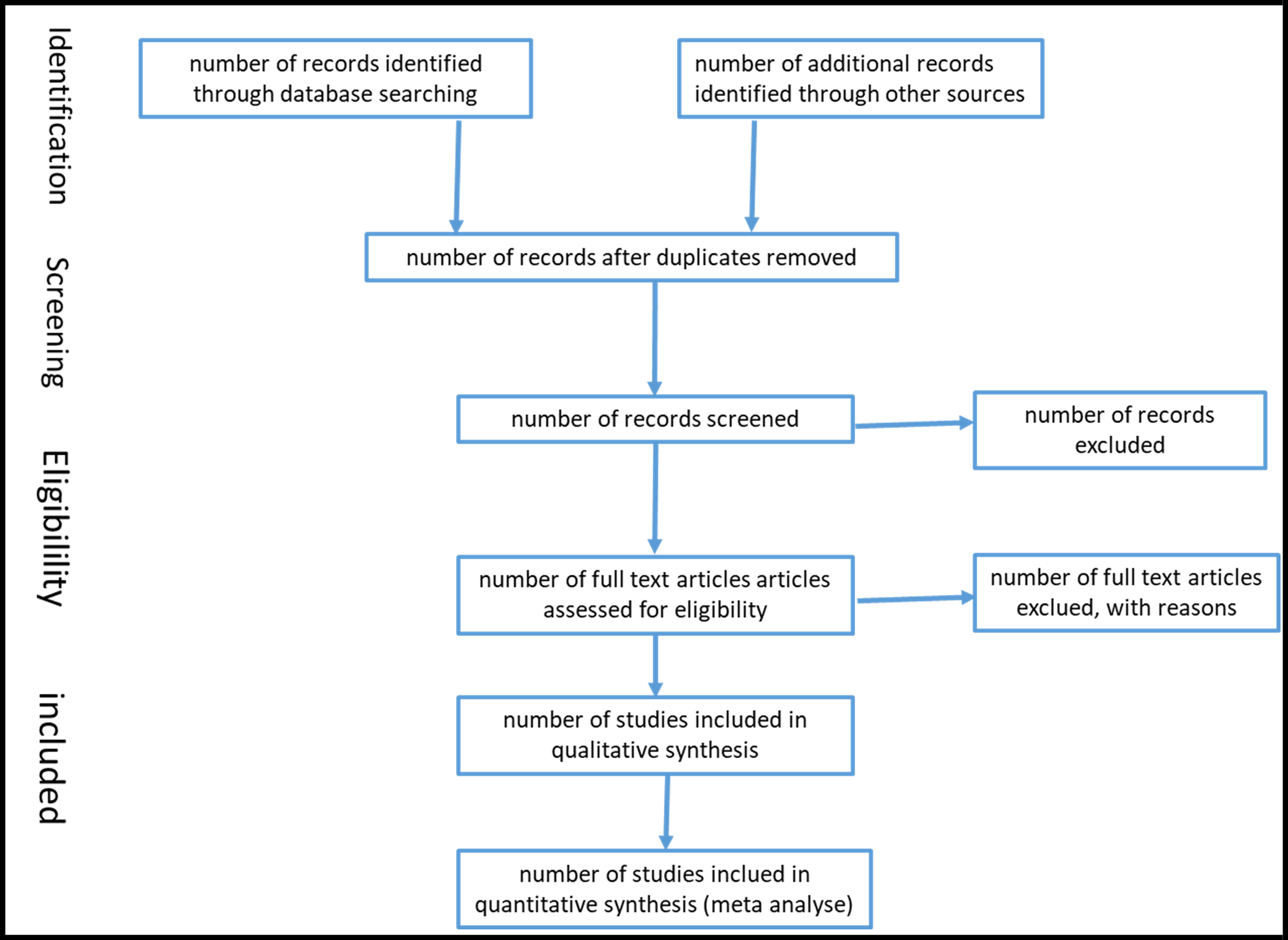
Figure 1: Flow of information through the different phases of a systematic review. / Flowchart of the study selection process.
5. Data management
5.1. Data extraction
The authors will extract data from selected studies. A predefined form will extract data from the selected studies; DKM and NM will carry out this operation. Initially, the state will be tested in several studies. The extraction form will then be modified if necessary to improve data collection. The extracted data will be represented in the form of tables. Authors DKM and NM will then identify themes in the data to build a narrative synthesis.
Data will be extracted according to the categories below by a reviewer and collatedTousing a standardized Excel extraction form. Discrepancies will be resolved by consensus or by arbitration of a third reviewer.
The extracted information will include the following information:
The data to be collected on each study will be Authors; Year of publication; Study title; Journal or publication journal; Population/environment; Country/region; Public health framework; Microorganism species; Total number of cases included; Sample representation; Time limit; Type of study; Purpose of the study.
