Mohammad Ali Fazeli1*, Malous Emadzadeh2
1The research center, Shohada Tajrish Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2ICORD research center, Department of Medicine, Division of Surgery, University of British Columbia. Canada
*Corresponding Author: Mohammad Ali Fazeli, The research center, Shohada Tajrish Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Abstract
Rasmussen’s encephalitis (RE) is an uncommon progressive inflammatory disease of the central nervous system. It is manifested with unilateral hemispheric atrophy, pharmacoresistant focal seizures, and advanced neurological deficits. The precise etiopathogenesis remains furtive. Brain imaging plays a vital role in diagnosis and follow-up. Fluctuation of lesions in brain imaging was described in a few cases. Here, we report an adult case of Rasmussen encephalitis with fluctuating changes in brain MRI. Our 25-year patient with Rasmussen encephalitis symptoms from 10 years old what was exacerbated with progressive hemiparesis and physical and mental significant recovery obtained dramatically After insertion of ventricular peritoneal shunt instead of invasive hemispherectomy.
Keywords: Rasmussen’s encephalitis, ventriculoperitoneal shunt
1. Introduction
Rasmussen's encephalitis (RE) was first defined in the late 1950s. It is an uncommon neurological disorder of childhood characterized by unilateral hemispheric atrophy, pharmacoresistant focal seizures, and progressive neurological deficits. The precise etiopathogenesis remains mysterious. Brain imaging plays a key role in the diagnosis and limitation of disease evolution. Few cases with unusual MRI characteristics of RE signified by the development and reoccurrence of signal irregularities were described.
We report on the clinical, electrophysiological, and imaging data of an additional pediatric RE case with unusual MRI features.
A 25-year-old man was diagnosed with epilepsy at 10 years old with no history of seizures in his family.
He had an episode of psychosis with paranoid ideations, irritability, aggression, auditory hallucinations, and decreased need for sleep. After that, his seizures exacerbated to 20 attacks a day. He stated that he had experienced auditory hallucinations several months ago. He could not remember its onset clearly. He has not worked during the past 3 months. He was referred to our hospital with right hemiparesis, aphasia, and a dystonic neck.
On the language testing, He had an apparent problem with fluency. In Visuoperceptual processing, he had borderline performance in figure-ground discrimination tasks. He demonstrated deficient capacity (below the 1st percentile) of recognition skills. On another more complex abstract verbal learning task, he showed weak immediate and delayed free recall but good recognition capacities. This pattern suggests suitable encoding but poor retrieval of the information in the verbal domain. In the visual memory domain, Musa's performance fell in the borderline range (the 3rd percentile) for immediate memory and after a delay. On another more complex abstract design learning task, he showed poor registration, recognition, and free recall capacities. This pattern suggests weak encoding, consolidation, and retrieval capacities of the information in the visual domain. Mental flexibility and executive function: The test result indicated poor performance in visual scanning and flexibility in the shift of cognitive sets.
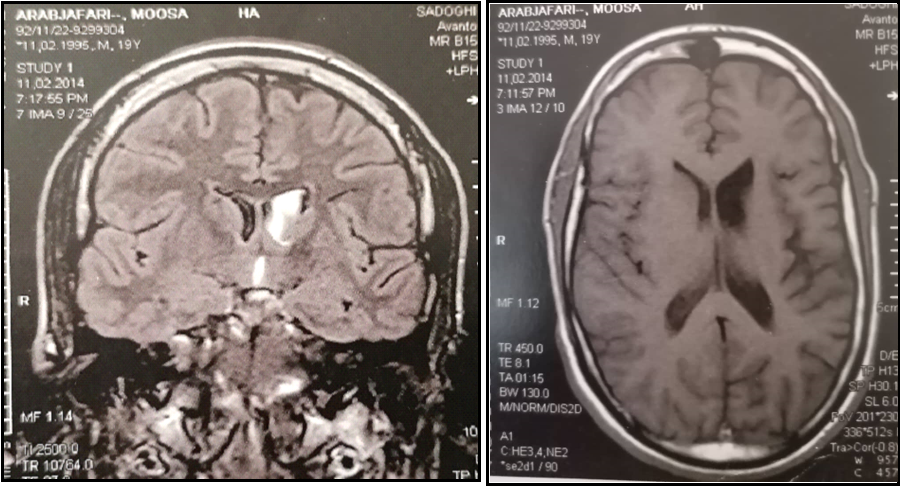
The brain MRI showed cortical hyperintensity on T2 and FLAIR images in left frontoinsular, left frontoinsular cortical atrophy with homolateral striatum atrophy, and dilatation of the ipsilateral ventricular system (Figure 2). Given the clinical course and MRI findings, the diagnosis of RE was performed.
