Bandar Ali M.D1*, Ahmad Alshihri M.D, Bassam Alhassan M.D1, Majed Alanazi M.D1, Mohammed Aljabaly M.D1, Khuloud Bukhari M.D2, Nasser Alzerwi M.D3
1Department of Surgery, Prince Sultan Military Medical City, Riyadh, Saudi Arabia
2Ministry of Health, Saudi Arabia
3Department of Surgery, College of Medicine, Majmaah University, Ministry of Education, Al-Majmaah City, Riyadh Region, Saudi Arabia
*Corresponding Author: Bandar Ali M.D, Department of Surgery, Prince Sultan Military Medical City, Riyadh, Saudi Arabia
Abstract
Median arcuate ligament syndrome is a rare disorder where there is compression on the celiac trunk by the median arcuate ligament of the diaphragmatic crura. It affects a very low percentage of the population. Usually, such disease diagnosis is delayed due to difficulty in differentiating signs and symptoms of median arcuate ligament syndrome from other similar presenting diseases such as superior mesenteric artery syndrome, anorexia nervosa, and bulimia. Also, to a lesser extinct, another differential diagnosis can be mistaken for MALS. In this case report, we are discussing a case in that diagnosis was delayed for 5 years before achieving a proper diagnosis of MALS. Plus, all the prior workup that should be achieved and how to increase the alertness for a possible diagnosis by either increasing the alertness for this disease and to keep an open eye for the radiological signs of MALS.
Case report
A 37-year-old male has been suffering from vague postprandial abdominal pain for several years. He visited health care facilities multiple times where he was misdiagnosed and mistreated. He was referred to psychiatry services with an impression of anorexia nervosa or bulimia. Presented to the Emergency Department with severe abdominal pain, nausea, and vomiting. With severe malnutrition. weight loss and acute renal failure. Underwent upper GI endoscopy that was suspicious for gastric volvulus. Underwent gastropexy with mild improvement in his case, where he kept complaining of the same pain episodes. He undergoes a computed tomography of the abdomen which showed signs of narrowing of the celiac trunk caused by median arcuate ligament compression. he underwent release of the ligament with gastric bypass, where he postoperatively improved dramatically.
Conclusion
Median arcuate ligament syndrome is a rare disease, challenging to diagnose and difficult to differentiate from similar presenting diseases. It requires a high suspicion from the treating physician, and an experienced radiologist to diagnose the findings on required radiological investigations. Early diagnosis cannot be stressed enough to avoid possible dire complications of such disease most importantly, gastroparesis, malnutrition, and kidney injury.
Keywords: Median arcuate ligament syndrome (MALS), celiac artery compression syndrome, celiac axis syndrome, celiac trunk compression syndrome, Dunbar syndrome
Introduction
Background
A 37-year-old male, medically and surgically free. Initially presented to the primary health care center in 2016 complaining of vague abdominal pain that started gradually, weaning on and off, associated with food intake, nausea, vomiting of food particles that was on and off. He was treated as a case of nonspecific abdominal pain. the patient did not improve with time, so he kept presenting again with the same complaint, patient was also referred to psychiatry service to rule out psychiatric disease-causing similar presentation. But he was getting worse over time. after two years the patient was referred to gastroenterology service complaining of severe abdominal pain that started after food intake and it got severe enough for him to visit the emergency department. At that time, the pain usually starts after food intake, starts epigastric, and radiates to the whole abdomen. The pain was colicky, non-radiating, the patient usually improves with vomiting, no other specific alleviating or exacerbating factors. The patient noted that he had suffered food fear and weight loss of 40 kg over the last 6 months.
On examination, he was vitally stable, looks in pain, leaning forward.
The abdomen was not distended, soft, and lax with mild epigastric tenderness. Lab works did show signs of dehydration in form of increased Urea and creatinine levels, consistent with acute renal injury secondary to dehydration. radiological investigations in form of abdominal X-rays were within normal limits.