Data to extract
The NRLs of the monitoring system evaluated:
To. Purpose of monitoring; b. Location and population; vs. Type of monitoring; d. Disease or syndrome under surveillance; e. Case definitions used; f. Data sources (including any data linkage)
Assessment Details
To. Assessment framework used (e.g., CDC)
b. Attributes of the system being assessed (for example, sensitivity or speed)
vs. Methods for evaluating each attribute
1. For example, how were the gold standards calculated for sensitivity and positive predictive value?
2. Were quantitative or qualitative methods used?
d. The main recommendations resulting from the evaluation
e. Document the identified strengths and weaknesses of the assessment
We will use the checklist available in the CDC guidelines as a model for measuring the scope of the evaluation and as a possible measure of the quality of the review. The CDC guidelines suggest six general tasks that should be part of the surveillance system evaluation process. While acknowledging that each system is different and that the methodological approach to undertaking each assignment will vary from system to system, we will establish how the assessment tried to address each of the six tasks.
5.2 Assessment of risk of bias in included studies
The tools for rating recommendations, development, and evaluation will be used to assess the overall quality of the studies.[23] Risk of bias assessments will be performed for each included paper using critical appraisal tools based on the GRADE (Grades of Recommendation, Assessment, Development, and Evaluation) and Cochrane approach.[24] Essential tools of appraisal will be selected based on the study design. The primary reviewer will perform the risk of bias assessment, with sample checks by the second reviewer. Other estimates, such as the assessment of conflict of interest or bias of study authors, will be included in the data extraction table. The quality of evidence will be assessed based on several factors, such as study limitations, indirectness of results, and publication or reporting bias. Proof for each outcome will be rated as high, moderate, low, or very low.[23] This tool is based on ten criteria or items plus an evaluation summary. Items 1-4 assess the study's external validity (domains are selection bias and nonresponse bias), items 5-10 assess internal validity (items 5-9 determine the extent of measurement bias and item 10 sets analysis bias). The summary of the overall risk of bias in the study is a subjective judgment made by the reviewers based on the responses to the first 10 items. This is based on the GRADE (Grades of Recommendation, Assessment, Development, and Evaluation) and Cochrane approach.[24]
5.3 Data summary
The expected data synthesis is a qualitative narrative synthesis. The review will describe how different studies have been applied to evaluate NRLs These results will be achieved by providing information in text and tabular form to summarize and explain the characteristics and outcomes of the included studies.
We will extract themes from the findings, discussion, and conclusions and present them in a narrative synthesis. The narrative synthesis will identify themes for discussion and decisions, especially those related to the methods used in the analysis, the strengths and weaknesses of the study, and the main conclusions.
5.4 Analysis of subgroups or subsets
Depending on the characteristics of the surveillance systems being assessed, we may analyze specific surveillance types or diseases separately—for example, influenza versus SARS-CoV2, hemorrhagic fever, etc.
6. Strength and limit
A limited number of studies relate to lessons learned from NRLs in Africa. The proposed systematic review will focus on observational studies that determine the status of NRLs in Africa, their general missions, and their characteristics. Few similar literature reviews have reported NRL assessments. However, this review may have a small number of studies meeting the entry criteria and result in a limited meta-analysis. Most authors should have reported on the missions and characteristics of the NRLs. Therefore, this systematic review will focus on studies separately reporting the tasks and features of NRLs in Africa.
A linguistic bias may exist since studies published in languages other than English and French will be excluded. This review may improve the capabilities of communicable disease surveillance tools.
7. Conclusion
The purpose of evaluating public health surveillance systems is to ensure that critical public health issues are monitored effectively and efficiently.[25]
Surveillance is divided into three types: (a) passive surveillance, (b) active surveillance, and (c) syndromic surveillance.[26],
[27] Infectious disease surveillance involves 3 sectors simultaneously: the health care delivery system, epidemiology, and the public health laboratory. Each of these sectors contributes to the four essential components of surveillance, namely (i) collection, (ii) analysis, (iii) dissemination, and (iv) response.[28]
The public health laboratory is a significant and vital link in the communicable disease surveillance system contributing to the results of the fight against infectious diseases.
Public health laboratories have a wide range of diagnostic facilities for communicable diseases, and today, many of them are computerized, making their databases easily accessible.[1] Using the information from these laboratories is valuable for monitoring viral infections since many are not reportable diseases, and accurate diagnosis often depends on laboratory identification of the disease. Viral agent.[29]
Therefore, establishing and using these NRLs have been considered to prevent communicable diseases. NRLs are associated with a significant reduction in the risk of infectious diseases and are effective in preventing the early onset of epidemics.
Evaluation work on specific LSPs has been carried out involving different NRLs.
However, an assessment of the characteristics and missions of NRLs in Africa in the fight against communicable diseases needs to be made.
This review will focus on articles reporting NRL evaluations of communicable disease control globally and in Africa.
Statement:
The dossier owner confirms that the information he has provided for this submission is accurate and complete and that he understands that the willful provision of inaccurate information or the omission of data may be construed as scientific misconduct.
Conflict of interest/funding sources/sponsors
DKM is a Ph.D. student at IPCI. The IPCI funds his research, but specific funding for this paper has yet to be used or provided.
Author Contributions
DKM, NKM, and FK sketched and prepared the draft protocol under TI's direction and MD's supervision. All authors contributed to the planning and design of the study. All authors have edited and approved the protocol.
