Anupama Barua FRCS, MD *, Richard Warwick FRCS
Department of Cardiothoracic Surgery University Hospitals North Midlands NHS Trust, UK
*Corresponding Author: Anupama Barua FRCS, MD, Department of Cardiothoracic Surgery University Hospitals North Midlands NHS Trust, UK
Abstract
Left ventricular free wall rupture is a catastrophic consequence of myocardial infarction and surgical intervention reduces the mortality. Here, we discuss a case of surgically repaired left ventricular free wall rupture who developed post-transfusion purpura in the postoperative period.
Introduction
Post-transfusion purpura (PTP) is a rare condition presented as severe thrombocytopenia and bleeding disorder [1]. It develops in 7-14 days after transfusion of blood products. The development of antibodies for human platelet-specific antigen (HPA) is responsible for this disorder. Detection of HPA and genotyping of platelet are essential to diagnose the disease. The estimated incidence is 1:50,000 - 100,000 transfusions [2]. It usually happens in a multiparous woman with a history of blood transfusion. PTP is rarely reported after cardiac surgery. Here we present a case of a female patient who underwent left ventricular free wall rupture repair and developed PTP after massive blood transfusions. To our knowledge, this is the first case of PTP following left ventricular free wall rupture.
Case Report
A 71 years old lady was admitted to the hospital with a history of chest pain and heaviness in the left arm. Her past medical history included osteoarthritis, breast cancer, type 2 diabetes, high blood pressure, and high cholesterol. On admission blood results revealed troponin level > 25,000, platelet 219, eGFR > 90, and the rest of the results were within the normal range. The coronary angiogram revealed mild disease in the left main stem, left anterior descending artery, right coronary artery, and blocked obtuse marginal branch. As she was pain-free, the plan was to monitor her and discharged her in a week for a further review in 6 weeks in the cardiac surgery clinic. On the day of discharge, she collapsed. Clinically, she was in cardiogenic shock and transthoracic echocardiography confirmed a large haemopericardium with a ruptured left ventricle (LV).
The decision was made to take her to the theatre for a salvage repair of LV. Intraoperatively, haemopericardium with large clots and rupture of the lateral wall of LV close to the apex were identified. The rupture was repaired with layers of the porcine pericardium and a BioIntegral surgical patch. BioGlue surgical adhesive (Cryolife) was applied to achieve a hemostatic patch. The repair was reinforced with Tisseel (Baxter), Floseal Haestatic Matrix (Baxter), and Surgicel (Ethicon). The patient was coagulopathic and required 12 units of FFP, and 4 units of platelets. Complete hemostasis was not achievable, the chest was packed and left open. An intraaortic balloon pump (IABP) was inserted to reduce the afterload and aid with coronary perfusion. The total Cross clamp was 47 min and the CPB time was 108 min.
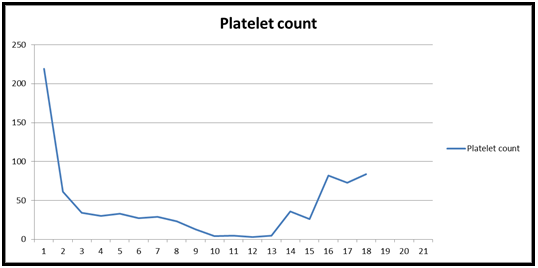
Her immediate post-operative period was stormy. She was on a high dose of inotropes. In 10th POD, the patient received multiple blood product transfusions including 17 units of RBC, 18 units of FFP, 6 units of cryoprecipitate, and 6 bags of platelets. Hematologists were involved and diagnosed the case as massive transfusion-related coagulopathy such as TTP, PTP, ITP, or HIT. HIT low probability score Probability of HIT (< 5 %) and HIT ELISA screen negative. The plasmic score was 4 which indicated a low probability of TTP. She did not show any sign of infection or sepsis. Anti-HPA1a antibodies were detected through MAIPA and platelet immunofluorescence, ADAMTS13 81 % (normal). The coagulopathy eventually was diagnosed as post-transfusion purpura. HLA-matched platelets 2 units were preserved but which could be ineffective due to the ‘bystander' effect. She received IV immunoglobulin for 2 consecutive days and responded slowly. The platelet count was rising from 5 to 82 on the 15th POD [Figures 1 & 2].
