Nazma Hamid1, Usman Ali2, Anwar Awan3*
1Department of Nursing, Shifa Tameer-e-Millat Islamabad-44000 Pakistan
2Department of Microbiology, Abasyn University Peshawer-25000 KPK-Pakistan
3Assistant Professor, Pakistan Institute of Medical Sciences Islamabad-04485 Pakistan
*Corresponding Author: Anwar Awan, Assistant Professor, Pakistan Institute of Medical Sciences Islamabad-04485 Pakistan.
Abstract
The current research study was focused on the identification, isolation, and antibiotic resistance of hospital environment microbes associated with HCAI in healthcare facilities in Islamabad, Pakistan. This research was conducted in the Microbiology Research Laboratory (MRL) at Pakistan Institute of Medical Sciences (PIMS) Islamabad from September 2021 to November 2022. 100 samples of environmental surfaces (e.g., lab instrument, dental chair, first aid room, and labor room delivery table) were collected from 18 government and 2 private hospitals. Various types of culture media Nutrient Agar, broth, MSA, and EMB were used to grow multiple microbes. Then, gram staining and biochemical tests were conducted for identification. The antibiotic analysis was conducted through a disk diffusion essay. This study was carried out with 4 sample portions, each heaving 25 sub-samples, which further sum up 100 samples. Out of the total 100 samples, 86 models were positive, while 14 models were negative. The isolated bacterial species were S. aureus (52), S. epidermidis (06), E. cloacae (34), E. aerogens (23), P. aurogensa (03), and E. coli (13). The Staphylococcus and Enterobacter acrogens) P. acrogens and E. coli were resistant against ETP, CAZ, FD, TZP, MEM, CFM, FOX, and AZM, while these bacteria were susceptible to UN2, CIP, and CRO. The use of antimicrobials in healthcare has the potential to reduce bacterial contamination of the healthcare environment and reduce HCAIs. It is recommended that the emergence of antibiotic resistance in HCAIs pathogenic bacteria is associated with a significantly increased frequency of adverse outcomes. So, Efforts should be directed toward early detection and prevention of the emergence of antibiotic resistance in HCAI bacteria and other pathogenic organisms.
Keywords: Isolation, Identification, Healthcare-Associated Infections (HCAIs), antibiotic resistance, and antimicrobials.
Introduction
Healthcare-associated infections (HcAIs) and nosocomial-associated infections harm medical outcomes in hospitalized admitted cases and are a major global problem. In hospitalized patients, (HcAIs) are the primary cause of morbidity and mortality rates in patients [1]. Infections that occur through or before treatment but remain non-existent or incubating during the patient's admittance to a clinic or other health-related facility are HAIs. Infections are the most common symptom among hospitalized patients and are often a deciding factor in patient outcomes [2]. (HcAIs), the main problems associated with critically ill patients are due to lowered host defense mechanisms, repeated use of invasive medical equipment, management of various medications, cross-transmission of pathogenic microbes between many patients and employees, and insufficient infection prevention and control procedures [3]. Healthcare pathogens have long been a concern for healthcare. Numerous clinics have put in place infection tracking and surveillance systems and effective prevention measures to reduce the incidence of hospital-acquired infections. Multidrug-resistant illnesses have been linked to healthcare diseases; as a result, their impact is felt at the level of the individual patient and the community. Identifying people with health risks is crucial to prevent and minimize hospital-acquired infections and multidrug-resistant illnesses [4].