Conceptualization: DKM, NKM, FK; Formal analysis: DKM, NKM, GKAN; Methodology: KM, NM; Writing – original draft revision and Edition: KM, NKM, DM, GKAN, TI
Availability of data and materials: Not applicable
Ethics approval and consent to participate: Not applicable
Consent for publication: Not applicable
Competing interests: The authors have no other competing interests to declare.
References
- Declich S, Carter AO (1994) Public health surveillance : historical origins, methods and evaluation, Bull. World Health Organ. 72(2): 285‑304.
- Hinman AR (1992) The laboratory’s role in prevention and control of infectious diseases, Clinical chemistry. 38(8B Pt 2): 1532-8.
- Guénel A (1999) The creation of the first overseas Pasteur Institute, or the beginning of Albert Calmette’s Pastorian career. Medical History. 43(1) : 1‑25.
- Teicher A (2020) Medical Bacteriology and Medical Genetics, 1880–1940 : A Call for Synthesis, Medical History. 64(3) : 325‑354.
- Boxrud D, Monson T, Stiles T, Besser J (2010) The role, challenges, and support of pulsenet laboratories in detecting foodborne disease outbreaks. Public Health Reports. 125 Suppl 2(Suppl 2) : 57-62.
- Lippi G, Plebani M (2020) The critical role of laboratory medicine during coronavirus disease 2019 (COVID-19) and other viral outbreaks. Clinical Chemistry and Laboratory Medicine (CCLM). 58(7) :1063‑1069.
- M’ikanatha NM, Lynfield R, Julian KG, Van Beneden CA, de Valk H (2013) Infectious disease surveillance: a cornerstone for prevention and control. Infectious disease surveillance. 1‑20.
- Organisation mondiale de la sante, Centre de Contrôle et de Prévention desMaladies (CDC), et l’Institut des N. C. et de Laboratoire (CLSI), Système de gestion de la qualité au laboratoire : manuel. Organisation mondiale de la Santé, 2013.
- World Health Organization (2005) Manuel de sécurité biologique en laboratoire, OMS.
- European Centre for Disease Prevention and Control (2010) Core functions of microbiology reference laboratories for communicable diseases. LU: Publications Office.
- de Lusignan S, Lopez Bernal J, Zambon M, Akinyemi O, Amirthalingam G, et al. (2020) Emergence of a Novel Coronavirus (COVID-19): Protocol for Extending Surveillance Used by the Royal College of General Practitioners Research and Surveillance Centre and Public Health England. JMIR Public Health and Surveillance. 6(2) :18606.
- RCGP, Research surveillance centre.
- Centers for Disease Control and prevention (1988) Guidelines for evaluating surveillance systems. MMWR supplements. vol. 37(5) : 1‑18.
- de Lusignan S, Dorward J, Correa A, Jones N, Akinyemi O, et al. (2020) Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network : a cross-sectional study. The Lancet Infectious Diseases. 20(9) :1034‑1042.
- Cronin P, Ryan F, Coughlan M (2008) Undertaking a literature review: à step-by-step approach. British journal of nursing. 17(1) : 38‑43.
- Institute of Medicine (US) Committee on Standards for Systematic Reviews of Comparative Effectiveness Research (2011) Finding What Works in Health Care : Standards for Systematic Reviews. Washington (DC) : National Academies Press (US).
- Moher D, Shamseer L, Clarke M, Ghersi D Liberati A, et al. (2015) Preferred reporting items for systematic review and meta- analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews. 4(1) :1.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, et al. (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 10(1) :89.
- Tarsilla M (2010) Cochrane handbook for systematic reviews of interventions. Journal of Multidisciplinary Evaluation. 6(14) : 142‑148.
- Aslam S, Emmanuel P (2010) Formulating a researchable question : A critical step for facilitating good clinical research. Indian J Sex Transm Dis AIDS. 31(1) :47‑50.
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan-a web and mobile app for systematic reviews. Syst Rev. 5(1) :210.
- Beller EM, Glasziou PP, Altman DG, Hopewell S, Bastian H, et al. (2013) PRISMA for Abstracts : reporting systematic reviews in journal and conference abstracts. PLoS Med. 10(4): e1001419.
- Mkhize PZ, Phoswa WN, Khaliq OP, Dorsamy V, Moodley J (2021) Aspirin in the prevention of preeclampsia: A protocol for systematic review and meta analysis. Medicine. 100(48) : e27916.
- Granholm A, Alhazzani W, Møller MH (2019) Use of the GRADE approach in systematic reviews and guidelines. British Journal of Anaesthesia. 123(5) :554‑559.
- CDC (2013) Evaluating an NCD-Related Surveillance System.
- ECDC, (2016) Self assessment questionnaire to assess communicable disease control and prevention in EU enlargement countries. Consulté le: 11 mai 2023. [En ligne].
- D. M. Morens, (1998) Principles of public health surveillance », Public Health Surveillance in the Pacific, p. 1.
- Thacker SB, Choi K, Brachman PS (1983) The surveillance of infectious diseases. Jama. 249(9) :1181‑5.
- Evans AS (1984) Surveillance and seroepidemiology. Viral infections of humans: epidemiology and control, p. 43‑64.



