Nucera A1*, Palumbo F1, Giovinazzo C1, Mazzuca E1, Di Bella A1, Micozzi E1, Arnò V1, Ferrazza G1, Piciucchi G1, Ballanti M1, Davato F1, Cardellini M1,2, Proietti G1, Ferlosio A1,3, Rizza S1,2, Federici M1,2
1Department of Medical Sciences, Policlinico Tor Vergata, Rome, Italy
2Department of Systems Medicine, University of Rome Tor Vergata, Italy
3Department of Experimental Medicine, University of Rome Tor Vergata, Italy
*Corresponding Author: Nucera A, Department of Medical Sciences, Policlinico Tor Vergata, Rome, Italy.
Abstract
Isolated pauci-immune pulmonary capillaritis (IPIPC) is a rare disorder characterized by small vessel vasculitis limited to alveolar capillaries in the absence of systemic manifestations. There are very few case reports of this disorder in the medical literature. We report here the case of a 35-year-old male with no known history of autoimmune pathology who was admitted to the hospital for evaluation and treatment of dyspnea and thoracalgia. Peripheral blood cultures, serum studies to detect Legionella and Pneumococcus antigens, and a nasopharyngeal swab test for SARS-CoV-2 were all negative. Chest imaging revealed bilateral pleural effusions from the base to the apices with concomitant atelectasis of the adjacent lung parenchyma. Although the results of an 18F-PET-CT scan revealed no pathological uptake, video-assisted thoracoscopy revealed diffusely edematous pleura with crater-like patches with new onset of venous vessel varicosities. The results of video-assisted thoracoscopic surgery revealed intra-alveolar hemorrhages associated with disordered vascularization, suggesting small vessel vasculitis. Histologic findings included widespread intra-alveolar hemorrhage with organizing injury, hemosiderin-laden macrophages, scattered intra-arterial thrombi, and diffuse perivascular neutrophilic infiltrates consistent with a diagnosis of capillaritis. Given the negative immune studies (save for a weakly-positive lupus anticoagulant [LAC] test) and no evidence for extra-pulmonary vasculitis (as determined by negative findings on retinal fluor angiography, gastroscopy, and computed tomography [CT]-angiography studies), the final diagnosis was isolated pauci-immune pulmonary capillaritis. The patient recovered in response to immunosuppressive/anti-inflammatory therapy.
Introduction
Pulmonary capillaritis is characterized histologically by fibrinoid necrosis of capillary walls with neutrophils and nuclear dust in the fibrin, interstitium, and blood within the alveoli. Inter-alveolar septal capillaries are typically occluded by fibrin thrombi in complexes with interstitial red blood cells or hemosiderin. The most common etiology of this disorder is a systemic autoimmune process.
Isolated pauci-immune pulmonary capillaritis (IPIPC) is a rare clinical entity involving small vessel vasculitis limited to alveolar capillaries in the absence of systemic manifestations.[1] Most cases are characterized by negative results from serological testing for antineutrophilic cytoplasmic antibodies (ANCA). Only eight cases of IPIPC have been reported in the literature [2] and no specific etiology or unique disease mechanisms have been identified. To the best of our knowledge, this is the first case report of a patient diagnosed with IPIPC who presented with clinical manifestations that include bilateral hemothorax and pleuro-pericarditis.