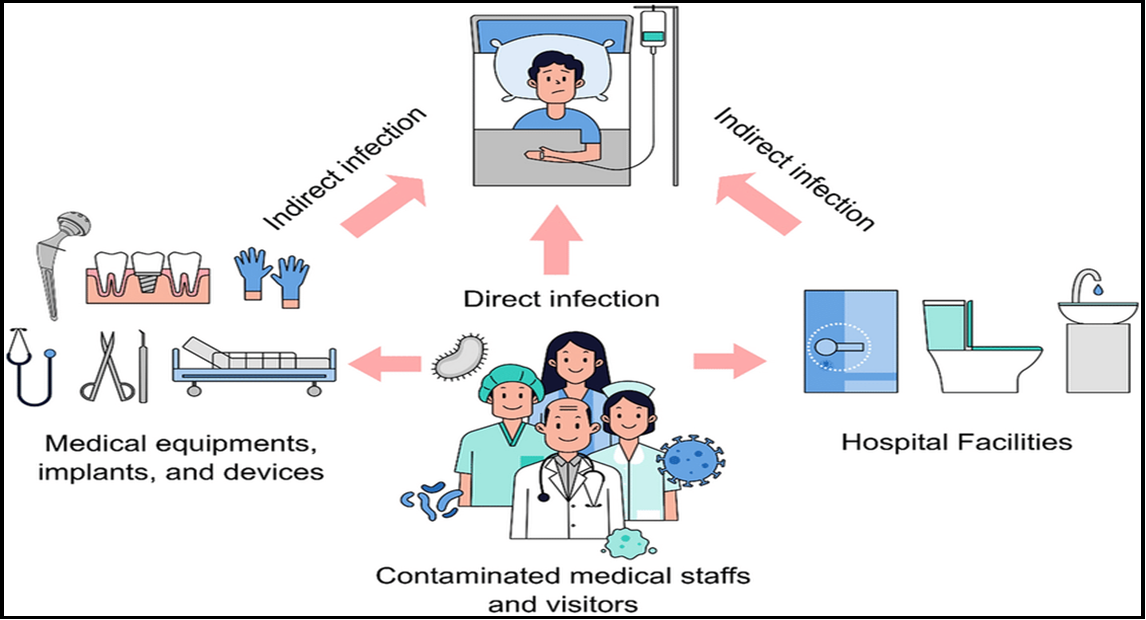
Figure 1: Diagram of the nosocomial infection or healthcare-associated infection (HCAI) transmission channels [3]
Infectious diseases people get while receiving medical treatment are called "health-care-associated infections" (HCAIs). Contagious diseases acquired in various environments where patients receive medical care have become covered by the word "HCAIs," such as protracted treatment, primary care health centers, home healthcare, and outpatient centers. The term "HCAIs" typically refers to those infectious diseases associated with hospitalization in a hospital setting (previously known as nosocomial infectious diseases) [5]. HCAIs include infections that initially develop two days or over after being admitted to the hospital or within thirty days of receiving hospital treatment [31]. Adverse medication events, HCAIs, and medical complications are the three most frequent categories of adverse outcomes impacting hospitalized patients, according to numerous publications. The USA Centre for Disease Prevention and Control estimates that every year, almost 1.68 million hospitalized patients experience Healthcare-associated infections after undergoing care for other health conditions and that approximately 97,700 of such patients (one each in 18) pass death owing to HCAI-related reasons [6]. HCAIs are among the top nine causes of death in the US, according to the Organization for Healthcare Standards and Improvement, and they are also one of the most frequent side effects of hospital treatment. Of every 100 admitted patients, 7 in developed nations and 10 in undeveloped countries suffer from HCAIs. Another research conducted in rising economies revealed that 5%-15 percent of hospitalized experience Healthcare-associated infections, which can harm patients in intensive care units (ICUs) by up to 37%. (ICUs) [7]. According to several research, the incidence rates of Healthcare-associated infections across all hospitals in Europe ranged from around 4.6% to 8.9%. Furthermore, as the Global Health Organization reported, global attention to Healthcare-associated infection often only arises within outbreaks. Since 3 million HCAI events are identified yearly in Emergency departments separately, HCAIs frequently affect seriously sick patients. Intensive care unit patients are more likely to be seriously ill and immunocompromised, making them more vulnerable to Health care-associated infections [8].
According to a review done in 193 American health facilities with 12,262 subjects, 6% of all patients had at least 1 HCAI, with Clostridium perfringens representing the most predominant pathogen. Respiratory, bacterial overgrowth and surgical-site infections (SSIs) represented the majority of infectious diseases. In research done by the same researchers two years prior, it was shown that 8% (54) of individuals developed Healthcare-associated infections, with the majority (69.97%) developing SSIs, Urinary infections, pneumonitis, and circulatory diseases. The bacterium that was found most commonly was S. aureus. In a paper comparing 2010 and 2015, the team discovered a significant statistical (P 0.05) decline in HCAIs in SSIs, Urinary infections, and catheter insertion infections, likely the result of a planned network [9].
