Mbogo N. Kija1,2*, Sharadhuli I. Kimera1, Ladslaus L. Mnyone2,3
1,2Epidemiologist, Department of Public Health, Sokoine University of Agriculture, P.O. Box 3015, Morogoro - Tanzania
1Professor of Veterinary Epidemiology & Infectious Diseases, Sokoine University of Agriculture, College of Veterinary Medicine and Biomedical Sciences, P.O. Box 3021, Morogoro - Tanzania
2Professor of Entomology, Institute of Pest Management, Sokoine University of Agriculture, P.O. Box 3110, Morogoro - Tanzania
3Division of Science, Technology and Innovation, Ministry of Education, Science and Technology, P.O. Box 10, Dodoma, Tanzania.
*Corresponding Author: Mbogo N. Kija, Epidemiologist, Department of Public Health, Sokoine University of Agriculture, P.O. Box 3015, Morogoro - Tanzania. Email: mbogo.ngwisabila@yahoo.com
Abstract
Background: Knowledge about malaria control and treatment-seeking behaviour is essential for devising effective area-specific malaria behavioural change communication (BCC) and control strategies.
Methods: A social survey was conducted on 398 randomly selected undergraduate students to assess the level of knowledge, control practices, and treatment-seeking behaviour about malaria across four higher education institutions (HEIs) in eastern Tanzania. Semi-structured questionnaires and face-to-face interviews were used for data collection. It was also supplemented with direct observation.
Results: The level of knowledge on malaria among the students varied significantly across the four HEIs. The respondents from Sokoine University (56.3 %) were more knowledgeable about malaria relative to Muslim University (36.8 %), Jordan University (31.2 %), and Mzumbe University (27.5 %); and females (23.4 %) were more knowledgeable compared to males (17.8 %). Only 36.7 % of the respondents correctly understood how malaria is transmitted to humans. About 24 % of respondents could name three diseases that present with fever. Approximately 32 % of the respondents correctly mentioned the five typical signs and symptoms of malaria in humans.
Conclusions: Results indicate an unexpectedly low level of knowledge about malaria among students of HEIs in eastern Morogoro. Therefore, regular BCC campaigns are necessary to raise awareness among HEIs in view of minimizing their vulnerability to malaria.
Keywords: malaria, knowledge, control practices, treatment-seeking behaviour
Introduction
Background
Targeting malaria mosquito vectors remain the most powerful approach for malaria control worldwide. Overall, this approach aims to reduce human-vector contact, protect individuals from infection, and reduce transmission at the community level. This is achieved through different control measures, including, among others, larval source management (LSM) [1,2], long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) [3,7], house improvements as well as topical and spatial repellents [8,11]. LLINs and IRS are the most powerful and widely deployed control measures [12,13]; their increased use has significantly reduced malaria transmission risk and burden in most malaria-endemic countries [14,15]. However, the effectiveness of LLINs and IRS is increasingly threatened by the development of resistance in the prominent malaria mosquitoes to almost all recommended classes of chemical insecticides, let alone their irregular and inappropriate use. Therefore, the improvement and complimentary use of other vector control measures with LLINs and IRS, as well as strengthening advocacy on their regular and appropriate deployment, are necessary, among other initiatives, for safeguarding and advancing the malaria control gains beyond the current margins.
Moreover, the combination of vector control measures with improved malaria diagnosis and treatment has increasingly made malaria control gains even more remarkable [16,17]. As such, the behaviour changes communication (BCC) and other types of efforts that emphasize early malaria diagnosis and case management are as important as those destined to sustain the effectiveness of vector control measures. Behavioural change communication (BCC) is a behaviour-shaping or -changing effort that mainly aims to use information education communication (IEC) to improve the knowledge level of the population, create a supportive social environment that is conducive to forming target behaviours, and provide material and moral support to help overcome factors that would affect target behaviours [18,19]. Behavioural change communications have helped improve the community's knowledge on malaria and its control strategies, improve the appropriate use of disease control measures, and shape the diagnosis and treatment-seeking behaviour in many endemic countries [20, 24]. Treatment-seeking behaviour is still challenging in many communities as many people still go for self-medication and traditional healers [25,26]. The treatment-seeking behaviour and adherence to malaria treatment are dictated by socio-demographic factors such as age, gender, income, education, culture, and environmental settings [27,29].
In order to develop area-specific and effective malaria control strategies and BCC, establishing the level of knowledge about malaria, prevailing vector control practices, and treatment-seeking behaviour is extremely vital. These and other allied parameters vary widely within and between spatiotemporal scales. Therefore, area-specific surveys are necessary in certain specific situations to ensure sustainable disease control outcomes, particularly in predisposed and vulnerable population segments such as in cases of school and college students. Despite these, very little is known about the reinstated parameters in higher education institutions in Tanzania, including those located within the Morogoro region. In view of the aforesaid, the current study assessed the level of knowledge, control practices, and treatment-seeking behaviour regarding malaria among students of higher education institutions (HEIs) in Morogoro urban district, eastern Tanzania. The findings discussed herein will help provide important insights for designing and implementing appropriate and responsive malaria control and behaviour communication strategies in HEIs and beyond.