Her CT scan on 16th POD confirmed the appearance of LV rupture at the inferolateral wall with contrast leaking into the pericardium and a rim measuring up to 2.7 cm. At the same time, she developed gastrointestinal bleeding and acute renal failure. She was on continuous venovenous hemofiltration (CVVH) for acute kidney injury. To date, she received 25 units of RBC, 10 units of platelets, 8 units of cryoprecipitate, and 20 units of FFP. Unfortunately, despite all efforts, she passed away on 17th POD due to multi-organ failure.
Discussion
Left ventricular free wall rupture (LVFWR) is one of the fatal complications following acute myocardial infarction. Conservative management leads to 80 % mortality and surgical mortality is also as high as 32-50 % [3]. In this case, the patient was diagnosed with LVFWR within six days of acute myocardial infarction. She was in cardiogenic shock before surgery and developed cardiac tamponade. Intraoperatively, an intra-aortic balloon was inserted to support the coronary perfusion and reduce the intracavitary pressure and volume in the left ventricle.
In our patient, PTP developed after getting blood transfusions following repair of LVFW repair. PTP develops when patients are exposed to HPA during pregnancy or massive blood transfusion. The antibodies annihilate the patients’ platelet and transfused platelet by cross-reactions.
Here, multiple factors contributed to the low platelet count and impaired platelet function. Firstly, she was admitted to the cardiology ward six days before surgery where she received low molecular weight heparin (LMWH) as prophylaxis of deep vein thrombosis. Although the incidence of heparin-induced thrombocytopenia (HIT) is less common in LMWH, thrombocytopenia is diagnosed in 0.065 % of patients of 1/1500 hospital admission [4]. Secondly, the patient was exposed to high dose heparin for cardiopulmonary bypass. She was in cardiopulmonary bypass for 108min which reduced platelet function. Thirdly, she was on IABP which also destroy the platelet. Finally, she had a massive blood transfusion (25 units of RBC, 10 units of platelets, 8 units of cryoprecipitate, and 20 units of FFP) to treat the coagulopathy and restore the hemostasis.
In our case, the patient was hemodynamically unstable and IABP was inserted twice and kept in situ for >10 days. IABP leads to a decrease in platelet count as the platelets are adherent to the balloon surface. The count usually returns to normal after 4 days of balloon insertion [5]. However, the low platelet count continued in our patient.
Our initial differential diagnosis was HIT which is not uncommon after cardiac surgery [6]. The incidence of HIT after open-heart surgery is as high as 3 % [7-10]. However, in this case, the platelet count was reduced by more than 50 % in 1st POD [Figure 1 & 2] and she did not show any clinical sign of thrombocytosis and ELISA revealed negative IgG for HIT. The platelet count dropped to as low as 4 in the 10th POD. It was confirmed at that time with PTP in presence of the HPA1a antibody in the serum. The main stem of the management of PTP is immunoglobulin [11]. She received the treatment and responded to it. It was documented in the literature to treat with plasmapheresis and prednisolone [12,13]. Plasmapheresis reduces the antibody load and can potentially decrease the need for transfusion of blood products [14].
Bleeding in the gastrointestinal system, intracranial bleeding, and bleeding in the urinary system are catastrophic complications in PTP. It was reported that 5 % of patients mortality following bleeding complications [13]. Our patient developed melena on the 15th day of diagnosis of PTP.
The absence of functional platelet resulted in continuous leakage and failure to seal the repair edge. On 16th POD, CT demonstrated the repair was leaking.
|
Post operative day |
Platelet count |
|
0 |
219 |
|
1 |
61 |
|
2 |
34 |
|
3 |
30 |
|
4 |
33 |
|
5 |
27 |
|
6 |
29 |
|
7 |
23 |
|
8 |
13 |
|
9 |
4 |
|
10 |
5 |
|
11 |
3 |
|
12 |
5 |
|
13 |
36 |
|
14 |
26 |
|
15 |
82 |
|
16 |
73 |
|
17 |
84 |
Figure 1. Table 1: Demonstrating Platelet count in post-operative days
Figure 2: Platelet count in post-operative days
Conclusion
LVFW rupture is a lethal complication and surgery is the gold standard of treatment. This case report demonstrated that a patient with surgical intervention in LVFWR could not have a successful outcome as the patient developed PTP, continued leakage due to no sealing of the repair, and ultimately a multi-organ failure and complication of PTP. The diagnosis of PTP should be considered in evaluating the patient with life-threatening thrombocytopenia after cardiac surgery.
