Ahmet Kor1*, Mert Ali Kaya2, Aynur Albayrak3, Kevser Gök4, Yüksel Maraş5
1Ankara Yıldırım Beyazıt University, Ankara City Hospital, Department of Rheumatology. https://orcid.org/0000-0002-5794-6951
2Ankara City Hospital, Department of Internal Medicine. https://orcid.org/0000-0003-0910-065X
3Ankara City Hospital, Department of Pathology. https://orcid.org/0000-0001-9665-9368
4University of Health Sciences, Ankara City Hospital, Department of Rheumatology. https://orcid.org/0000-0003-1610-3867
5University of Health Sciences, Ankara City Hospital, Department of Rheumatology. https://orcid.org/0000-0001-9319-0955
*Corresponding Author: Ahmet Kor, Ankara Yıldırım Beyazıt University, Ankara City Hospital, Department of Rheumatology. https://orcid.org/0000-0002-5794-6951.
Abstract
Background and aims: Rheumatic musculoskeletal symptoms may be associated with lymphoproliferative malignancies. Rheumatic symptoms may mask the original neoplastic disease, and therefore the differential diagnosis of most of the rheumatic syndromes associated with Malig- nancy from primary rheumatic disorders can sometimes be challenging. We aim to draw attention to some problems that may be experienced in diagnosis and treatment since lymphoproliferative conditions may cause symptoms similar to various rheumatological diseases. We also em- phasized the need for histological biopsy to rule out coexisting neoplasia in patients with unexpected arthritis or atypical rheumatic symptoms until more accurate data.
Case presentations: In this article, we describe two male patients, aged 21 and 26, diagnosed with axial spondylitis and started treatment in other health centers with complaints of inflammatory low back pain. B-cell neoplasm mimicking axial spondylitis was detected in these two patients who applied to Ankara City Hospital Rheumatology Department for an axial spondylitis treatment plan.
Conclusion: As in this case report, it may be challenging to diagnose Malignancy at the onset of symptoms due to the low number of signs of Malignancy and its similarity with rheumatological diseases. Atypical features such as asymmetric pauciarticular pattern and absence of morning stiffness should alert clinicians to perform a comprehensive diagnostic study, including a synovial biopsy or magnetic resonance examination of the involved joints.
Keywords: Sacroiliitis, neoplasm, lymphoma
Introduction
Relationships between Malignancy and rheumatic symptoms have been well defined before. Malignant neoplasms can cause rheumato-logical findings by direct tumor invasion into bones and joints and paraneoplastic syndromes or by various mechanisms as a side effect of cytokine therapy. Musculoskeletal involvement in patients with lymphoproliferative malignancies has been previously reported in many cases. In lymphoid malignancies, when rheumatic symptoms are the first sign of the disease, it may be difficult to diagnose because there may be no signs of Malignancy at the first application. [1,2]
In this study, we describe two malignant lymphoproliferative cases that initially presented with rheumatic musculoskeletal symptoms, which we describe as interesting cases due to the difficulty in distin-guishing between the findings of rheumatic diseases and lymphopro-liferative diseases. In addition, we aimed to emphasize the importance of Malignancy mimicking rheumatic conditions and to review the literature.
Case Presentations
Case report 1
A 21-year-old male patient was diagnosed with axial spondyloarthri-tis according to ASAS diagnostic criteria upon the detection of bilat-eral sacroiliitis in sacroiliac magnetic resonance imaging (MRI) (Figure 1) at a physical therapy clinic, where he went to a physical therapy clinic with a complaint of low back pain with an inflamma- tory character for more than six weeks. Sulfasalazine 2x1000 mg and indomethacin 3x25 mg were started. In laboratory tests in this period white blood cell (WBC): 12000x10^9/L, neutrophil: 10000 x10^9/L, hemoglobin (Hmg): 9.1 g/dL, erythrocyte sedimentation rate (ESR): 48 mm/hour, C-reactive protein (CRP) : 52 mg/L detected. Organo- megaly and lymphadenopathy were not detected in abdominal ultra- sonography. The patient, who developed dizziness with sulfasalazine two months after the start of treatment, was admitted to our rheuma- tology clinic.

Figure 1: Pathological signal increase in bilateral sacral wing and iliac bone medulla, narrowing in both sacroiliac joint spaces, and findings suggestive of bilateral sacroiliitis in the sacroiliac MRI COR-STIR of the first case.
On physical examination, sacroiliac compression, FABER, and FA- DIR tests were found to be positive; there were pale skin, hepatomeg-aly, and lymphadenopathies in the cervical, axillary and inguinal re-gions. There was no history of heel pain, uveitis, oral and genital aph-thae, psoriatic skin findings, pulse and blood pressure difference be- tween extremities, the murmur of vascular structures by listening, chronic diarrhea, and urinary tract infection. There were no features in his family history.