Methods
Study area
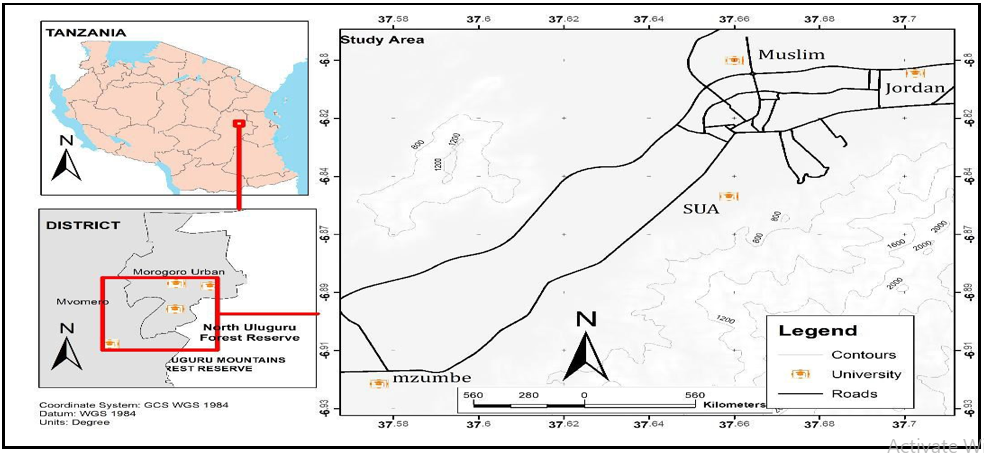
The study was conducted in four higher education institutions (HEIs) located within the Morogoro Urban district in the Morogoro region, Tanzania. The HEIs were Sokoine University of Agriculture (SUA) (6.8278ºS, 37.6591 ºE), Mzumbe University (MU) (6.9239ºS, 37.5691ºE), Muslim University of Morogoro (MUM) (6.8288ºS, 37.6612ºE) and Jordan University College (JUCO) (6.8068ºS, 37.7024ºE) (Figure 1). Morogoro Urban district is about 200 km west of Dar es Salaam and lies between latitudes 5º7' and 10º00' south of the Equator and longitudes 35°6' and 39°5' east of Greenwich. Like the rest of the Morogoro region, this district experiences two main seasons, the wet and dry seasons. The wet season runs from March-May and October–December, with April and December being the wettest months. The dry seasons run from June – September, and January – February, with July being the driest month. The area experiences an average annual rainfall of 935 mm and a temperature of 24.6°C. The nature of economic activities across the district is variable; however, the major ones include crop cultivation, livestock keeping, and micro-business. For Sokoine University, located about 3 km from Morogoro town centre, small-scale livestock keeping, and agriculture, mainly for training purposes, are done within and proximal to university premises. For Mzumbe University, located about 16 km at the outskirts of Morogoro town centre, small-scale agriculture is done within and proximal to university premises involving the cultivation of maize, rice, sweet potatoes, cassava, and nuts. Similarly, for Muslim University, located about 4.9 km from Morogoro town centre, comparatively small-scale cultivation of rice and vegetables is done mainly within the university premises. Jordan University premises do not have any agricultural activities within the campus. However, the surrounding community is substantially involved in agriculture of food crops as those of Mzumbe.
Figure 1. A map of the Morogoro region showing the Higher Education Institutions (HEIs) that were involved in the study.
Determination of sample size
The sample size of respondents was determined based on the number of undergraduate students in each institution: SUA (n=12,609), MU (10,762), MUM (n=4,320) and JUCO (n=2,414). As such, the study respondents below were selected from an overall student population of about 30,105. The overall number of respondents in all four HEIs and per each institution was calculated as indicated below (Conroy) [10].
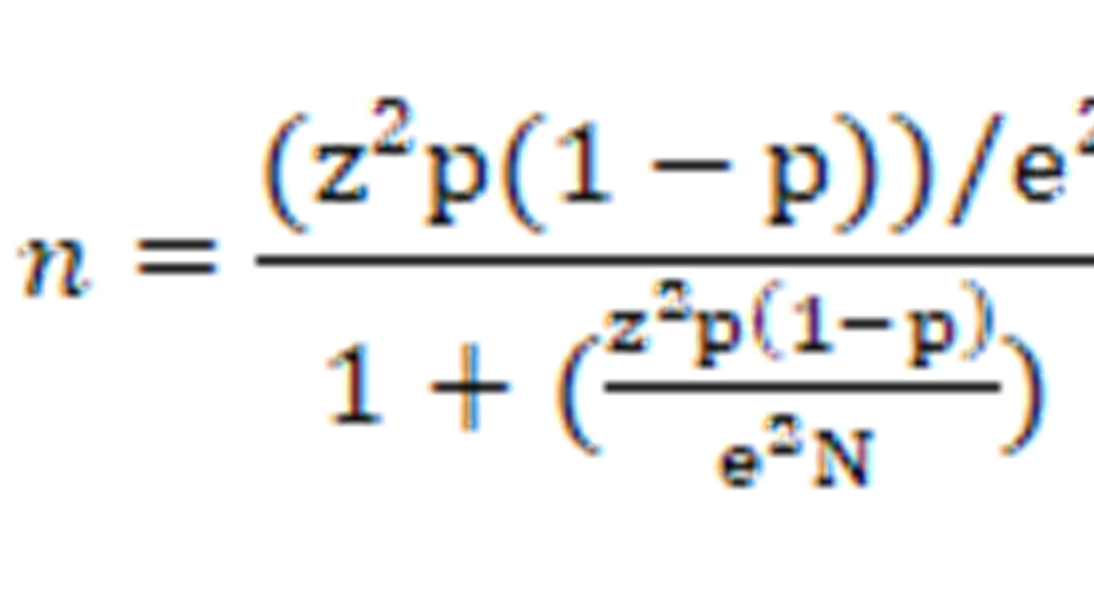
Whereas:
N= Population size (30,105),
e= Margin of Error (0.05)
z= Confidence Level (1.96)
P= Malaria prevalence (0.61)
n = Total sample size for the study (398 respondents)
The proportional to size of sample size per HEI was determined using the formula described (Kothari) [19].
ni=nPi
Where;
ni= number of required respondents per institution
n = total sample size required for the study
Pi = proportion of the population per institution
Based on the above, the sample size of respondents in each HEIs was as follows:
SUA = 398*(12609/30105); had 167 respondents.
JUCO = 398*(2414/30105); had 32 respondents.
MUM = 398*(4320/30105); had 57 respondents; and
MU = 398*(10762/30105); had 142 respondents
Study population and design
The study employed a cross-sectional survey that ran from January to August 2021 and involved undergraduate students across four study HEIs that had been in the respective institutions for not less than a year. In each HEIs, the study respondents were selected from two on-campuses and two off-campuses using a simple random technique: SUA = 167, MU 142, MUM 57, and JUCO 32. Semi-structured questionnaires and face-to-face interviews were used for data collection. It was also supplemented with direct observation and a separate sheet for parameters regarding managerial questions was used. The questionnaire consisted of four questions about malaria: knowledge, control practices, treatment-seeking behaviour, and transmission risk factors. The questionnaire was developed in English and translated to the Kiswahili language to allow respondents to provide answers using their language of choice. The questionnaire was piloted and revised before being employed for the data collection. A team of qualified interviewers did the questionnaire and face-to-face supplementary interviews. All respondents gave verbal and written consent before participating in the study.
Statistical analysis
Responses to the questionnaire were appropriately coded, entered, and cleaned in Ms. Excel and eventually transferred to SPSS for detailed statistical analysis. The Data analysis was done using Statistical Package for Social Sciences (SPSS) version 22 IBM and Ms. Excel computer program. Descriptive statistics are presented in tables and graphs. The test results were considered statistically significant at a p-value < 0.05.