Antibiotic mismanagement and overdoing in medication and agriculture have resulted in the emergence of multidrug-resistant (MDR) microorganisms, which are increasingly recognized as a foremost source of hospital-related infections [30]. MDR bacterial infections are linked to an upper hazard of reduced clinical consequences and loss and an increased financial burden on patients [10]. For appropriate treatment, it's critical to understand the medical features, occurrence, and spread of nosocomial-related infections produced by MDR microorganisms. Multiple current studies demonstrate that environment-related contaminations are significant in the nosocomial spread of MDROs, viruses, mycobacteria, and fungi [11]. Several nosocomial pathogens are proven to present and occur in the environment for long periods. A further precise explanation of MDRs has been offered as 'non-sensitivity (resistance, intermediate-ability, or non-susceptibility outcome) to a minimum one mediator in four or additional antimicrobial groups. Pan-drug resistance (PDR) was described as "non-susceptibility to all agents in all antimicrobial categories" in the same research [12]—methicillin-resistant Staph aureus (MRSA). The Salmonella species, Shigella species, Campylobacterium, Helicobacter pylori, Mycobacterium bio vis, E. coli strains, Listeria monocytes, Actinobacter baumannii, Streptos pyogenes, Strepto mutants, Staph aureus, Vibrio cholerae, and Yersinia pestis are some of the bacteria found in healthcare-associated Infections. Due to a weakened immune system and various other factors, these bacterial species cause opportunistic infections in individuals with healthcare-associated disorders [13].
MDR organisms show resistance to more than one antimicrobial agent in vitro antimicrobial susceptibility testing in the strictest meaning. MDR microorganisms are Gram-positive and harmful microorganisms that resist two or more antibiotic groups [14]. Antibiotics become less effective due to antimicrobial resistance, making therapy more difficult, expensive, and, in some circumstances, impossible to treat the infection. As a result, the society faces more serious problems and illnesses. Antibiotics of various generations are now utilized against pathogenic bacteria, reducing antibiotic resistance to some extent. Consuming alternative medicine is a beautiful method to avoid antimicrobial resistance [15,29]. Antibiotic resistance quickly increases in areas where people lack good hygiene and take medications inappropriately. One of the key reasons is the rapid movement of individuals from one country to another for various reasons, particularly from areas where resistant bacteria, such as XDR and ESBL, occur [16]. Bacteria that produce MDR are found in Asia, Africa, and South America. According to the most recent combined technical study from collaboration with the Acts of EDA on Antimicrobial sensitivity, at least 3.7 million European patients die each year from infections caused by ESBL and MDR bacteria [17].
The objectives were to isolate and Identify bacteria in a hospital environment associated with healthcare-associated infections. Furthermore, to explore Antibiotic susceptibility Profiles profile of the isolated bacteria.
Research Methodology
The current research study was focused on the identification, isolation, and antibiotic resistance of hospital environment microbes associated with HCAI in healthcare facilities in Islamabad, Pakistan. This research was conducted in the Microbiology Research Laboratory (MRL) at Pakistan Institute of Medical Sciences (PIMS) Islamabad from September 2021 to November 2022. This study focused on identifying hospital environment microbes associated with HCAI in healthcare facilities, assessment, and antibiotic profiles. From September to November 2021, samples of 100 environmental surfaces (e.g., lab instrument, dental chair, first aid room, and labor room delivery table) were collected from healthcare institutions through standard vaccine carriers. The samples were collected from various hospitals in Islamabad.
Different types of culture media were utilized for the growth of various microorganisms isolated from multiple materials [28]. According to [18], isolated pure colonies were evaluated after incubation and subculture on fresh culture media for additional confirmation. On nutritional agar, pathogenic bacteria were isolated using a serial dilution approach. Pathogenic bacteria were isolated and formed into a colony, from which distinct bacterial species were isolated based on their morphology, Gram's reactivity, and biochemical tests [19]. The identification was achieved through gram staining [20] and microscopy [21]. The subsequent biochemical identification tests were performed to check the presence of microorganisms [22]. According to CLSI 2015, indole [24], Triple Sugar Iron (TSI) [25], urease, oxidase [26], catalase [27], and coagulase [28] are all enzymes.