Abbreviations
IABP: Intra-aortic balloon pump; ICU: Intensive care unit; POD: Post-operative day, Post transfusion purpura (PTP), Left ventricular free wall rupture (LVFWR), human platelet specific antigen (HPA), left ventricle (LV), continuous venovenous hemofiltration (CVVH).
Competing interests: The authors declare that they have no competing interests.
Informed consent: Consented
Authors’ contributions
AB collected data and drafted the manuscript. RW was the surgeon of the case and conceived the case report. All authors read and approved the final manuscript.
Funding: None
Conflict of Interest: None
International Review Board approval or waiver: None
Clinical trial registration: Not required.
References
- Shtalrid M, Shvidel L, Vorst E, Weinmann EE, Berrebi A, et al. (2006) Post-transfusion purpura: a challenging diagnosis. Isr Med Assoc J. 8(10): 672-4.
- Taaning E, Tønnesen F (1999) Pan-reactive platelet antibodies in post-transfusion purpura. Vox Sang. 76(2): 120-3.
- Figueras J, Alcalde O, Barrabés JA, Serra V, Alguersuari J, et al. (2008) Changes in hospital mortality rates in 425 patients with acute ST-elevation myocardial infarction and cardiac rupture over a 30-year period. Circulation. 118(25): 2783- 2789.
- Dhakal B, Kreuziger LB, Rein L, Kleman A, Fraser R, et al. (2018) Disease burden, complication rates, and health-care costs of heparin-induced thrombocytopenia in the USA: A population- based study. Lancet Haematol. 5(5): e220–e231.
- Vonderheide RH, Thadhani R, Kuter DJ (1998) Association of thrombocytopenia with the use of intra-aortic balloon pumps. Am J Med. 105(1): 27-32.
- Hogan M, Berger JS (2020) Heparin-induced thrombocytopenia (HIT): Review of incidence, diagnosis, and management. Vascular Medicine. 25(2): 160-173.
- Seigerman M, Cavallaro P, Itagaki S, Chung I, Chikwe J (2014) Incidence and outcomes of heparin-induced thrombocytopenia in patients undergoing cardiac surgery in North America: An analysis of the nationwide inpatient sample. J Cardiothorac Vasc Anesth. 28(1): 98–102.
- Pishko AM, Cuker A (2017) Heparin-induced thrombocytopenia in cardiac surgery patients. Semin Thromb Hemost. 43(7): 691– 698.
- Lillo-Le Louët A, Boutouyrie P, Alhenc-Gelas M, Le Beller C, Gautier I, et al. (2004) Diagnostic score for heparin-induced thrombocytopenia after cardiopulmonary bypass. J Thromb Haemost. 2(11): 1882–1888.
- Kestin AS, Valeri CR, Khuri SF, Loscalzo J, Ellis PA, et al. (1993) Platelet function defect of cardiopulmonary bypass. Blood. 82(1): 107–117
- Rankin JS, Stratton CW (2014) Efficacy of immunomodulation in the treatment of profound thrombocytopenia after adult cardiac surgery. The Journal of Thoracic and Cardiovascular Surgery. 147(2): 808-13; discussion 813-5.
- Puig N, Sayas MJ, Montoro JA, Villalba JV, Pla A (1991) Post- transfusion purpura as the main manifestation of a trilineal transfusion reaction, responsive to steroids: flow-cytometric investigation of granulocyte and platelet antibodies. Ann Hematol. 62(6): 232-4.
- Kroll H, Kiefel V, Mueller-Eckhardt C (1993) Post-transfusion purpura: clinical and immunologic studies in 38 patients. Infusionsther Transfusionsmed. 20(5): 198-204.
- Welsby IJ, Um J, Milano CA, Ortel TL, Arepally G (2010) Plasmapheresis and heparin reexposure as a management strategy for cardiac surgical patients with heparin-induced thrombocytopenia. Anesth Analg. 110(1): 30-35.