Results
Demographic characteristics of respondents
398 respondents, 198 males and 200 females, were finally interviewed across the four study HEIs. The proportion of respondents across the institutions was SUA 42.0 % (n=167), MU 35.7 % (n=142), MUM 14.3% (n=57) and JUCO 8 % (n=32). In each HEIs, 50 % of the respondents came from in-campus and off-campus. The details of demographic characteristics are provided in Table 1.
Table 1: Demographic characteristics of respondents across the four study HEIs
|
Name of Institution |
Campus |
Total |
Percent (%) |
|||
|
on campus |
off campus |
|||||
|
Jordan |
|
Male |
8 |
8 |
16 |
4.0 |
|
Female |
8 |
8 |
16 |
4.0 |
||
|
Total |
16 |
16 |
32 |
8.0 |
||
|
Muslim University |
|
Male |
14 |
14 |
28 |
7.0 |
|
Female |
14 |
15 |
29 |
7.3 |
||
|
Total |
28 |
29 |
57 |
14.3 |
||
|
Mzumbe University |
|
Male |
35 |
35 |
70 |
17.6 |
|
Female |
36 |
36 |
72 |
18.1 |
||
|
Total |
71 |
71 |
142 |
35.7 |
||
|
Sokoine University |
|
Male |
41 |
43 |
84 |
21.1 |
|
Female |
40 |
43 |
83 |
20.9 |
||
|
Total |
81 |
86 |
167 |
42.0 |
||
|
Total |
|
Male |
98 |
100 |
198 |
49.7 |
|
Female |
98 |
102 |
200 |
50.3 |
||
|
Total |
196 |
202 |
398 |
100 |
||
Knowledge on malaria among the respondents
The level of knowledge on malaria among the students varied significantly across the four HEIs (Table 2). Overall, only 41.2 % of the interviewed students were desirably knowledgeable about malaria across the four HEIs within Morogoro Municipality; females (23.4 %) were more knowledgeable than males (17.8 %). Sokoine University (56.3 %) had the highest proportion of students who were relatively more knowledgeable about malaria, followed by Muslim University (36.8 %), Jordan University (31.2 %), and Mzumbe University (27.5 %) (Table 3). However, the level of knowledge did not vary between the on-campus and off-campus students (Appendix 1).
Only 36.7 % of the respondents correctly understood how malaria is transmitted to humans. The remaining most significant proportion was unaware that the female Anopheles mosquito only transmits malaria. About 24 % of respondents were able to name three diseases that present with fever as compared to 30.9 % who mentioned two conditions and 42.2 % who mentioned only one disease, the diseases mentioned were malaria, UTI, typhoid fever, pneumonia, flu, diarrhoea, pressure, covid 19 and anemia. Furthermore, approximately 32 % of the respondents correctly mentioned the five typical signs and symptoms of malaria in humans. The rest of the respondents mentioned at least three (41.5 %) and at least two (26.6 %). A small proportion (18.6 %) of respondents mentioned at least five typical risk factors for malaria transmission. The rest mentioned at least three (35.7 %) and two (45.7 %).
About the knowledge of the critical time/hours that a person can easily be infected with malaria, the responses were categorized as; Don't know, Early morning (0500-0800 am), Late evening (1700-1900 pm), Early night (1900-2300 pm) and Mid and late-night (2300 pm - 500 am) had the percentages of 20.4, 2.5, 11.6, 15.3 and 50.2 respectively, giving the impression that the majority know that they can easily get malaria at mid and late-night (2300 pm - 500 am).
Table 2: One-way ANOVA output indicating variation in knowledge level on malaria across the four study HEIs
|
Name of Institution |
Significance level |
95 % Confidence Interval |
||
|
Lower Bound |
Upper Bound |
|||
|
Sokoine University |
Jordan University |
.001 |
2.19 |
11.07 |
|
|
Muslim University |
.019 |
.43 |
7.49 |
|
|
Mzumbe University |
.000 |
.4.55 |
9.80 |
Table 3: Knowledge level on malaria across the four HEIs with respect to gender
|
Name of Institution |
Knowledge level |
Total |
|||
|
Low knowledge on malaria |
High knowledge on malaria |
||||
|
Jordan University |
|
Male |
13 |
3 |
16 |
|
Female |
9 |
7 |
16 |
||
|
Total |
22(68.8 %) |
10 (31.2) |
32 |
||
|
Muslim University |
|
Male |
20 |
8 |
28 |
|
Female |
16 |
13 |
29 |
||
|
Total |
36(63.2 %) |
21(36.8) |
57 |
||
|
Mzumbe University |
|
Male |
53 |
17 |
70 |
|
Female |
50 |
22 |
72 |
||
|
Total |
103(72.5 %) |
39(27.5) |
142 |
||
|
Sokoine University |
|
Male |
41 |
43 |
84 |
|
Female |
32 |
51 |
83 |
||
|
Total |
73(43.7 %) |
94(56.3) |
167 |
||
|
Total |
|
Male |
127 |
71 |
198 |
|
Female |
107 |
93 |
200 |
||
|
Total |
234(58.8 %) |
164(41.2 %) |
398 |
||
Malaria control practices
Overall, the use of LLINs, was the other common malaria control approach reported across the four study HEIs. The proportion of respondents that reported to sleep under long-lasting insecticidal bed nets (LLINs) ranged from 34.1 – 56.1 %. Muslim University had the highest proportion of respondents that reported to sleep under LLINs (56.1 %) relative to Jordan (50 %), Mzumbe (43.7 %) and Sokoine (34.1 %). As such, Sokoine registered the lowest proportion of respondents who reported to sleep under LLINs. The commonly mentioned reasons for not using LLINs in the order of magnitude across the four HEIs were: dislike (22.8 %), no money (20.6 %) and suffocation or health problems (14.6 %) (Table 4). The responses within the respective HEIs indicate that the majority of students did not sleep under LLINs just because they disliked them. The details on proportion of students that reported to sleep under LLINs and the reasons for using and not using an LLIN across the individual HEIs are presented in Table 4.