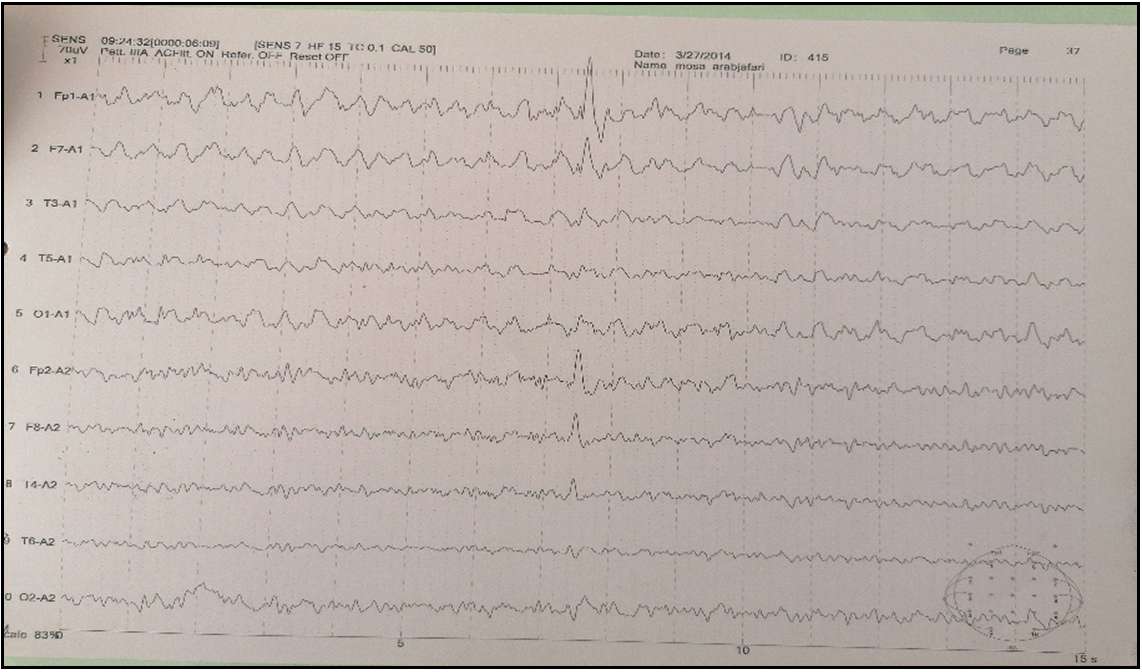
Figure 1: EEG showing asymmetric background activity with the left frontotemporal intercritical discharges.
Figure 2: Serial MRIs in T1 and FLAIR sequences showing hyperintensity in the left caudate nuclei one year after the first MRI, persistence of hyperintensity, left fronto-insular cortical and homolateral striatum atrophy, and dilatation of the ipsilateral ventricular system
2. Discussion
Rasmussen’s encephalitis is an immune-mediated condition categorized by drug-resistant focal epilepsy and progressive neurological and cognitive deficits related to unilateral hemispheric atrophy [1,2]. RE is commonly found in young children. The histopathological hallmarks of RE are cortical inflammation, neuronal loss, and gliosis confined to one cerebral hemisphere [3]. Surgery plays a crucial role in treating childhood RE and targets controlling seizures. Because most lesions are restricted to one hemisphere, Hemispherectomy is presented as the final effective treatment. As well as the intractable seizures, these patients usually suffer from many other disabling symptoms such as Hemparesis, Cervical dystonia, and Cognitive impairment, which are not curable by current approaches.