The patient was referred to the gastroenterology department at that time to rule out peptic ulcer diseases, Gastroesophageal reflux disease, gastric outlet obstruction, or malignancy causing such presentation. also, the patient underwent upper gastrointestinal endoscopy at the time. As per the gastroenterologist, there was a high suspicion of gastric volvulus.
General surgery service was contacted and involved at this point. After a discussion of the case, the decision was to take the patient for laparoscopic exploration.
Intraoperatively, no gastric volvulus was clear, the stomach was dilated otherwise nonspecific finding. The decision was to do gastropexy for him.
The patient tolerated the procedure well, stayed in the hospital for 10 days, and was discharged home after he started taking orally with no direct complications noted.
During his outpatient follow-up two weeks after discharge, he was doing well, tolerating orally, no vomiting, he was gaining weight. Also, he was on regular follow-up with the nephrology service for the acute renal injury that showed to progress to chronic renal injury.
The patient presented again to the Emergency department after one-month complaining of similar pain episodes before his surgery.
After insuring his stability, computed tomography “CT” scan was ordered for him that showed narrowing of the celiac access with “hooking appearance “which is typical in cases of median arcuate ligament syndrome, after careful review of this CT and the prior CT that was done a couple of years earlier, it showed a similar finding of narrowing of the celiac trunk.
Diagnosis of median arcuate ligament syndrome was made at this time as shown in Figure1 and 2.
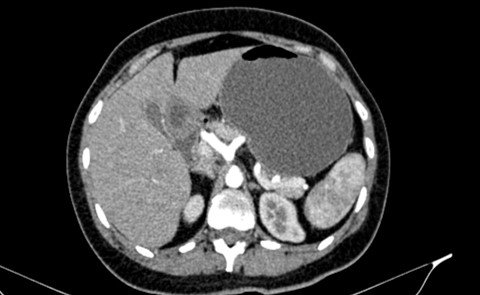
Figure 1: Axial CT abdomen with IV contrast showing the hooking appearance.
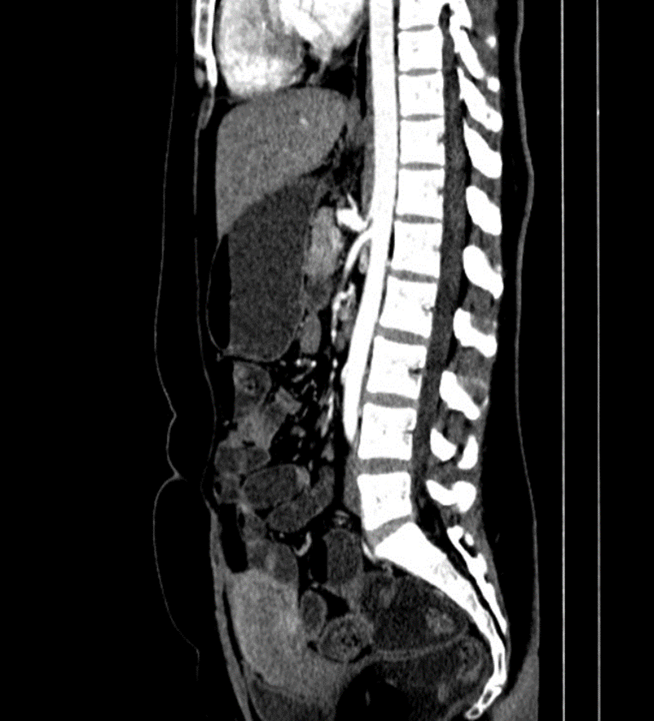
Figure 2: Sagittal (lateral) view of CT abdomen showing acute narrow angle of the celiac trunk.
At this point, a decision was to take the patient for median arcuate ligament release and to do Roux-En-Y gastric bypass for the patient. The rationale for doing the Roux- En-Y bypass at this time is that patient has been suffering from this disease for a long period, with the chronic gastric dilation that he suffered, he developed gastroparesis. The patient underwent the procedure and tolerated it well, postoperatively he stayed in the hospital for 14 days and was discharged home in good health.