Table 4: Proportion of respondents that used long-lasting insecticidal net (LLIN) and reasons for using and not using an LLIN across the four study Higher Education Institutions (HEIs)
|
Name of institution |
Reason for using or not using LLINs |
Total |
|||||
|
Prevent Malaria |
No money |
Suffocation/other health problems |
Dislike |
||||
|
Jordan University |
Sleep under LLIN |
Yes |
16 |
0 |
0 |
0 |
16 |
|
No |
0 |
7 |
3 |
6 |
16 |
||
|
Total Percent |
16 50 |
7 21.9 |
3 9.4 |
6 18.7 |
32 100 |
||
|
Muslim University |
Sleep under LLIN |
Yes |
32 |
0 |
0 |
0 |
32 |
|
No |
0 |
13 |
5 |
7 |
25 |
||
|
Total Percent |
32 56.1 |
13 22.8 |
5 8.8 |
7 12.3 |
57 100 |
||
|
Mzumbe University |
Sleep under LLIN |
Yes |
62 |
0 |
0 |
0 |
62 |
|
No |
0 |
27 |
18 |
35 |
80 |
||
|
Total Percent |
62 43.7 |
27 19.0 |
18 12.7 |
35 24.6 |
142 100 |
||
|
Sokoine University |
Sleep under LLIN |
Yes |
57 |
0 |
0 |
0 |
57 |
|
No |
0 |
35 |
32 |
43 |
110 |
||
|
Total Percent |
57 34.1 |
35 21.0 |
32 19.2 |
43 25.7 |
167 100 |
||
|
Total |
Sleep under LLIN |
Yes |
167 |
0 |
0 |
0 |
167 |
|
No |
0 |
82 |
58 |
91 |
231 |
||
|
Total Percent |
167 42.0 |
82 20.6 |
58 14.6 |
91 22.8 |
398 100 |
||
Wearing long clothes was the most commonly used approach for malaria control before bedtime. Approximately 39 % of the respondents from the four HEIs reported that they were putting on long cloth during personal studies and other night-time activities to protect themselves against mosquito bites and consequently malaria. The rest (~61 %) did not consider putting on long clothing to control malaria; therefore, did not bother doing that (Table 5).
Personal mosquito repellents were the following most commonly used malaria mosquito control approach. This approach was used before bedtime, mainly when the students were on individual studies and other night-time activities. Overall, only 7.8 % of all respondents from the four HEIs reported using repellents to prevent mosquito bites and control malaria thereof. Of the four HEIs, Muslim university registered the highest proportion of respondents who reported using personal mosquito repellents (14 %), followed by Mzumbe (7 %), Sokoine (6.6 %), and Jordan (6.3 %), as presented in Table 5.
The use of insecticidal sprays in a sleeping room was another mosquito control method practiced. The students used this approach a time before bed or when away from a sleeping room for about half an hour or more, and it was practiced mainly by those who stayed off-campus. Overall, only 27.1 % of all respondents from the four HEIs reported using mosquito repellents in their sleeping rooms to self-protect from mosquito bites. Of the four HEIs, Mzumbe University recorded the highest proportion of respondents who used mosquito repellents in their sleeping rooms (31.0 %), followed by Sokoine (26.3 %), then Jordan (25.0 %), and finally Muslim university had the least (21.1 %) (Table 5).
Table 5: Use of mosquito insecticidal sprays, repellents, and protective clothing before bedding time across the four studies HEIs
|
|
Name of Institution |
Total |
|||||
|
Jordan |
Muslim |
Mzumbe |
Sokoine |
||||
|
a) Use of Personal mosquito repellents before bedding |
Yes (%) |
2 6.3 |
8 14.0 |
10 7.0 |
11 6.6 |
31 7.8 |
|
|
No (%) |
30 93.7 |
49 86.0 |
132 93.0 |
156 93.4 |
367 92.2 |
||
|
Total |
32 |
57 |
142 |
167 |
398 |
||
|
b) Use of insecticidal sprays or mosquito repellents in a sleeping room |
Yes (%) |
8 25.0 |
12 21.1 |
44 31.0 |
44 26.3 |
108 27.1 |
|
|
No (%) |
24 75.0 |
45 78.9 |
98 69.0 |
123 73.7 |
290 72.9 |
||
|
Total |
32 |
57 |
142 |
167 |
398 |
||
|
c) Type of clothes worn at night before bedding |
Long (%) |
11 34.4 |
26 45.6 |
51 35.9 |
66 39.5 |
154 38.7 |
|
|
Short (%) |
21 65.6 |
31 54.4 |
91 64.1 |
101 60.5 |
244 61.3 |
||
|
Total |
32 |
57 |
142 |
167 |
398 |
||
Treatment-seeking behaviour towards malaria
Approximately 31 % of the respondents in the four study institutions reported visiting health facilities for malaria diagnosis and treatment after noticing fever. The majority (69 %) said they often did not consider visiting health facilities but instead opted for self-remedies such as prayers, sleep, drinking much fluid, eating fruits and herbal leaves, exercising, and taking anti-pains/antipyretics (Table 6). Furthermore, the proportion of respondents who reported visiting health facilities for diagnosis and malaria treatment after noticing fever was Jordan (53.1 %), Muslim (47.4 %), Mzumbe (25.4 %), and Sokoine (25.1 %). As such, Jordan had the highest proportion of students that considered visiting health facilities in the events of fever compared to the other three HEIs.
About 92 % of the respondents who reported visiting health facilities for diagnosis used antimalarial drugs after a positive malaria diagnosis. The most commonly used brand of antimalarial drugs in the order of magnitude included ALU (66.3 %), Metakelfin or Laefin (13.8 %), Quinine (4.0 %), others (3.8 %), Fansider/SP (2.0 %) and Artequick (1.0 %) (Table 6). It is worth noting that 5.8 % of the respondents reported using traditional herbs for malaria treatment. Most respondents claimed to complete an entire course of the dose of antimalarial drugs (Table 6).