Laboratory evaluation of the patient: WBC: 28000 x10 ^ 9/L, neutro-phil: 23000 x10 ^ 9/L, thrombocyte (Plt): 48000 x10 ^ 9/L, Hmg: 8.6 g/dL, ESR: 110 mm/hour, CRP: 130 g/L detected. HLA-B27 was neg-ative. Reactive leukocytosis was present in the peripheral blood smear; there were no atypical cells. When the patient had a fever up to 39 °C in vital follow-up quantifier, serial blood cultures, brucella tube, slide agglutination, syphilis tests, TORCH panel, parvovirus, and EBV panel were found to be negative. No vegetation was detected in transesophageal echocardiography.
Whole-body Positron Emission Tomography (PET-CT) was per- formed to investigate the etiology of fever of unknown origin and leu-kocytosis. On PET CT, pathologically increased F-18 FDG uptake in cervical, supraclavicular, axillary, mediastinal, inguinal, and intra-ab- dominal lymph nodes and extensive hypermetabolism increase in ver- tebral, costal and pelvic bone medullary structures were observed (Figure 2). In the pathology of bone marrow biopsy performed for histopathological tissue sampling, hypercellularity was observed in all bone marrow series, and no atypical blast cells were detected. In the histological tissue sample of supraclavicular tru-cut lymph node biopsy, PAX5, CD30, and CD15 positive giant cells that were not ex- pressed by LCA, CD20, and CD3 were detected (Figure 3). This bi- opsy result was evaluated as compatible with Hodgin's lymphoma.
Figure 2: Involvement areas of lymphoma in the iliac bone and sacroiliac joint in PET-CT of the first case taken two months after the diagnosis of axial spondyloarthritis.
Fıgure-3: a) Reed-Sternberg cells are anti-PAX5 positive (anti-PAX5, X200). b) Reed-Sternberg cells are anti-CD45 negative (anti-CD45, X200). c) Reed-Sternberg cells are anti-CD30 positive (anti-CD30, X200). d) Tumor composed of eosinophil leukocytes and Reed-Sternberg cells (HE, X200).
Case report 2
A 26-year-old male patient went to a thermal spa to treat his back and hip pain for ten months. The patient whose pain became more severe in the thermal spa was applied to a primary health care institution. In the laboratory tests examined here CRP: 300 g/L, ESR: 60 mm/hour WBC: 10000x10^9/L, neutrophil: 7000 x10^9/L, Hmg: 12.5 g/dL were detected. In contrast sacroiliac, MRI was taken to the patient, scattered areas of contrast material in both iliac bones and sacrum were seen (Figure 4), axial spondyloarthritis was diagnosed, and non-steroidal anti-inflammatory drug treatment was considered was initi-ated. The patient was directed to the rheumatology clinic for subse-quent follow-up.
The patient who was evaluated in our clinic had increased waist and hip pain, especially at night, there was no morning stiffness, and the pain continued all day and increased with movement. On physical ex- amination, FABER, FADIR, and sacroiliac compression tests were positive, and the right shoulder joint was painful with active and pas-sive motion. Lymphadenopathy was palpable in the cervical and ax-illary region. Laboratory studies showed ESR: 78 mm/hr, LDH: 1328 U/L, WBC: 10500x10^9/L, neutrophil: 7100 x10^9/L, Hmg: 12 g/dL, and PLT: 450000x10^9/L. HLA-b27 genetic mutation was found neg-ative.
Figure 4: At the diagnosis of axial spondyloarthritis, hypointense lesions in T1A (left picture) with scattered localization in both iliac bones and sacrum, hypointense in fat-suppressed T2A (right image), and peripheral hyperintense irregular shaped lesions in sacroiliac MR.
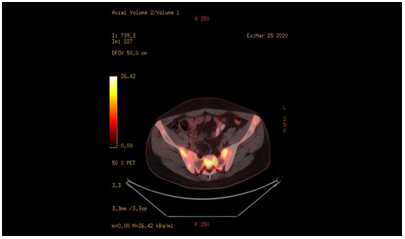
Very high CRP, LDH values, and the presence of lymphadenopathy were evaluated as atypical findings for axial spondyloarthritis, and the patient underwent whole-body PET-CT. Submandibular lymphade-nopathies with moderate F-18 FDG uptake and pathologically in- creased FDG uptake in the whole skeletal bone system were detected in PET-CT (Figure 5). Bone marrow biopsy was performed for his- topathological tissue diagnosis was reported as high-grade B-cell lymphoma.