The patient presented to the emergency department again after two months complaining of abdominal distention, pain, nausea, vomiting, and inability to pass bowel motion for 1 day. He was diagnosed as adhesive small bowel obstruction with a transitional zone at the jujeno-jujenal anastomotic site, where he went laparoscopic exploration and was found to have a band at the anastomotic site, where it was released. The patient stayed for 1 week and was discharged home in good health.
The patient was being followed as an outpatient, during his visit’s patient showed a dramatic improvement in his overall condition. He started to tolerate orally well, gained weight, his kidney functions improved and normalized. With no signs of Nausea, vomiting, or postprandial abdominal pain.
Discussion
Current reports on median arcuate ligaments are rare. Generally, such patients present with the complexity of symptoms in form of postprandial epigastric pain, nausea, and vomiting. It is typically a diagnosis of exclusion [1].
It was first noted in 1917 by noticing that in some postmortem dissection, it was found that the celiac trunk was compressed by the diaphragmatic crura [1].
Prevalence is not well known in the general population until the time being [2].
Pathophysiology of this disease is not well known, but it is hypothesized that it is due to one of either: decreased blood flow through celiac trunk due to compression, leading to foregut ischemia that leads to this periodic pain, but the issue is that it is a chronic disorder and development of collateral blood vessels usually inhibit progression to ischemia and pain [2]. Another hypothesis is that two simultaneous disease processes in the forms of compression on the celiac trunk and overstimulation of the celiac ganglion led to irritation and sympathetic pain fibers, leading to splanchnic vasoconstriction and chronic ischemic changes [2].
Because symptoms of MALS usually are like other abdominal diseases, it remains an area of controversy to diagnose. Usually, it is a diagnosis of exclusion.
Such patients usually undergo extensive work up in form of CT, MRI, UPPER ENDOSCOPY, US, HIDA SCAN all to reach a diagnosis of this devastating complaint [3]. Once suspicion of MALS is in mind, The duplex US of the abdomen can create an anatomical and physiological basis for a diagnosis of MALS [1].
It is important to mention the celiac trunk track superiorly after taking off from the aorta, leading to the understanding that in such disease where the is stenosis, it is expected to have a higher velocity, also the deflection angle is expected to be 50. US duplex has a sensitivity of 83 % and specificity of 100 %, creating a good rationale for it to be used as a first-line non-invasive diagnosis tool in high suspicion cases of MALS [1].
Furthermore, CT and MRI with contrast can also identify such disease, with a finding of narrowing of the celiac trunk that appears as “hooking appearance “which helps in differentiating MALS from another similar presenting disease. The gold standard for diagnosing MALS is mesenteric angiography with breathing maneuvers, which has high specificity and sensitivity to show narrowing of the celiac trunk and the celiac angle [4].
Adjunct to these prior mentioned investigations, gastric emptying studies, and tonometry help to identify delayed gastric emptying, gastroparesis, or chronic gastric ischemia caused by MALS [5].
Another procedure that was thought to be of significance to identify which patients with MALS would benefit most from surgical intervention, is the percutaneous ganglion block. The idea of this rose after understanding that symptoms of MALS result from chronic compression of the celiac trunk which serves to relay the pain through celiac ganglion, it showed promising results in the mayo clinic experience. 75 % of involved patients had stated that there was pain improvement with this procedure. Which is the case showed excellent outcomes after surgical intervention [1].
In this algorithm (Figure 3), there are well-established diagnosing and treatment guides.