Table 6: Proportion of respondents that reported visiting health facilities for malaria diagnosis, taking antimalarial after a positive diagnosis, and completing the dose
|
Name of Institution |
||||||
|
|
|
Jordan |
Muslim |
Mzumbe |
Sokoine |
Total |
|
a) Decision made after noticing fever
|
Go to hospital |
17(53.1) |
27(47.4) |
36(25.4) |
42(25.1) |
122(30.7) |
|
Self-remedies |
15(46.9) |
30(52.6) |
106(74.6) |
125(74.9) |
276(69.3) |
|
|
Total |
32 |
57 |
142 |
167 |
398 |
|
|
b) Use of antimalarials
|
Yes |
28 |
54 |
132 |
150 |
364(91.5) |
|
No |
4 |
3 |
10 |
17 |
34(8.5) |
|
|
Total |
32 |
57 |
142 |
167 |
398 |
|
|
c) Malaria dosage completion |
Yes |
28 |
47 |
124 |
136 |
335(84.2) |
|
No |
4 |
10 |
18 |
31 |
63(15.8) |
|
|
Total |
32 |
57 |
142 |
167 |
398 |
|
|
d) Brand of antimalarial drugs used
|
ALU |
16 |
36 |
96 |
116 |
264(66.3) |
|
Fansidar or SP |
0 |
1 |
2 |
5 |
8(2.0) |
|
|
Metakelfin or Laefin |
7 |
7 |
23 |
18 |
55(13.8) |
|
|
Duocortexin |
3 |
4 |
3 |
3 |
13(3.3) |
|
|
Artequick |
0 |
1 |
0 |
3 |
4(1.0) |
|
|
Quinine |
0 |
4 |
7 |
5 |
16(4.0) |
|
|
Traditional |
6 |
3 |
9 |
5 |
23(5.8) |
|
|
Others |
0 |
1 |
2 |
12 |
15(3.8) |
|
|
Total |
32 |
57 |
142 |
167 |
398 |
|
Malaria transmission risk factors
Environmental factors
Places that were commonly used for self-studies by students across the four HEIs in the order of preference included classes (33.7 %), sleeping rooms (31.9 %), other self-study spaces in hostels (19.8 %), libraries (12.6 %), and lecture halls (2.0 %) (Table 7).
Table 7: Places commonly used for self-studies across the four Higher Education Institutions (HEIs)
|
|
Name of Institution |
Total |
||||
|
JORDAN |
MUSLIM |
MZUMBE |
SUA |
|||
|
Places used for self-studies in terms of preference |
Classes Percent |
8 25.0 |
25 43.9 |
68 47.9 |
33 19.8 |
134 33.7 |
|
Library Percent |
3 9.4 |
6 10.5 |
16 11.3 |
25 15.0 |
50 12.6 |
|
|
Halls Percent |
1 3.1 |
0 0.0 |
6 4.2 |
1 0.6 |
8 2.0 |
|
|
Hostel Percent |
8 25 |
7 12.3 |
20 14.1 |
44 26.3 |
79 19.8 |
|
|
Room Percent |
12 37.5 |
19 33.3 |
32 22.5 |
64 38.3 |
127 31.9 |
|
|
Total |
32 |
57 |
142 |
167 |
398 |
|
The proportion of respondents who reported living in rooms with screened windows was 87.5 %, 77.2 %, 84.5 %, and 64.7 % for Jordan, Muslim, Mzumbe, and SUA respectively (Table 8). The proportion of respondents who reported that they lived in rooms with doors and roofs with limited possibility of mosquito entry was 87.5 %, 82.5 %, 61.3 %, and 62.9 % for Jordan, Muslim, Mzumbe, and SUA respectively.
About 18.6 % of respondents across the four HEIs correctly mentioned five possible risk factors for malaria transmission. The rest managed to mention at least three (35.7 %) and two (45.7 %) risk factors (Table 9)
Table 8: Proportion of respondents that reported sleeping in rooms with screened windows and well-fixed doors and roofs
|
|
Name of Institution |
Total |
||||
|
JORDAN |
MUSLIM |
MZUMBE |
SUA |
|||
|
a) Sleeping rooms with screened windows |
Yes (%) |
28 87.5 |
44 77.2 |
120 84.5 |
108 64.7 |
300 75.4 |
|
No (%) |
4 12.5 |
13 22.8 |
22 15.5 |
59 35.3 |
98 24.6 |
|
|
Total |
|
32 |
57 |
142 |
167 |
398 |
|
b) Sleeping rooms with mosquito entry limited doors and roofs |
Yes (%) |
28 87.5 |
47 82.5 |
87 61.3 |
105 62.9 |
267 67.1 |
|
No (%) |
4 12.5 |
10 17.5 |
55 38.7 |
62 37.1 |
131 32.9 |
|
|
Total |
|
32 |
57 |
142 |
167 |
398 |
Table 9: Proportion of respondents managed to mention malaria risk factors
|
Name of Institution |
Total |
|||||
|
|
|
JORDAN |
MUSLIM |
MZUMBE |
SUA |
|
|
Five possible risk Factors |
Fair % |
15 (46.9) |
22 (38.6) |
71 (50.0) |
74 (44.3) |
182 (45.7) |
|
Moderate % |
11 (34.4) |
19 (33.3) |
57 (40.1) |
55 (32.9) |
142 (35.7) |
|
|
Correct % |
6 (18.8) |
16 (28.1) |
14 (9.9) |
38 (22.8) |
74 (18.6) |
|
|
Total |
32 |
57 |
142 |
167 |
398 |
|
Human behaviour-related malaria transmission risk factors.
Most respondents (90.1 %) across the four HEIs altogether reported starting self-studies before 2300 pm. Likewise, most respondents (90.7 %) said they usually went to bed after 2300 pm (Table 10). Furthermore, a considerable proportion of respondents reported waking at late hours of the night for prayers or other activities at least once (47.0 %), two times (22.6 %), three times (6.8 %), and four times (3.0 %) (Table10). Moreover, 80.4 % of the respondents were storing buckets with water inside sleeping rooms, with 43.7 % of those leaving the buckets unclosed (Table 11).