The Kirby-Bauer disc diffusion technique was used to test antibiotic sensitivity. The Clinical Laboratory Standards Institute (CLSI) 2015 standard protocols were followed. Antibiotics of various concentrations (amoxicillin groups, cyclosporine, Vancomycin, Gentamycin, cephalosporin, Tazobactam, Cefotaxime, Ciprofloxacin, Doxycycline, Chloramphenicol, Carbapenam, tetracycline) were tested. Antibiotics were tested against isolated pathogenic bacteria depending on the type of bacteria recovered [23].
Results
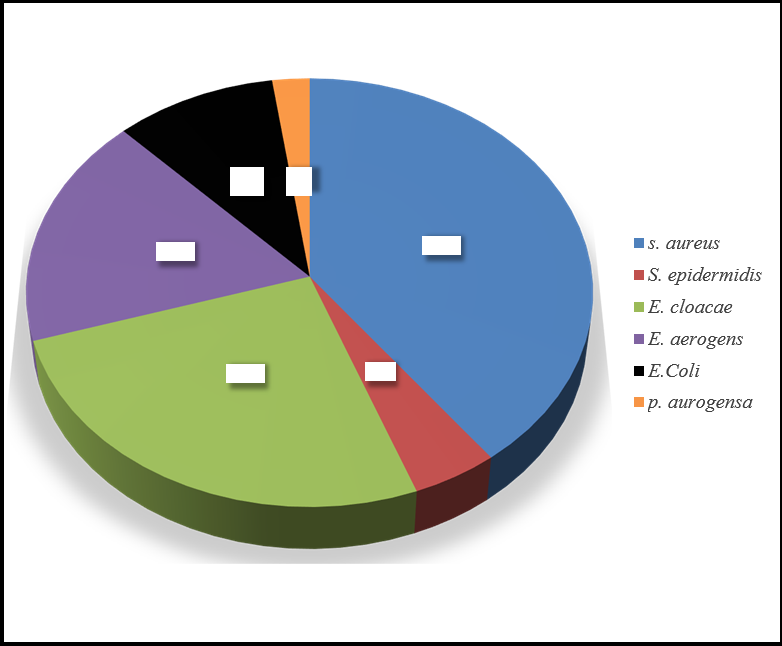
The current research study was focused on the identification, isolation, and antibiotic resistance of hospital environment microbes associated with HCAI in healthcare facilities. This study was carried out with 4 sample portions, each heaving 25 sub-samples, which further sum up 100 samples. Out of the 100 samples, 86 models were positive, while 14 were negative. S. aureus (52), S. epidermidis (06), E. Coli (13), E. cloacae (34), E. aerogens (23) and P. aurogensa (03). Similarly, A cross-sectional study by Gashaw, Abtew, & Addis [14] was conducted from March 2013 to May 2013 at 7 health centers that are found around Gondar town, Addis Ababa Ethiopia isolated Staphylococci, S. aureus, E. cloacae. Citrobacter and E. coli were the most frequently isolated bacteria. Another study conducted by Tajeddin [5] isolated K. pneumoniae, S. aureus, S. aureus, P. aeruginosa, E. coli, A. baumannii, S. epidermidis, and Streptococciin healthcare-associated infections.
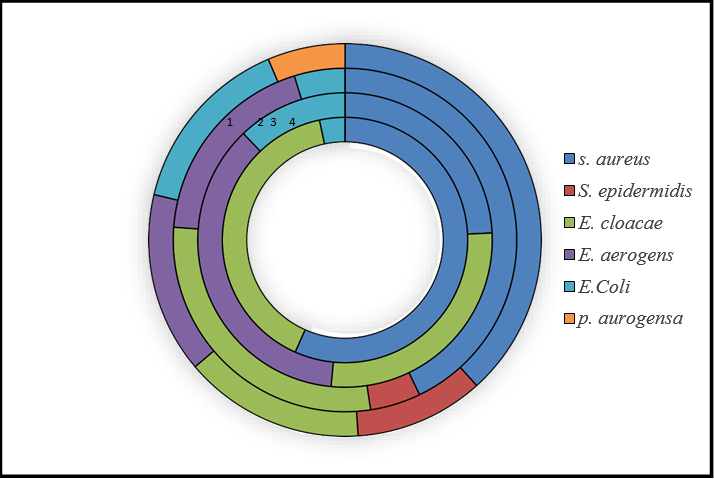
In the first 25 sub-samples, the PRL has one, RDB has 1, and RLN has 2 negative samples. RSK has all positive models, while the TOG series has two negative samples on Nutrient agar and broth. Then, each illustration was cultured through MSA and EMB media and with different biochemical examinations in which staphylococcus aureus 17, Enterobacter cloacae 12, and E. coli were 1. These were the microbial isolation and identification outcomes. Bacteria were isolated with nutrient agar and broth, while isolated bacterial species were identified through biochemical tests with MSA and EMB.