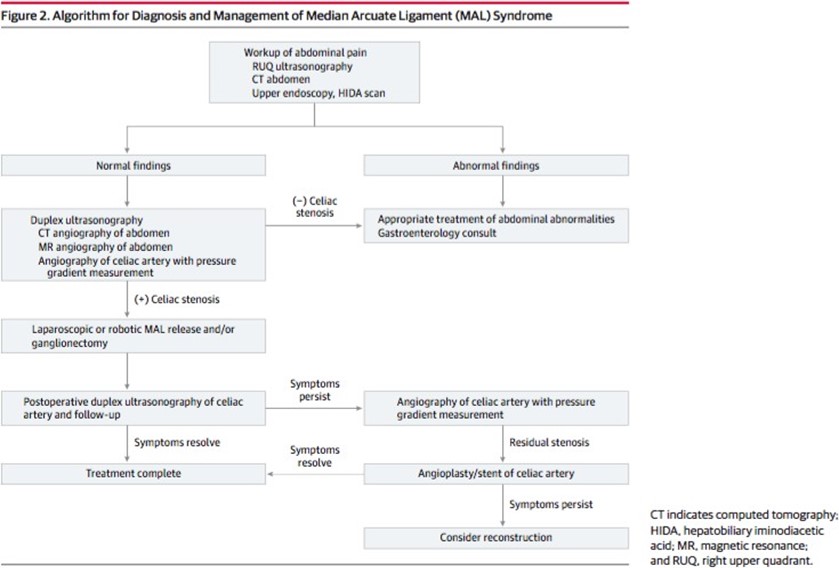
Figure 3: Algorithm for diagnosis and management of MALS.
regarding the surgical approach, the basis of it is to release the compression of the celiac trunk and restore the blood flow through the celiac trunk to normal velocity. it is also recommended to either do neurolysis or to excise the affected celiac plexus to address the issue of chronic pain [1].
This can be done either by open approach or laparoscopic approach. Also, a novel approach via robotic-assisted surgery is an emerging technology [2].
Either if it was open or laparoscopic, the idea is to reach the celiac trunk after careful dissection and then to incise the diaphragmatic crura 5 cm superiorly, exposing a 5 cm segment of the aorta, then to ensure the release of the ligament and restoration of the blood flow, intraoperative ultrasound should be used to assess celiac trunk blood flow velocity and that it reached normal numbers [2].
Careful inspection is to be done intraoperatively to check for any chronic stenosis in a segment of the celiac trunk. In such cases, release alone will not be enough to improve the outcome and vascular reconstruction is needed.
Laparoscopic MAL release is standard surgical intervention currently, but it has the disadvantage of the inability to control hemorrhage from the celiac trunk, the inability to undergo vascular repair or bypass procedures, and the possibility of incomplete release of MAL [1].
Endovascular interventions alone are not considered to be of benefit to such patients, since the pathology is external pressure over the celiac trunk, but it has been used as adjuvant therapy in some cases. Also, it can be of benefit to inpatients that underwent MAL release with relapse of their symptoms, where balloon-expandable stents were used to dilate the diameter of the celiac trunk [4].
Conclusion
MALS is a rare disease that can be challenging to diagnose and treat. High suspicion is of immense importance to identify such cases and avoid possible complications.
Surgical intervention is the mainstay of the management of this disease. Multiple surgical approaches are acceptable, laparoscopic MAL release being the gold standard, but open repair might be immanent depending on the case.
Conflict of Interest: The authors declare no conflict of interest
Funding: Self-funded by the authors
References
- Kim EN, Lamb K, Relles D, Moudgill N, DiMuzio PJ, et al. (2016) Median Arcuate Ligament Syndrome—Review of This Rare Disease. JAMA Surgery. 151(5): 471-7.
- Nguyen T, Neale M, Lane R, Schiavone V, Samra JS, et al. (2012) Laparoscopic management of the median arcuate ligament syndrome. ANZ Journal of Surgery. 82(4): 265-8.
- Sun Z, Zhang D, Xu G, Zhang N (2019) Laparoscopic treatment of median arcuate ligament syndrome. Intractable & Rare Diseases Research. 8(2): 108-112.
- Dalag L, Patel M, Kang L, Lorenz J (2017) Comparing CT angiography with Doppler ultrasound evaluation in the diagnosis of median arcuate ligament syndrome. Journal of Vascular and Interventional Radiology. 28(2): S195.
- Randhawa S, Patil A M, Kelkar AB, Kelkar AB (2015) Median arcuate ligament syndrome: A diagnosis on CT abdominal angiography in cases of non-specific abdominal pain. Medical Journal of Dr. D.Y. Patil University. 8(5): 645-648.