Table 10: Night self-study start and bedtime across the four HEIs
|
|
Name of Institution |
Total |
||||
|
JORDAN |
MUSLIM |
MZUMBE |
SUA |
|||
|
a) Self-study starting time |
Before 2300 pm % |
31 96.9 |
50 87.7 |
127 89.4 |
152 91.0 |
360 90.1 |
|
After 2300 pm % |
1 3.4 |
7 12.3 |
15 10.6 |
15 9.0 |
38 9.9 |
|
|
Total |
32 |
57 |
142 |
167 |
398 |
|
|
b) Bedding time |
Before 2300 pm % |
7 21.9 |
10 17.5 |
8 5.6 |
12 7.2 |
37 9.3 |
|
After 23 pm % |
25 78.1 |
47 82.5 |
134 94.4 |
155 92.8 |
361 90.7 |
|
|
Total |
32 |
57 |
142 |
167 |
398 |
|
Table 11: Proportion of students storing buckets with water (open or closed) in sleeping rooms and frequency of waking up at night out of bed and or room
|
|
Name of Institution |
Total |
||||
|
JORDAN |
MUSLIM |
MZUMBE |
SUA |
|||
|
a) Storing buckets with water in a sleeping room |
Yes (%) |
18 56.3 |
46 80.7 |
120 84.5 |
136 81.4 |
320 80.4 |
|
No (%) |
14 43.7 |
11 19.3 |
22 15.5 |
31 18.6 |
78 19.6 |
|
|
Total |
32 |
57 |
142 |
167 |
398 |
|
|
b) Water buckets normally open |
Yes (%) |
11 34.4 |
38 66.7 |
52 36.6 |
73 43.7 |
174 43.7 |
|
No (%) |
21 65.6 |
19 33.3 |
90 63.4 |
94 56.3 |
224 56.3 |
|
|
Total |
32 |
57 |
142 |
167 |
398 |
|
|
c) Frequency of waking at night out of bed and or room |
0 |
5 |
9 |
36 |
32 |
82(20.6) |
|
1 |
13 |
29 |
58 |
87 |
187(47.0) |
|
|
2 |
13 |
14 |
32 |
31 |
90(22.6) |
|
|
3 |
0 |
5 |
7 |
15 |
27(6.8) |
|
|
4 |
1 |
0 |
9 |
2 |
12(3.0) |
|
|
Total |
32 |
57 |
142 |
167 |
398 |
|
The information gained through direct observation on human and environmental factors associated with malaria transmission.
Upon direct observation, only a small proportion of students were shown to own bed nets and abide by other control measures. Also, most students were not wearing long-sleeved clothes at night except for females at the Muslim University. Across the four study HEIs, it was observed that after self-studies at night, a considerable proportion of students spend 1-2 hours for stories from colleagues and or watching movies and other games. They also wake at night at around 0500 am for prayers and exercises, especially for Muslim University and Jordan, and small proportions for Mzumbe and SUA.
Jordan is surrounded by an open space that is watery with tall grass and shrubs at a distance of about 70 and 90 meters to the study premise and female hostel respectively, and the surrounding community is mainly involved in agricultural activities. Muslim has a paddy field within the campus about 30 meters from the male hostel and the nearby community cultivate maize, rice and vegetables. For SUA, most agricultural activities are within the campus as part of training. Mzumbe has five big sewerage ponds about 250 meters from the study premises and the surrounding community is highly involved in agricultural activities such as maize, nuts, cassava, rice, and vegetables.
SUA had many potential mosquito breeding sites (74) adjacent to the campus, followed by MUM (37), MU (37), and JUCO (33). Nonetheless, buildings are not sprayed with residue insecticides targeting mosquitoes, and the possible surrounding breeding sites are not well handled. Instead, the institutions practice only an annual fumigation targeting other insects such as bed bugs, cockroaches, ticks, flies, etc.
Discussion
Knowledge on malaria
The overall result of this study has demonstrated a considerable low level of knowledge on malaria among respondents (58.8 %), whereby females were shown to have high knowledge by 46.5 percent compared to 35.9 percent for males (Table 3). This corresponds to the study in the rural community of Tanzania that revealed 36.8 % of the study subjects were knowledgeable, for which women had more knowledge than males (38.8 % and 33.7 %, respectively) [16]. Respondents from SUA differed significantly in knowledge on malaria compared to other Institutions (Table 2), and there was no significant difference between students living on campus and those living off-campus (Appendix 1). This difference could be attributed to the nature of courses offered with respect to the Institutions.
Also, the majority of respondents understand that they can easily get malaria infection in mid and late-night (2300 pm - 500 am) and do not bother about the early morning and late evening mosquito bites. This corresponds with the study done in South-eastern Tanzania [13], for which all respondents believed the critical time at which a person can easily be infected with malaria was between midnight and 200 am ('usiku wa manane’ a Swahili slogan). Likewise, the study carried out in the Morogoro, and Dodoma regions of Tanzania reported a 78.8 % of respondents were unaware that early mosquito bites could cause malaria [20].
Also, less than half of the respondents understand correctly how malaria is transmitted to humans, and about a quarter of respondents understand precisely three diseases that present with fever (Table 9). This relates to a study conducted in Eastern Tanzania that revealed low knowledge level among respondents on non-malaria illnesses presenting with fever [9]. About 31.9 % of respondents understand correctly the typical signs and symptoms of malaria in humans. This resembles a study in Nigeria [1] that reported a 26.4 % of respondents were knowledgeable on the common signs and symptoms of malaria, and upon mentioning severe symptoms, the knowledge level declined drastically to 13.1 %, 13.5 %, and 7.9 % for convulsion, coma, and anemia respectively.
Control practices
The use of mosquito nets among all study participants was 42 % (Table 4) and for individual Institutions was 50 %, 56.1 %, 43.7 %, and 34.1 % for Jordan, Muslim, Mzumbe, and SUA respectively. Those who do not use mosquito nets presented with some reasons; No money (20.6 %), Suffocation or health problems (14.6 %), and Dislike (22.8 %). Likewise, the study in South-East Nigeria found that 40.5 % of respondents slept under mosquito nets (both treated and untreated) [8] and 22.1 % reported by Ojurongbe Taiwo Adetola1 [1], 18 % in Kavango East, Namibia [14] and 44.7 % for bed nets use and 4.5 % for body spray or ointment on daily bases while the rest infrequently use cream or spry or do not bother at all [27].
This is contrary to a study conducted in Mainland Tanzania that reported 70 % of primary school children used mosquito nets [6]. This difference might be due to the fact that pupils are under close parental care and usually obey their parents' orders. Nevertheless, children seem to be keen when are informed of a possible danger compared to young adults who are more likely to neglect on some matters.
The widespread use of Personal mosquito repellents before bedding among students in all respondents was 7.8 percent, the use of insecticides or mosquito repellents in a sleeping room was 27.1 %, and the type of clothes worn by students during the night before going to sleep was 38.7 % and 61.3 % for long and short garments respectively (Table 5).