The 2nd sample consisted of 25 sub-samples, divided into 5 series: K, RCK, RTS, TBF, and W, each having 5 pieces. On MSA, the K series had two samples positive for staphylococcus aureus, RCK samples were negative on MSA, and RTS and TBF were 2 samples positive each for S. aureus. The W has only one selection that showed growth on MSA. K samples have 4 positive on the EMB medium, which has two types of bacteria, Enterobacter aerogens and Enterobacter cloacae. RCK two pieces were positive for Enterobacter aerogens and three samples for E. coli. RTS and TBT have all positive models with two types of bacteria, Enterobacter aerogens and Enterobacter cloacae, while the W also has all positive samples on EMB and W have Enterobacter aerogens, Enterobacter cloacae and E.Coli. Each model series was cultured through MSA and EMB media and with different biochemical examinations in which staphylococcus aureus 7, Enterobacter aerogens were 11, Enterobacter cloacae 9, and E.Coli were 4. The 3rd sample also has 5 series, namely GMN, RS, RBL, RCB, and RNA, each having 5 pieces. The GMN series samples were negative on MSA, RS samples had two positive, and three were negative on MSA having staphylococcus aureus while RBLsamples were all negative, and the RCB two pieces were positive for S. aureus. The RNA samples showed growth on MSA, having two S. aureus and one staphylococcus epidermidis. On the EMB medium, GMN has only one piece cheerful, which have bacterial specie Enterobacter cloacae, while RS has 4 samples positive for Enterobacter cloacae. RBL one samples positive having E. coli. RCB and RNA both have two positive pieces, each with bacteria Enterobacter aerogens and Enterobacter cloacae. The isolated bacterial species were staphylococcus aureus 6, staphylococcus epidermidis 1, Enterobacter erogens 4, Enterobacter cloacae 5, and E.Coli were 1 through MSA and EMB media and with different biochemical examinations.
In the last and final 4th sample, the series names PMC, PKG, TBL, RKKS, and TGH. PMC samples have two staphylococcus aureus and two staphylococcus epidermidis through MSA. On EMB, PMC has 4 positives: Enterobacter cloacae and Enterobacter aerogens. PKG has 4positive pieces consisting of staphylococcus aureus and two staphylococcus epidermidis, and 4 positives on EMB, which consists of Enterobacter aerogens and E. coli. TBL has 3 positive samples on MSA and 5 positive samples on EMB, with three types of bacteria: staphylococcus aureus, E. coli, and Pseudomonas acrogens. The RKKS series has one selection adverse on MSA, and all are positive on EMB, consisting of staphylococcus aureus, Enterobacter cloacae, and E. coli. The TGH series has one sample negative and 4 positive on MSA 3 staphylococcus aureus, and one staphylococcus epidermidis, and all are positive on EMB consisting of Enterobacter cloacae and E.Coli.
Figure 2: shows overall bacterial species distribution.
Figure 3: shows bacterial species distribution in sample series.