Case Report
The patient was a 35-year-old male who was admitted to the emergency room (ER) of our hospital for evaluation of dyspnea and thoracalgia. Approximately one month before admission, his chest radiograph revealed pulmonary thickening. His primary care physician diagnosed suspected pneumonia and prescribed antibiotics (third generation cephalosporins for one week) and corticosteroids. When evaluated in the ER, the patient was reported to be in a generally good condition. He was awake and alert although feverish (38 °C) and on oxygen therapy (24 % via venti mask [VMK]). His vital signs included a blood pressure of 145/85 mmHg, a heart rate of 120 beats per minute, oxygen saturation (spO2) of 97 % on oxygen (VMK 24 %), and a Glasgow Coma Scale (GCS) score of 15. His cardiac exam revealed tachycardia, with clear tones and free pauses. His lung exam was notable for a vesicular murmur that was diffusely reduced bilaterally and absent at both bases. His skin was pale and dry without lower limb edema. Hepatic and renal function tests, blood cell counts, and leukocyte differentials were all within the normal range. Cardiac enzyme tests were all negative and coagulation parameters were within normal limits, except for a significant elevation of D-dimer (2654 ng/ml, [normal range 100-250 ng/ml]). Inflammation indices were also high (i.e., serum C-reactive protein at 14.20 mg/dl [normal range 0-3 mg/dl]). Chest computed tomography (CT) revealed bilateral pleural effusions that extended from the lung bases to the apices with a maximum thickness of approximately 95 mm at the right lung base and atelectasis of the adjacent lung parenchyma. CT also revealed a thin pericardial film that reached a maximum thickness of 10 mm in the posterior region. Peripheral blood cultures and tests for Legionella and Pneumococcus serum antigens were negative as was a nasopharyngeal swab polymerase chain reaction test for SARS-CoV-2. Arterial blood gas values while on a VMK at 24 % oxygen included a pH of 7.48, pCO2 of 32 mmHg, pO2 of 67 mmHg, and arterial lactate of 1.3 mmol/L, P/F ratio of 279, and bicarbonate anion (HCO3-) concentration of 23.8 mmol/L. Given our suspicion of acute pericarditis, a cardiac ultrasound was performed which revealed a non-dilated VSn with normal wall thickness and no overt changes in segmental movements. Other findings included an ejection fraction of 55 % and a left atrium of normal size. Cardiac valves were not evaluated, and the right heart was within limits. The ultrasound also revealed circumferential pericardial detachment at a maximum of 5 mm.
In the ER, the patient received 800 mg of ibuprofen (3 cp per day) and 0.5 mg colchicine (2 cp per day) for three days to treat presumptive pericarditis and was later transferred to the Division of Internal Medicine. Upon transfer, the patient appeared to be alert, cooperative and oriented to time and space. The lung examination remained abnormal, with a bilateral vesicular murmur that was diffusely reduced and fully absent at the bases. The results of an abdominal examination were within normal limits. The patient was no longer febrile; cardiac activity included normal sinus rhythm and heart sounds. Both remote pathological history and family pathological history were negative. However, the patient reported that he smoked cigarettes (1 pack per day for about 15 years) but did not abuse illicit drugs or alcohol and otherwise tried to follow a healthy lifestyle. There was no history of recent or past toxic inhalations of hydrocarbons, heavy metals, or drug use. [4]
In light of the clinical and laboratory findings that were indicative of pericarditis accompanied by massive bilateral pleural effusions, diagnostic investigations were performed to evaluate the possibility of an infectious, neoplastic, or autoimmune etiology (Table 1). [3] All microbiological, virological, and special coagulation tests were negative, except for the test for lupus anticoagulant (LAC), which remained weakly positive.