Treatment seeking behaviour
About 30.7 % of respondents visit health care facilities after noticing a fever, while the rest, 69.3 %, opt for self-remedies such as praying, sleeping, drinking fluids, especially water, eating fruits and leaves, exercising and taking anti-pains/antipyretics. About 91.5 % used antimalarial medicines after being diagnosed of having malaria (Table 6), for which ALU/MSETO was the most used drug (66.3 %) among others (Table 10), and the majority seemed to complete their full courses of the doses. This is similar to the study done in the Rural Northwest Tanzania [21] and in Kilosa District-Morogoro [9]. However, males are more likely to use antimalarial medicines without medical advice than females (52.2 % and 38 %, respectively) [16]. Also, a study in Mozambique showed a low level of treatment-seeking habits [5], and the study was done in South-East Nigeria revealed a 63.0 % of respondents purchased any antimalarial medicine available at a shop, and 85.2 % of the women would complete their prescribed dose [8].
Generally, people buy drugs from nearby shops or stores when sick and tend to go to the hospital when the condition is unbearable, as revealed in Nigeria [22].
Malaria transmission risk factors
Environmental factors
The most favourable places for self-studies were as follows; Classes (33.7 %), Room (31.9 %), Hostel (19.8 %), Library (12.6 %), and Hall (2.0 %), as shown in Table 7. About 75.4 % of respondents seemed to live in sleeping rooms having windows fixed with mosquito-proof wire mesh, 67.1 % sleep indoors and roofs fixed properly not to allow mosquito entry and 18.6 % of respondents understand correctly the common risk factors for malaria transmission in human (Table 9).
Generally, living in houses that are not sprayed or with no indoor mosquito control measures employed, together with low understanding on transmission risk factors increase the risk for malaria infection [30,26].
Human factors: The majority of respondents (90.1 %) demonstrated starting their self-studies at a time before 2100 pm, for which 90.7 % of respondents shown to bed after 2100 pm (Table 10) and waking at night for prayers, washroom services, or other activities out of bed at; One time (47.0 %), Two times (22.6 %), Three times (6.8 %) and Four times (3.0 %) as summarized in Table 11. This corresponds with a study in South‑Eastern Tanzania on outdoor malaria transmission risks and a social life that increased risk with increasing time spent outdoors, mostly at night [23]. About 80.4 % of respondents used to store water in buckets within their sleeping rooms, while 43.7 % of respondents left their buckets unclosed. This correlates with the study done in South-East Nigeria, where 33.1% of respondents kept water in their sleeping rooms with their containers closed [8].
Conclusions
The results from this study have revealed the prevailing low level of knowledge on malaria among respondents of the selected higher education institutions within Morogoro Municipality. The low level of knowledge attributes to poor adherence to malaria control measures such as use of mosquito nets, repellents of all types and delay in seeking medical care, and the presence of multiple positive mosquito breeding sites around the institutions. All these situations pose a great risk of exposure for malaria transmission among students within respective institutions and the surrounding community. Thus, there is a need for urgent measures to be taken in order to achieve a healthy and malaria free community.
Recommendation
From the study findings, we would recommend that higher education institutions should design imperative awareness campaigns and control strategies that are most appropriate to students and should be updated regularly; the following suggestions can help;
- Initiation of a special course on malaria that should be integrated in the usual curriculum and be mandatory to every student or be imbedded as one of the essential topics in general studies.
- Offer special packages to students on malaria control measures upon registration. The package should include a treated mosquito net and mosquito repellents (creams, coils/mats, or spray); it should be offered at the same time when a student is given a living room, bed, and mattress. In case the institution can't afford to provide it, it can think of adding some few amounts to the "other fee section" and or find some stakeholders who can chip in to rescue this younger generation.
- The Government should allocate special funds and or formulate suitable policies that will help to reduce the risk of malaria transmission among university students.
- Institutions should consider spraying their buildings with Residue Insecticides (RI) and larvicides that will target the elimination of adult mosquitoes and the control of surrounding mosquito breeding sites with appropriate means.
List of abbreviations
ALU: Artemether-lumefantrine
BBC: Behavioural Change Communication (BCC)
CL: Confidence Level
EASTC: The Eastern Africa Statistical Training Centre
HEIs: Higher Education Institutions
IEC: Information Education Communication
JUCO: Jordan University College
mRDT: Malaria Rapid Diagnostic Test
MU: Mzumbe University
MUM: Muslim University College of Morogoro
N: Number
P: Level of significance
SUA: Sokoine University of Agriculture
WHO: World Health Organization
Ethics approval and consent to participate
The ethics approval for conducting this study was obtained from the Research and Publication Committee of Sokoine University of Agriculture (SUA) (Ref. SUA/DPRTC/R/06). The study was conducted upon permission by the senior management of all study HEIs. The respondents were introduced to the purpose and data collection procedures before participating in the study. All respondents gave verbal and written consent before participating in the study. To ensure maximum confidentiality, questionnaires and respondents were assigned unique identification numbers instead of their actual names.
Availability of data and materials
The dataset used and analyzed, and the materials collected during the current study are available from the corresponding author on reasonable request.
Competing interests: The authors declare that they have no competing interests.
Funding: This study was self-funded.
Authors' contributions
Mbogo N. Kija (MNK) and Ladslaus L. Mnyone (LLM) conceived and designed the study. MNK, Sharadhuli I. Kimera (SIK), and LLM analyzed the data and coordinated the work. MNK wrote the initial draft of the manuscript, and LLM and SIK critically revised the manuscript. All authors have read and approved the final version of the manuscript.
Acknowledgments
We would like to thank all respondents for agreeing to participate in the study. We also thank the senior management of all study HEIs for allowing us to conduct the study in their respective institutions and their cooperation throughout the study. The authors wish to especially thank the staff from the study HEIs, College of Veterinary Medicine and Biomedical Sciences, and Institute of Pest Management (IPM), who contributed to this study in one way or another. Also, thanks to the interviewer's team for their excellent job, namely Mr. Castory Cosmas, Mr. Sadick Kakomo, Mr. Zam Mdindikasi, Mr. Samson Mateo and Mr. Samuel Josephat. Finally, special thanks and appreciation be to Dr. Majaliwa John from ESTC for his valuable contribution in data analysis.
References
- Adetola OT, Aishat II, Olusola O (2014) Perception and treatment practices of malaria among tertiary institution students in Oyo and Osun states, Nigeria. Journal of Natural Sciences Research. 4(5): 33-42.
- Adinan J, Damian DJ, Mosha NR, Mboya IB, Mamseri R, et al. (2017) Individual and contextual factors associated with appropriate healthcare seeking behaviour among febrile children in Tanzania. PLoS One. 12(4): e0175446.