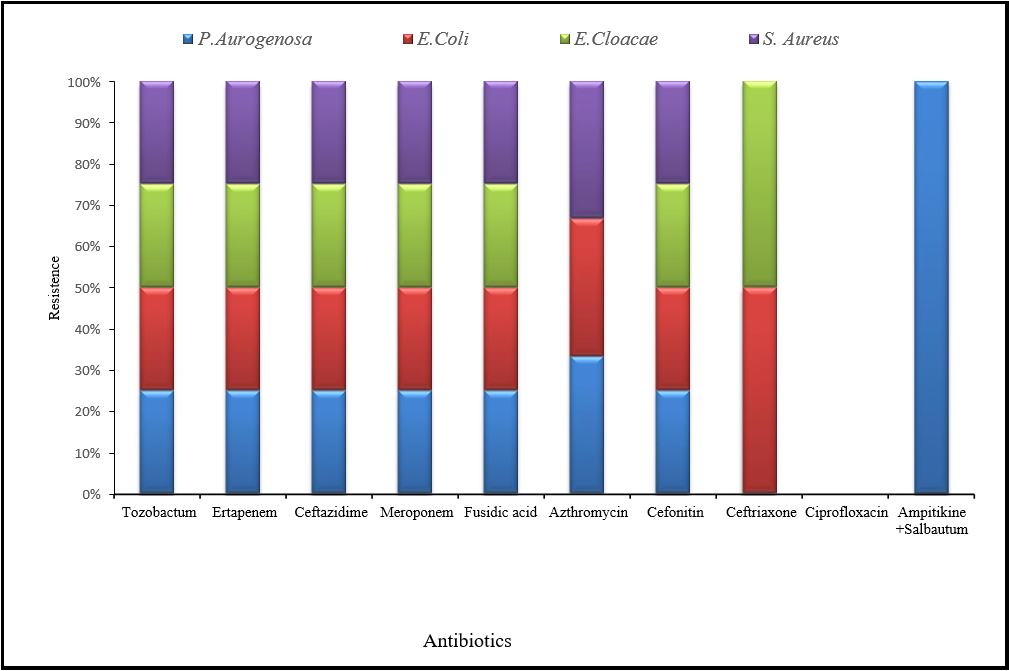
After isolating bacteria through nutrient medium and for further differentiation, MSA, EMB, Gram staining, and biochemical examination were performed. The antibiotic susceptibility was performed against selected bacterial species. The antibiotics were used in this current microbiological study were Ampitikine+Salbautum (UN2), Meroponem (MEM), Ceftriaxone (CRO), Cefixime (CFM), ciprofloxacin (CIP), Azthromycin (AZM), Ceftazidime (CAZ), Ertapenem (ETP) Fusidic acid (FD) Tazobactum (TZP) and were Cefonitin (FOX). Antibiotic discs were checked against staphylococcus in Petri plates, which were showed to be fully resistant against Ertapenem (ETP), Fusidic acid (FD), Tazobactum (TZP), Meropenem (MEM), Cefixime (CFM), Cefoxitin (FOX) and Azithromycin (AZM). Staphylococcus was susceptible or showed no resistance against Ampitikine +Salbautum (UN2), which produced a 10mm zone of inhibition, and Ceftriaxone (CRO), which had a 17mm zone of inhibition. The Enterobacter species showed fully resistant Ertapenem (ETP), Fusidic acid (FD), and Tazobactum (TZP) in all samples. E.Coli were resistant against Ceftazidime (CAZ), Cefixime (CFM), Meropenem (MEM), Azithromycin (AZM), and Cefonitin (FOX), while susceptible to Ceftriaxone (CRO) produce 17mm zone of inhibition, Ampitikine +Salbautum (UN2) was created 22 mm zone of inhibition and ciprofloxacin (CIP) was build 35 mm zone of inhibition. Enterobacter cloacae were showed resistance against Cefixime (CFM), Meropenem (MEM), and Cefonitin (FOX), which susceptible against ciprofloxacin (CIP) was produced 17 mm zone of inhibition, Azithromycin (AZM) was created 10 zones of inhibition and Ceftriaxone (CRO) had 22 mm zone of inhibition.
Figure 4: shows the resistance against all antibiotics used in the current study for isolated bacteria.
Pseudomonas autogenous was resistant against Meroponem (MEM), Ampitikine +Salbautum (UN2), Azithromycin (AZM), Cefixime (CFM), and Cefonitin (FOX), while susceptible to Ceftriaxone (CRO) which was produced 32 mm zone of inhibition and ciprofloxacin (CIP) which was built 17 mm zone of inhibition. Similarly, a study by Xia, Gao, & Tang [10] stated that Staphylococcus aureus are the most common nosocomial infection-related pathogens and are resistant to a wide range of medicines, including penicillins, ertapenem, and cefixime. A study conducted by Anago et al. in [32] revealed that the E. coli were resistant to antibiotics and were imipenem followed by ceftriaxone. Another study in Iran by Hashemi et al. [7] stated that Enterobacter 98.8% of them were resistant to ampicillin, but just 3.7% were to piperacillin. Most isolates were also resistant to cefixime, cefazolin, and co-trimoxazole. The most resistance to ceftriaxone and the least resistance to ceftizoxime were found among third-generation cephalosporins. Ipenem resistance was present in 19.3% of isolates. Nosocomial and community-acquired infections were resistant to ciprofloxacin to a corresponding extent of 33% and 4.1% in fluoroquinolones. Rezai et al. [23] Stated that Enterobacter cloacae showed high resistance to Cefonitin and was susceptible to cefixime (99%), colistin (82%), and ciprofloxacin (76%).