RE is a progressive chronic inflammatory disease of the central nervous system. It was first described by Theodore Rasmussen in 1958 [1]. It is categorized by focal intractable seizures, progressive neurological deficit, and cognitive deterioration, with unihemispheric brain atrophy found in our patient [2]. The etiopathogenesis of RE is unidentified. Suggested including viral infections, an autoimmune phenomenon involving circulating antibodies against glutamate receptors, and cytotoxic T cells [3,4]. Diagnosis of RE is based on distinctive clinical, radiological, and pathological features. Diagnostic criteria were established by Bien et al. in 2005 [5]. Three stages have been anticipated.
The prodromal stage is manifested with mild signs (low seizure frequency and mild hemiparesis). The acute stage is manifested with frequent focal seizures, progressive hemiparesis, and cognitive deterioration. The residual stage is characterized by steadiness of neurological deficits and persistence of seizures, but less frequent than in the acute stage [6]. In some patients, less frequent manifestations such as unilateral movement disorders, including hemiathetosis and hemidystonia, have been reported [7]. Our patient had hemidystonia. Brain MRI is an essential imaging tool for diagnostic evaluation and follow-up in RE [2,5]. The majority of cases show, at an earlier stage, unilateral enlargement of the ventricular system, which is more dominant in the insular and periinsular regions. A T1/FLAIR hyperintense signal is often noticed in the cortical or subcortical areas.
Afterward, unihemispheric atrophy sets in and predominates typically in the perisylvian region, as in our case. Most of the tissue loss occurs during the first 12 months after the initiation of symptoms in most patients. Atrophy of the ipsilateral head of the caudate nucleus is a distinctive feature. Gadolinium enhancement is very uncommon in RE [5]. Serial MRIs reveal the progression of signal change and atrophy. Enhancement and one reoccurrence of signal abnormalities characterize an atypical MRI aspect of RE. Recurrence of high signal intensity was related to clinical seizure deterioration, as shown in our case. This fluctuation is indicative of the inflammatory process [8]. In the literature, 9 patients of RE displayed regression afterward recurrence of lesions on serial MRIs [4,8–10]. In primary disease stages, electroencephalograms (EEG) may help diagnose RE. Several abnormalities are observed in patients with RE. Some unihemispheric results, such as diminishing of background activity with persistent polymorphic delta waves and sleep spindles, focal slow activity, subclinical ictal discharges, and multifocal ictal discharges, are potently representative of RE [2,3]. The EEG of our patient depicted inter critical left frontotemporal discharge continuing during sleep.
Brain biopsy can also support the diagnosis, but it is unnecessary in all RE cases. The typical histopathological characteristics are microglial and lymphocytic nodules, neuronal loss, neuronophagia, and perivascular cuffing, limited to one cerebral hemisphere with frontoinsular predilection [2,5].
RE can be treated by antiepileptic drugs, immunosuppressive and immunomodulator regimens, and surgery. These modalities decrease seizure severity and advance motor and cognitive performance [2,5]. Often, seizures are resistant to antiepileptic drugs [11]. Patients receiving immunotherapy positively affected seizure frequency and delayed aggravation [12].
Plasmapheresis sound effects on seizures and neurological functions. It contributes to evaluating mental and residual motor function before surgery. The frequency was three to six single-volume exchanges on consecutive or alternate days every 2 to 8 weeks [13].
We report an adult case of RE with fluctuating changes in brain MRI. Our patient was a 25-year-old male who presented with disabling progressive right hemiparesis, intractable seizures (20-30 seizing per day), cervical dystonia, and aphasia initiated at the age of 10 and exacerbated at the age of 18. moreover, he suffered from frequent episodes of psychosis with paranoid ideations, irritability, aggression, and insomnia. His social function deteriorated. No improvements were seen after non-surgical therapies.