Table 1: Panel of laboratory tests performed on the patient
|
Laboratory test |
Result |
|
Mycobacterium tuberculosis |
Negative sputum, urine, and bronchial washings |
|
Quantiferon |
Negative |
|
Parvovirus B-19 |
Negative |
|
Coxsackievirus |
Negative |
|
Adenovirus |
Negative |
|
Echovirus |
Negative |
|
Leptospira |
Negative |
|
Toxoplasma IgM |
Negative |
|
Epstein-Barr virus (EBV) IgM |
Negative |
|
Typical and atypical mycobacteria |
Negative bronchial washings |
|
Fungi |
Negative bronchial washings |
|
Pneumocystis carinii |
Negative bronchial washings |
|
Microscopy and culture for aerobic and anaerobic bacteria |
Negative on bronchial washing, blood cultures, pleural fluid |
|
Hepatitis B and hepatitis C viruses |
Negative |
|
Treponema pallidum |
Negative |
|
|
|
|
Anti-neutrophil cytoplasmic antibodies (cANCA); proteinase 3 |
Negative |
|
Anti-neutrophil cytoplasmic antibodies (pANCA); myeloperoxidase |
Negative |
|
Anti-nuclear antibodies |
Negative |
|
Anti-mitochondrial antibodies |
Negative |
|
Anti-smooth muscle actin antibodies (ASMA) |
Negative |
|
Anti-liver kidney microsome type 1 antibodies (LKM) |
Negative |
|
Anti-citrulline |
Negative |
|
Anti-endomysium IgG and IgA |
Negative |
|
Anti-C1q IgG |
Negative |
|
Anti-nuclear antibodies (ANA) |
Negative |
|
Complement factors C3-C4 |
Negative |
|
Rheumatoid factor |
Negative |
|
Lupus Anticoagulant (LAC) |
Weakly positive |
|
Anti-cardiolipin IgG |
Negative |
|
Anti-cardiolipin IgM |
Negative |
|
anti-histone antibodies |
Negative |
|
Anti-endothelial antibodies (indirect fluorescence assay) |
Negative |
|
Anti-glomerular basement membrane antibodies |
Negative |
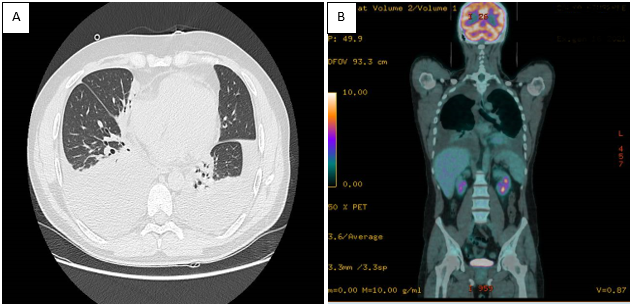
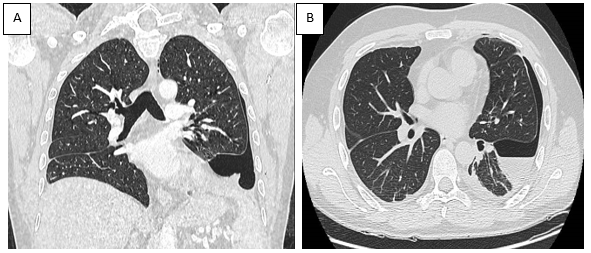
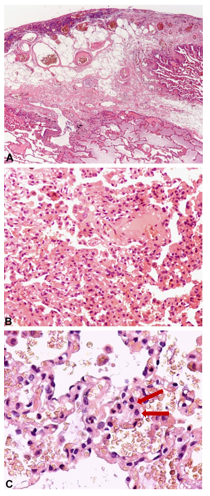
A follow-up CT scan (Figure 1) revealed extensive pleural effusions, while an 18F-positron emission tomography (PET)-CT scan (Figure 2) was negative for pathological uptake. No murmur was evident on pulmonary auscultation. Pulmonary ultrasound documented the extent of the atelectatic parenchyma bilaterally at both lung bases in association with the pleural effusion. Ultrasound-guided thoracentesis was performed which resulted in the aspiration of approximately 1000 ml of the bloody fluid. Cytologic, microbiologic, and physiochemical examinations performed on the thoracocentesis fluid were unrevealing. No neoplastic cells were detected in an evaluation of three different samples. The tests documenting autoimmune responses were also all negative. As the thoracocentesis had no impact on the physical examination, the patient underwent a video thoracoscopy procedure that was performed by an experienced thoracic surgeon. The surgeon reported a diffusely edematous pleura with crater-like patches that was hyper vascularized by newly formed varicose venous vessels. The surgeon aspirated 1200 ml of blood pleural fluid during the VATS procedure and performed multiple biopsies of the pleura and lung parenchyma. This evaluation was followed by a chest CT scan (Figure 2, panels A and B) which documented a reduction of the pleural effusion at the drainage flap on the right and multiple areas of altered density. These latter regions have a ground glass appearance, more evident in the right lower lobe, and are consistent with the findings of intra-alveolar hemorrhages and disordered vasculature. Taken together, these findings are compatible with a diagnosis of small vessel vasculitis. Tissue histology revealed widespread intra-alveolar hemorrhage (Figure 3, panels A–C) with organizing injury, hemosiderin-laden macrophages, scattered intra-arterial thrombi, and diffuse perivascular neutrophilic infiltrates suggesting capillaritis within the right lung. A left-sided thoracentesis was performed to promote complete lung expansion. Based on findings that include pulmonary vasculitis with negative immune studies and no evidence for extra-pulmonary vasculitis (as determined by negative retinal fluor angiography, gastroscopy, and CT-angiography), the patient was diagnosed with IPIPC. Once the diagnosis was established, ibuprofen was discontinued. The patient continued treatment with colchicine (0.5 mg two times daily) together with a corticosteroid (prednisone 25 mg per day) on the advice of the consulting rheumatologist. The patient was discharged after 49 days in good physical condition, with rheumatological follow-up, including a chest CT and evaluation of respiratory function (global spirometry, diffusing capacity of the lungs for carbon monoxide [DLCO], and walking tests).