Conclusion and Recommendation
It is concluded that from a total of 100 samples, 86 samples were positive while 14 models were negative, and the isolated bacteria were S. aureus (52), S. epidermidis (06), E.Coli (13), E. cloacae (34), E.aerogens (23) and P. aurogensa (03).
It was determined that staphylococcus and Enterobacter were resistant against ETP, FD, TZP, MEM, CFM, FOX, and AZM, while staphylococcus and Pseudomonasautogenous were susceptible to UN2, CIP, and CRO. It is recommended that an extremely high frequency of unfavorable results is connected with establishing antibiotic resistance in HCAIs pathogenic microorganisms. Efforts should focus on early diagnosis to avoid the evolution of antibiotic resistance in HCAIs, bacteria, and other harmful organisms. To combat this resistance, well-functioning laboratories, surveillance of AMR (antimicrobial resistance), and proper antibiotic tracking systems are needed. Maintaining the efficacy of antibiotics depends on controlling the spread of resistant bacteria and using antimicrobial medications as directed. Studies on the multi-drug resistant bacteria isolates and ongoing monitoring are crucial actions. A national strategic approach to using antibiotics is also vital if we want to maintain their efficacy in the future.
References
- Antony HA, Parija SC (2016) Antimalarial drug resistance: An overview. Trop Parasitol. 6(1): 30-41.
- Arenz S, Wilson DN (2016) Blast from the past: Reassessing forgotten translation inhibitors, antibiotic selectivity, and resistance mechanisms to aid drug development. Mol Cell. 61(1): 3-14.
- Falagas ME, Koletsi PK, Bliziotis IA (2006) The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa. J Med Microbiol. 55(Pt 12): 1619-1629.
- Yayan J, Ghebremedhin B, Rasche K (2015) Antibiotic resistance of Pseudomonas aeruginosa in pneumonia at a single university hospital center in Germany over a 10-year period. Plos one. 10(10): e0139836.
- Tajeddin E, Rashidan M, Razaghi M, Javadi SS, Sherafat SJ, et al. (2016) The role of the intensive care unit environment and health-care workers in the transmission of bacteria associated with hospital acquired infections. Journal of infection and public health. 9(1): 13-23.
- Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, et al. (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard defnitions for acquired resistance. Clin Microbiol Infect. 18(3): 268-81.
- Hashemi SH, Esna-Ashari F, Tavakoli S, Mamani M (2013) The prevalence of antibiotic resistance of Enterobacteriaceae strains isolated in community-and hospital-acquired infections in teaching hospitals of Hamadan, west of Iran. Journal of Research in Health Sciences. 13(1): 75-80.
- Sokol D (2008) A guide to the Hippocratic Oath.
- Beveridge WIB (1957) The art of scientifc investigation. Chapter IX. Revised Edition. WW Norton & Company, New York. 114.
- Xia J, Gao J, Tang W (2016) Nosocomial infection and its molecular mechanisms of antibiotic resistance. Bioscience trends. 10(1): 14-21.
- McCannon CJ, Berwick DM, Massoud MR (2007) The science of large-scale change in global health. JAMA. 298(16): 1937–9.
- McCannon CJ, Hackbarth AD, Griffin FA (2007) Miles to go: an introducetion to the 5 Million Lives Campaign. Jt Comm J Qual Patient Saf. 33(8): 477–84.
- Benyus J (2018) Brainy Quote.
- Gashaw M, Abtew D, Addis Z (2014) Prevalence and antimicrobial susceptibility pattern of bacteria isolated from mobile phones of health care professionals working in Gondar town health centers. International Scholarly Research Notices. 2014(5): 1-6.