Fıgure-5: Diffuse involvement areas of lymphoma in the iliac bone and sacroiliac joint seen in PET-CT taken five months after the diagnosis of axial spondyloarthritis.
Discussion
The types of Malignancy with common rheumatic symptoms are ne- oplastic diseases of the lungs, breasts, ovaries, and lymph nodes, which are generally seen in the elderly population. Malignancy-re- lated musculoskeletal findings may be similar to rheumatic symptoms in areas such as joints, fascia, muscles, vascular structures, or bones. Rheumatological results are associated with solid malignancies at ap- proximately 65 %, but they can also be seen in hematological malig- nancies. Generally, a diagnosis of Malignancy is made within two years after rheumatic symptom presentation. [3] The first of our cases were diagnosed with lymphoma approximately four months after the onset of rheumatological symptoms and the second ten months after the onset of rheumatologic symptoms.
A wide variety of osteoarticular, muscular, and skeletal symptoms can be seen among rheumatic symptoms in patients with lymphoprolifer- ative malignancies. In these malignancies, patients usually complain of rheumatic symptoms in the late stages of the disease. However, one should be aware that rheumatic symptoms may be the first presenta- tion of lymphoproliferative Malignancy. In general, a wide variety of lymphoproliferative conditions may be associated with rheumatic symptoms. [4] However, lymphadenopathy and hepatosplenomegaly are often absent at the first presentation. Musculoskeletal complaints in lymphoma patients are not uncommon, but clinical features of pe- ripheral joint involvement are scarce. Some patients may present with monoarticular or polyarticular arthritis. [5] It has been reported that 7 %-25 % of musculoskeletal lesions develop during NHL.[6] Both of our cases applied to the primary health care institution with the com- plaint of inflammatory low back pain. Organomegaly and lymphade- nopathy were not detected in both of our cases at the time of admis- sion. Treatment was initiated considering axial spondyloarthritis in patients.
In the pathogenetic mechanism of bone and joint involvement seen in malignant diseases, direct infiltration of malignant cells into bone or synovial tissue, synovial reaction due to periosteal or joint capsule infiltration, or development of immune complex synovitis has been demonstrated. Periarticular metastases are not uncommon and may present as acute arthritis. This is observed with the development of bone involvement resulting from neoplastic metastatic invasion of the joint or non-neoplastic reactions without joint degeneration. Direct synovial involvement by malignant lymphoid cells is less common and has not been demonstrated in all cases undergoing synovial bi- opsy.[7] In our first case, although diffuse hypermetabolism was ob- served in vertebral, costal, and pelvis bone medullary structures in PET CT, no atypical blast cells were detected in bone marrow biopsy. In our second case, pathologically increased FDG uptake was de- tected in the whole skeletal bone system on PET CT, and bone mar-row biopsy pathology performed for histopathological tissue diagno- sis was reported as bone marrow involvement of high-grade B-cell lymphoma.
There are 8 cases of sacroiliitis associated with hematological Malig- nancy that we can detect in the literature. Two young female cases were diagnosed with Hodgkin's disease approximately 5 and 12 months after reported sacroiliitis. [2] In contrast to this, in one case, rapid-progressive sacroiliitis and enthesopathy developed after diag- nosing well-differentiated lymphocytic lymphoma. The first clinical presentations of our cases were similar to sacroiliitis. In our cases, the period between the clinic of sacroiliitis and the detection of sacro- iliitis-like findings on MRI and the final diagnosis of Hodgkin's lym- phoma was 6 and 10 months. [8] Another case is a patient with acute lymphoblastic leukemia in which a complete blood count shows pan- cytopenia. [9] The first of our cases had neutrophil-predominant leu- kocytosis that did not respond to broad-spectrum antibiotic therapy; in our second case, LDH levels were above 1000 U/L. Bureau et al. presented a case with sacroiliitis diagnosed with Hodgkin's disease by MR-guided biopsy of the sacroiliac joint. [10] Similar to our cases, another case was diagnosed with Hodgkin lymphoma after diagnos- ing sacroiliitis. The ESR level remained above 100 mm/hour despite using non-steroidal anti-inflammatory drugs and sulfasalazine. [11] In another case, a 28-year-old female patient was diagnosed with sa- croiliitis and myelodysplastic syndrome and with acute myeloid leu- kemia one month later. [12] In the last case, paraneoplastic sacroiliitis developed due to acute myeloid leukemia in a young man who was followed up with peripheral arthritis. While this case was followed for one year with arthritis in the knee and wrist, the patient developed concurrent sacroiliitis and acute myeloid leukemia. [13]
Conclusion
It may be challenging to diagnose Malignancy at the onset of symp- toms due to the typical findings of Malignancy and its similarity with rheumatological diseases, similar to the cases presented here. Atypi- cal features such as asymmetric pauciarticular pattern and absence of morning stiffness should alert clinicians to perform a comprehensive diagnostic study, including a synovial biopsy or magnetic resonance examination of the involved joints. It should be known that atypical findings for rheumatic diseases such as severe joint pain dispropor- tionate to physical examination findings, absence of morning joint stiffness, poor response to conventional antirheumatic therapy, signif- icant osteopenia, or lytic lesions in the early period are distinctive for paraneoplastic arthritis. It is essential to make the differential diagno- sis of early non-radiographic stage sacroiliitis detected in MRI and infection, fracture, or neoplastic infiltration, which can produce symptoms and images similar to rheumatologic sacroiliitis. Findings such as non-anatomical inflammation and the presence of lytic bone lesions in the pelvic bone or spine should be interpreted with caution. On the other hand, FDG-PET imaging can significantly support the diagnosis of Malignancy and more accurate staging. Significantly in- creased levels of LDH should be a warning sign to distinguish hema- tological malignancies from other cases.