Figure 1. Panel A: Pulmonary CT scan revealed extensive pleural effusion; Panel B: Total body 18F-PET-CT scan documents the absence of radiopharmaceutical uptake.
Figure 2. Coronal (Panel A) and transverse (Panel B) plane CT scan after VATS and subsequent drainage of the pleural effusion. Residual left-sided pneumothorax was revealed post-procedure (at arrow).
Figure 3. Representative histology documenting extensive fibrin deposition and chronic pleuritis with fat metaplasia associated with an underlying edematous and hemorrhagic lung parenchyma. (Panel A) Original magnification, 40x. (Panels B and C) Original magnification, 200x and 400x, respectively). The images in Panels B and C include erythrocytes associated with chronic lung hemorrhage and hemosiderin-laden macrophages (at arrow).
Discussion
Alveolar hemorrhage has been reported in a wide variety of dysfunctional states including autoimmune disorders (e.g., systemic vasculitis, Goodpasture syndrome, antiphospholipid antibody syndrome, connective tissue disease), toxic exposures (e.g., trimellitic anhydride, isocyanates, cocaine/crack, and some pesticides) [5], adverse drug reactions (e.g., propylthiouracil, diphenylhydantoin, amiodarone, methotrexate, nitrofurantoin, bleomycin, montelukast, infliximab), and coagulation disorders. Isolated pauci-immune pulmonary capillaritis is diagnosed in the absence of these findings. Notably, this case highlights the differences in the lung parenchyma on chest CT scan before and after VATS, as the extensive pleural effusion obscured the lesions that are typical of hemorrhagic alveolitis. Based on the patient’s past medical history (including his smoking history), we concluded that the previous bout of pneumonia triggered the hemorrhagic alveolitis associated with IPIPC, which in this case presented with an atypical manifestation that included a hemothorax and pleuro-pericarditis. To the best of our knowledge, this is the first case of IPIPC reported in the literature that presented these atypical findings.
Conflicts of Interest: The authors declare that there are no conflicts of interest regarding the publication of this article.
References
- Thompson G, Klecka M, Roden AC, Specks U, Cartin-Ceba R (2016) Biopsy-proven pulmonary capillaritis: A retrospective study of aetiologies including an in-depth look at isolated pulmonary capillaritis. Respirology. 21(4): 734-8.
- Collard HR, King T, ISchwarz M (2010) Diffuse alveolar hemorrhage and rare infiltrative disorders of the lung. in Murray and Nadel’s Textbook of Respiratory Medicine.
- Marchiori E, Zanetti G, Hochhegger B (2011) Diffuse alveolar hemorrhage in infectious diseases. Chest. 139(1): 228–229.
- Oh JS, Wong U, Bajaj D, Hines SE (2020) Isolated Pauci-Immune Pulmonary Capillaritis Associated with Hydrocarbon Inhalation and Marijuana Smoking: An Unusual Case of Severe Hypoxemia. Case Reports in Pulmonology. 2020: 1264859.
- Lara AR, Schwarz M I (2010) Diffuse alveolar hemorrhage. Chest. 137(5): 1164–1171.