- Iqbal A, Muhammad R, Khan BB, Jamshed A, Rehman MI, et al. (2023) Comparative study of C-reactive protein and complete blood count in cancer and non-cancer patients followed by antibiogram analysis of isolated bacterial pathogens. J Bacteriol Mycol Open Access. 11(1): 1-4.
- Sydnor ER, Perl TM (2011) Hospital Epidemiology and Infection Control in Acute-Care Settings. Clin Microbiol Rev. 24(1): 141– 173.
- Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, et al. (2014) Multistate point-prevalence survey of health care- associated infections. N Engl J Med. 370(13): 1198–208.
- Magill SS, Hellinger W, Cohen J, Kay R, Bailey C, et al. (2012) Prevalence of Healthcare-Associated Infections in Acute Care Hospitals in Jacksonville, Florida. Infect Control Hosp Epidemiol. 33(3): 283–91.
- Magill SS, Wilson LE, Thompson DL, Ray SM, Nadle J, et al. (2017) Reduction in the Prevalence of Healthcare-Associated Infections in U. S. Acute Care Hospitals, 2011. Open Forum Infect Dis. 4(Suppl 1): S49.
- Cai Y, Venkatachalam I, Tee NW, Tan TY, Kurup A, et al. (2017) Prevalence of Healthcare- Associated Infections and Antimicrobial Use among Adult Inpatients in Singapore Acute- Care Hospitals: Results From the First National Point Prevalence Survey. Clin Infect Dis. 64(suppl_2): S61–S67.
- Cassini A, Plachouras D, Eckmanns T, Abu Sin M, Blank HP, et al. (2016) Burden of Six Healthcare-Associated Infections on European Population Health: Estimating Incidence-Based Disability-Adjusted Life Years through a Population Prevalence- Based Modelling Study. PLoS Med. 13(10): e1002150.
- Kritsotakis EI, Kontopidou F, Astrinaki E, Roumbelaki M, Ioannidou E, et al. (2017) Prevalence, incidence burden, and clinical impactof healthcare-associated infections and antimicrobial resistance: a national prevalent cohort study in acute care hospitals in Greece. Infect Drug Resist. 10: 317–328.
- Jamshed A, Iqbal A, Ali S, Ali S, Mamoon (2023) A quick review on the applications of nanomaterials as adsorbents. MOJ Eco Environ Sci. 8(3): 86-89.
- Ling ML, Apisarnthanarak A, Madriaga G (2015) The Burden of Healthcare-Associated Infections in Southeast Asia: A Systematic Literature Review and Meta-analysis. Clin Infect Dis. 60(11): 1690–99.
- Askarian M, Yadollahi M, Assadian O (2012) Point prevalence and risk factors of hospital acquired infections in a cluster of university-affliated hospitals in Shiraz, Iran. J Infect Public Health. 5(2): 169–176.
- Ali S, Ali B, Khan BB, Khan M, Ali S, et al. (2023) Sero- prevalence of hepatitis-c virus among blood donors in northern Pakistan. MOJ Public Health. 12(1): 37-41.
- Santajit S, Indrawattana N (2016) Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. Biomed Res Int. 2016: 2475067.
- Ali A, Sherani MZ, Khan J, Muhammad N, Ullah R, et al. (2020) Resistance of Bacterial Pathogen of Dental clinic against the Antibiotics. Adv. Biores. 11(4): 49-53.
- Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, et al. (2009) Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 49(1): 1–45.
- Iqbal A, Ali S, Asad M, Saeed M, Ullah N, et al. (2022) Perspectives and Applications of Plant Microbe Interactions in Post Genomic Era. Annals of the Romanian Society for Cell Biology. 26(01): 1216-1226.
- Altaf R, Ullah Z, Darko DA, Iqbal A, Khan MS, et al. (2022) Molecularly imprinted polymers for the detection of chlorpyrifos (an organophosphate pesticide). ASEAN Journal of Science and Engineering. 2(3): 257-266.
- Anago E, Ayi-Fanou L, Akpovi CD, Hounkpe WB, et al. (2015) Antibiotic resistance and genotype of beta-lactamase producing Escherichia coli in nosocomial infections in Cotonou, Benin. Annals of clinical microbiology and antimicrobials. 14: 5.