- Awolola TS, Adeogun A, Olakiigbe AK, Oyeniyi T, Olukosi YA, et al. (2018) Pyrethroids resistance intensity and resistance mechanisms in Anopheles gambiae from malaria vector surveillance sites in Nigeria. PloS One. 13(12): e0205230.
- Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, et al. (2015) The effect of malaria control on Plasmodium falciparum in Africa between 2000 and2015. Nature. 526(7572): 207-211.
- Cassy A, Saifodine A, Candrinho B, Martins MDR, da Cunha S, et al. (2019) Care-seeking ehavior and treatment practices for malaria in children under 5 years in Mozambique: a secondary analysis of 2011 DHS and 2015 IMASIDA datasets. Malaria journal. 18(1): 115.
- Chacky F, Runge M, Rumisha SF, Machafuko P, Chaki P, et al. (2018) Nationwide school malaria parasitaemia survey in public primary schools, the United Republic of Tanzania. Malaria Journal. 17(1): 452.
- Challet GL (1994) Mosquito abatement district programs in the United States. Gaoxiong Yi Xue Ke Xue Za Zhi. 10(Supp l): S67–73.
- Chinweuba AU, Agbapuonwu NE, Onyiapat JE, Israel CE, Ilo CI, et al. (2017) Determinants of malaria prevention and treatment seeking behaviours of pregnant undergraduates resident in University Hostels, South-East Nigeria. Journal of Pregnancy. 2017: 3653874.
- Chipwaza B, Mugasa JP, Mayumana I, Amuri M, Makungu C, et al. (2014) Community knowledge and attitudes and health workers’ practices regarding non-malaria febrile illnesses in eastern Tanzania. PloS Neglected Tropical Diseases. 8(5): e2896.
- Conroy R (2015) Sample size a rough guide.
- Dale PE, Carlson DB, Easton CS (2008) Four degrees of latitude: mosquito control on the “right” coasts of Australia and Florida, USA. J Am Mosq Control Assoc. 24(3): 427–37.
- Deressa W, Yihdego YY, Kebede Z, Batisso E, Tekalegne A, et al. (2014) Effect of combining mosquito repellent and insecticide treated net on malaria prevalence in Southern Ethiopia: a cluster-randomised trial. Parasit Vectors. 7: 132.
- Finda MF, Moshi IR, Monroe A, Limwagu AJ, Nyoni AP, et al. (2019) Linking human behaviours and malaria vector biting risk in south-eastern Tanzania. PloS One. 14(6): e0217414.
- Jacob V, Nuuyoma V (2019) Knowledge, Attitudes and Practices of the University Students on Malaria Prevention in Kavango East, Namibia. Global Journal of Health Science. 11(2): 102-109.
- Kessy ST, Mnyone LL, Nyundo BA, Lyimo IN (2020) Passive Outdoor Host Seeking Device (POHD): Designing and Evaluation against Outdoor Biting Malaria Vectors. The Scientific World Journal. 2020: 4801068.
- Kigodi, K. N. and Komanya, M. (2006). Malaria and anti-malarial drugs ehavior on among adults in a rural coastal community of Tanzania: Knowledge, attitude and practice study. Medical Students’ Journal 14(1): 4 – 9.
- Killeen GF, Kiware SS, Okumu FO, Sinka ME, Moyes CL, et al. (2016) Going beyond personal protection against mosquito bites to eliminate malaria transmission: population suppression of malaria vectors that exploit both human and animal blood. BMJ Global Health. 2 :e000198
- Killeen GF, Tatarsky A, Diabate A, Chaccour CJ, Marshall JM, et al. (2017) Developing an expanded vector control toolbox for malaria elimination. BMJ Global Health. 2(2): e000211.
- Kothari CR (2004) Research Methodology. (Second Edition). New Age International Publishers. New Delhi. India. 418.
- Mathania MM, Kimera SI, Silayo RS (2016) Knowledge and awareness of malaria and mosquito biting ehavior in selected sites within Morogoro and Dodoma regions Tanzania. Malaria Journal. 15(1): 287.
- Mazigo HD, Obasy E, Mauka W, Manyiri P, Zinga M, et al. (2010) Knowledge, attitudes, and practices about malaria and its control in rural northwest Tanzania. Malaria Research and Treatment. 2010: 794261.
- McCloud RF, Okechukwu CA, Sorensen G, Viswanath K (2016) Beyond access: barriers to internet health information seeking among the urban poor. Journal of the American Medical Informatics Association. 23(6): 1053-1059.
- Moshi IR, Manderson L, Ngowo HS, Mlacha YP, Okumu FO, et al. (2018) Outdoor malaria transmission risks and social life: A qualitative study in South-Eastern Tanzania. Malaria Journal. 17(1): 397.
- Msugupakulya BJ, Kaindoa EW, Ngowo HS, Kihonda JM, Kahamba NF, et al. (2020) Preferred resting surfaces of dominant malaria vectors inside different house types in rural South-Eastern Tanzania. Malaria Journal. 19(1): 22.
- Muangphrom P, Seki H, Fukushima EO, Muranaka T (2016) Artemisinin-based antimalarial research: application of biotechnology to the production of artemisinin, its mode of action, and the mechanism of resistance of Plasmodium parasites. Journal of Natural Medicines 70(3): 318-334.
- Naing PA, Maung TM, Tripathy JP, Oo T, Wai KT, et al. (2017) Awareness of malaria and treatment-seeking ehavior among persons with acute undifferentiated fever in the endemic regions of Myanmar. Tropical Medicine and Health. 45(1): 31.
- Nyahoga Y, Bochkaeva Z (2018) Cross-study of malaria prevalence in history, bed net utilization, and knowledge about the disease among Tanzanian College Students. Malaria Research and Treatment. 2018: 8137051.
- Kassile T, Lokina R, Mujinja P, Mmbando BP (2016) Determinants of delay in care seeking among children under five with fever in Dodoma Region, Central Tanzania: A cross-sectional study. Malaria Journal 13: 348.
- USAID. President’s Malaria Initiative Tanzania (Mainland) Malaria Operational Plan FY 2019. Malaria Operational Plan Report. United States Agency for International development, Geneva 134pp.
- West PA, Protopopoff N, Rowland M, Cumming E, Rand A, et al. (2013) Malaria risk factors in Northwest Tanzania: The effect of spraying, nets and wealth. PloS One. 8(6): e65787.
- WHO (2019) World Malaria Report. World Health Organization, Geneva. 232pp.
- WHO (2020) World Malaria Report. World Health Organization. Geneva. 134pp.