Our patient was treated by Numerous regimens of antiepileptic drugs were prescribed (valproic acid 1000mg/day, carbamazepine 1200 mg/day, levetiracetam 2000 mg/day, lamotrigen 200 mg/day),
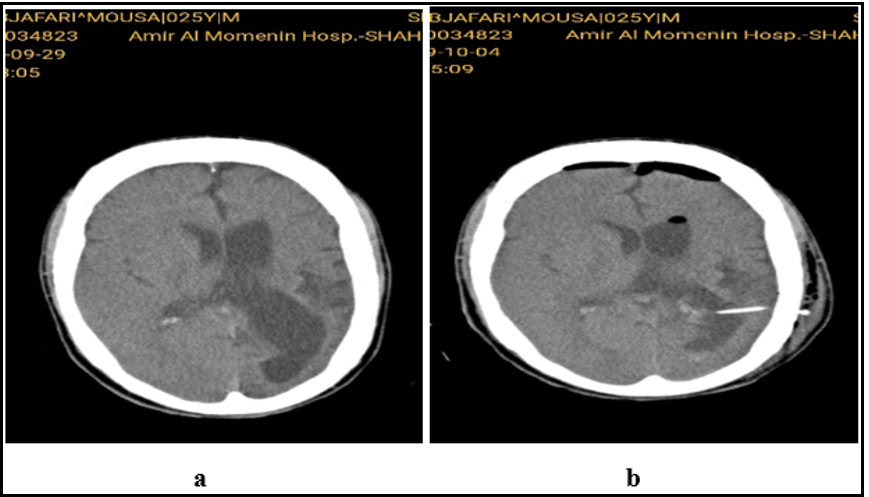
The patient underwent external ventricular drainage (EVD) insertion for probable response to decompression. A dramatic reaction to EVD was achieved in the form of fast recovery of hemiparesis, aphasia, and a significant reduction of the seizure frequency that urged us to replace EVD with a ventriculoperitoneal shunt (VPS). About 24 hours after VPS implantation, the frequency of seizures significantly reduced from 30 to 3 to 5 times daily. After 20 days, not only did the frequency of seizures decline dramatically to 8 seizures in 72hrs, but also the type of seizure changed from generalized tonic-colonic to Focal, lasting for 15 seconds. After three months, seizures Occure just 1-2 times daily in the awakening state. More dystonia and hemiparesis showed a dramatic recovery. The patient could walk and do his daily activities independently. Over a 2-year follow-up, all the cognitive and psychiatric impairments recovered.
Based on the current guidelines, despite all the deteriorating functional complications due to the complete disconnection of the affected hemisphere, Hemispherectomy is the only available approach to reducing the drug-resistant seizures caused by RE [5]. The patient selection criteria are limiting, and the Risk of Failure always must be considered.
We are introducing VPS as a less invasive approach to RE treatment for the first time.
In contrast with Hemispherectomy, VPS is a less invasive procedure without neurological sequels, no restrictive criteria, and no specific pre/post Operation consideration. It is easy to perform in any basic neurosurgical OR with a shorter hospitalization.
Our patient experienced rapid cognition and function improvement also, a full recovery from a long-term debilitating hemiparesis, as well as a significant persistent seizure reduction.
Our Theories
1. Ventriculomegaly might cause the CSF dynamic impairment.
2. The compression effect caused by Ventriculomegaly results in sensory and motor disorder (contralateral hemiparesis).
Replacing a complicated surgical procedure with an accessible, less invasive, safer, and more effective surgical approach can be a great success if confirmed by further investigations. We are so hopeful that this approach can be extended to other Brain atrophic malformations with intractable seizures like corpus callosum agenesis. Surgery (anatomic Hemispherectomy, functional Hemispherectomy, perisylvian Hemispherectomy, trans-sylvian Hemispherectomy, and central/vertical Hemispherectomy) appears to be the only cure for seizures and to advance cognitive prognosis. However, inevitable sequelae (hemianopia, hemiparesis, and aphasia in the dominant hemisphere) should be measured [2]. A rehabilitation approach should be considered. It may improve the quality of life of RE patients.