Ethical Standards Compliance: Consent was obtained from the patients by ethical standards.
Conflict of Interest
The authors declare that they have no known competing financial in- terests or personal relationships that could have appeared to influence the work reported in this paper.
Author Contributions
AK cared for the patients, wrote the first manuscript draft, and was responsible for the overall supervision of the study. YM cared for the patients and was responsible for the general management of the study. MAK cared for the patients. KG cared for the patients. AA was re-sponsible for the pathological evaluation of tissue samples and image acquisition from tissue preparations.
Funding Support: The author(s) received no financial support for this article's research, authorship, and publication.
References
- Sinigaglia R, Gigante C, Bisinella G, Varotto S, Zanesco L, et al. (2008) Musculoskeletal manifestations in pediatric acute leukemia. J Pediatr Orthop. 28(1): 20-28.
- Saviola G, Abdi-Ali L, Trentanni C, Notarangelo LD, Desiati F, et al. (2003) Sacroiliitis as a manifestation of Hodgkin's disease in young females. Clin Exp Rheumatol. 21(2): 270-270.
- Wen J, Ouyang H, Yang R, Bo L, Zhang Y, et al. (2018) Malignancy dominated with rheumatic manifestations: A retrospective single-center analysis. Sci Rep. 8: 1786.
- Ehrenfeld M, Gur H, Shoenfeld Y (1999) Rheumatologic features of hematologic disorders. Curr Opin Rheumatol. 11(1): 62-67.
- Birlik M, Akar S, Onen F, Ozcan MA, Bacakoglu A, et al. (2004) Articular, B-cell, non-Hodgkin's lymphoma is mimicking rheumatoid arthritis: synovial involvement in a small hand joint. Rheumatol Int. 24(3): 169-172.
- Braunstein EM, White SJ (1980) Non-Hodgkin lymphoma of bone. Radiology. 135(1): 59-63.
- Rudwaleit M, Elias F, Humaljoki T, Neure L, Knauf W, et al. (1998) Overexpanded B cell clone mediating leukemic arthritis by abundant secretion of interleukin‐1β: A case report. Arthritis & Rheumatism: Arthritis Rheum. 41(9): 1695-1701.
- Cohen MR, Carrera GE, Lundberg J (1993) Rapidly progressive sacroiliitis in a patient with lymphocytic lymphoma. Ann Rheum Dis. 52(3): 239-240.
- Moghadam A, Talebi-Taher M, Dehghan A (2010) Sacroiliitis as an initial presentation of acute lymphoblastic leukemia. Acta Clin Belg. 65(3): 197-199.
- Bereau M, Prati C, Wendling D (2011) Sacroiliac edema by MRI does not always indicate spondylarthritis. Joint Bone Spine. 78(6): 646-646.
- Yalbuzdag SA, Erol AM, Celik C, Sarifakioglu AB, Karabulut B (2014) Hodgkin's lymphoma: case report of mimicking sacroiliitis/Sakroiliiti taklit eden Hodgkin lenfoma: olgu sunumu. Turkish Journal of Physical Medicine and Rehabilitation. 60(4): 353-356.
- Hoshino T, Matsushima T, Saitoh Y, Yamane A, Takizawa M, et al. (2006) Sacroiliitis as an initial manifestation of acute myelogenous leukemia. Int J Hematol. 84(5): 421-424.
- Mondal S, Sinha D, Ete T, Goswami RP, Bardhan J, et al. (2016) Foe Incognito: Paraneoplastic Sacroiliitis. J Med Cases. 7(8): 341-343.