This study introduced another less invasive approach with no sequelae, hoping to replace Hemispherectomy.
Figure 3: Brain CT before and after VP shunt
3. Conclusions
Rasmussen’s encephalitis is an advanced inflammatory disease of one cerebral hemisphere characterized by frequent focal seizures, hemiparesis, and mental deterioration. Brain imaging findings about electroencephalogram and clinical data may show early diagnosis and could be indicative of outcome. Our understanding of uncharacteristic radiological features is essential to make the correct diagnosis. Rapid diagnosis and management can alter the progression of the disease. VP shunting is a less invasive approach introduced in Rasmussen encephalitis treatment. I can imagine some theories justify the recovery of these patients, including:
1.Ventriculomegaly causes CSF dynamic disorder and can be recovered by VP shunting.
2.Ventriculomegaly can pressure the thalamus, Basal ganglia and Posterior limb of the internal capsule, resulting in sensory and movement disorders.
References
- Rasmussen T, Olszewski J, Lloydsmith D (1958) Focal seizures due to chronic localized encephalitis. Neurology. 8(6): 435-45.
- Varadkar S, Bien CG, Kruse CA, Jensen FE, Bauer J, et al. (2014) Rasmussen’s encephalitis: clinical features, pathobiology, and treatment advances. The Lancet Neurology. 13(2): 195–205.
- Varghese B, Aneesh M, Singh N, Gilwaz P (2014) A case of Rasmussen encephalitis: the differential diagnoses and role of diagnostic imaging. Oman Medical Journal. 29(1): 67–70.
- Pradeep K, Sinha S, Mahadevan A, Saini J, Arivazhagan A, et al. (2016) Clinical, electrophysiological, imaging, pathological and therapeutic observations among 18 patients with Rasmussen’s encephalitis. Journal of Clinical Neuroscience. 25: 96–104.
- Bien CG, Granata T, Antozzi C, Cross JH, Dulac O, et al. (2005) Pathogenesis, diagnosis and treatment of Rasmussen encephalitis: a European consensus statement. Brain. 128(3): 454–71.
- Olson HE, Lechpammer M, Prabhu SP, Ciarlini PD, Poduri A, et al (2013) Clinical application and evaluation of the Bien diagnostic criteria for Rasmussen encephalitis. Epilepsia. 54(10): 1753–60.
- Frucht S (2002) Dystonia, athetosis, and epilepsia partialis continua in a patient with late-onset Rasmussen’s encephalitis. Movement Disorders. 17(3): 609–612, 2002.
- Yamazaki E, Takahashi Y, Akasaka N, Fujiwara T, Inoue Y (2011) Temporal changes in brain MRI findings in Rasmussen syndrome. Epileptic Disorders. 13(3): 229–39.
- Nakasu S, Isozumi T, Yamamoto A, Okada K, Takano T, et al. (1997) Serial magnetic resonance imaging findings of Rasmussen’s encephalitis. Neurologia Medico-Chirurgica. 37(12): 924–928.
- Avberšek A, Miserocchi A, McEvoy AW, Patel AV, Aronica E, et al. (2015) Multiphasic presentation of Rasmussen’s encephalitis. Epileptic Disorders. 17(3): 315–320.
- Caraballo RH, Fortini S, Cersósimo R, Monges S, Pasteris MC, et al. (2013) Rasmussen syndrome: an argentinean experience in 32 patients. Seizure. 22(5): 360–367.
- Bien CG, Tiemeier H, Sassen R, Kuczaty S, Urbach H, et al. (2013) Rasmussen encephalitis: incidence and course under randomized therapy with tacrolimus or intravenous immunoglobulins. Epilepsia. 54(3): 543–550.
- Granata T, Fusco L, Gobbi G, Freri E, Ragona F, et al. (2003) Experience with immunomodulatory treatments in Rasmussen’s encephalitis. Neurology. 61(12): 1807–1810.