Matjaž Kopač1*, Zvonka Rener-Primec2
1Department of Nephrology, Division of Paediatrics, University Medical Centre, Bohoričeva 20, 1000 Ljubljana, Slovenia, EU.
2Department of Child, Adolescent and Developmental Neurology, Division of Paediatrics, University Medical Centre, Bohoričeva 20, 1000 Ljubljana, Slovenia
Faculty of Medicine, University of Ljubljana, Slovenia
*Corresponding Author: Matjaž Kopač, Department of Nephrology, Division of Paediatrics, University Medical Centre, Bohoričeva 20, 1000 Ljubljana, Slovenia, EU.
Abstract
Aim: to study the influence of parasympathetic stimulation with vagus nerve stimulation (VNS) device on blood pressure (BP) and heart rate (HR) in patients with pharmacoresistant epilepsy.
Methods: 24-hour ambulatory blood pressure monitoring was done in two patients before and after the VNS device's surgical implantation.
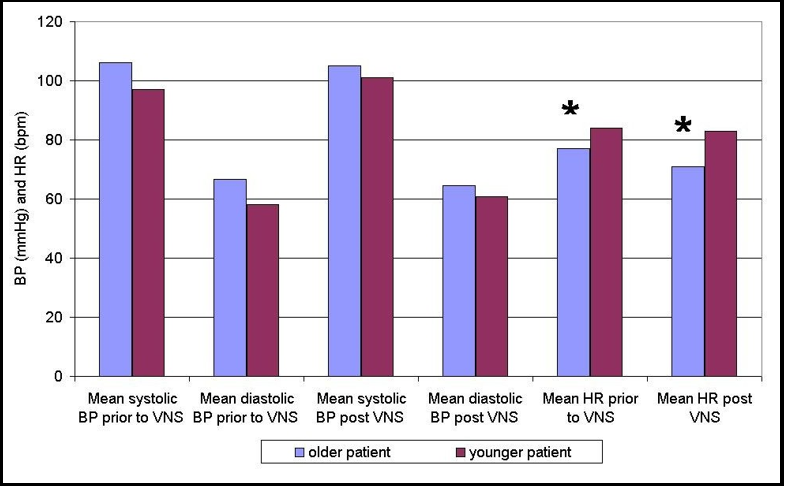
Results: Mean BP in older patients was 106.1/66.8 mmHg before and 105.0/64.5 mmHg post VNS device implantation. Mean BP in younger patients was 97.1/58.1 mmHg before and 101.0/60.8 mmHg post-implantation. The difference in mean BP was statistically non-significant in both patients.
Mean HR in the older patients was 77 beats per minute (bpm) before and 71 bpm post-VNS device implantation, a statistically significant difference (p=0.009). Mean HR in the younger patients was 84 bpm before and 83 bpm post-implantation, a statistically non-significant difference.
Conclusions: The vagus nerve stimulation did not result in a significant change of BP in both patients. However, it causes a substantial reduction in HR in one of them.
Keywords: Blood pressure; Heart rate; Epilepsy; Parasympathetic stimulation; Surgical implantation; Vagus nerve
Introduction
Arterial hypertension is a vital healthcare problem worldwide. However, awareness and recognition of the situation by clinicians sometimes remain insufficient. Prevalence estimates vary due to differing standards, populations, and blood pressure (BP) measurement techniques. Estimates in the United States range from 0.3 % to 4.5 %. [1]
In certain patients with essential hypertension, BP cannot be controlled with medications and non-pharmacological measures; this is called pharmacoresistant hypertension. These cases are rare during childhood but present a significant challenge in clinical practice. It is undoubtedly recognized that untreated or uncontrolled hypertension is a leading risk factor for subsequent myocardial infarction, cerebrovascular insult, and kidney failure. [2,3] 24-hour ambulatory blood pressure monitoring (ABPM) is extremely valuable to diagnosing and managing hypertension. It provides many indications, such as confirmation of hypertension before starting drug treatment and monitoring response to therapy, especially n chronic kidney disease and other conditions in which rapid and episodic elevations of BP are challenging to detect in the office. [1,3]
Its use in clinical trials is considered especially important due to the smaller number of children with hypertension.[4] They must be treated with long-term and sometimes life-long medications. This is often a cause for concern for pediatric patients, their parents, and physicians, as some drugs adversely affect glucose and lipid metabolism. The long-term significance of these metabolic changes is still debated and imperfectly understood.[5]
The study explores the potential use of vagus nerve stimulation (VNS) beyond already established treatment options for pediatric epilepsy patients, such as hypertension. The vagus nerve is the longest and the most complex of all the cranial nerves. It helps transmit messages to the internal organs (including the heart). The parasympathetic nervous system helps in the relaxation of the heart. Therefore, any treatment aimed towards stimulating the parasympathetic response of the heart (through the vagus nerve) should theoretically reduce the blood pressure and heart rate. This study aims to determine the treatment's practical efficacy when medication is no longer effective. This is the unique aspect of the study.
Materials and Methods
Two patients (both were girls) with drug-resistant epilepsy, aged 18 and 8 years, treated in a tertiary care children's hospital, were evaluated prospectively. Written informed consent was obtained from patients (older than 14 years) and their parents. ABPM was done both before and after the VNS device's surgical implantation. Altogether, 205 BP and heart rate (HR) measurements were obtained, 90 in the older patient and 115 in the younger one, at 20- min intervals during the day and 30-min intervals at night. Both patients completed a diary (with the assistance of their parents, if necessary), recording their activities and events to evaluate their influence on BP and HR. Additionally, the intake of anti-epileptic drugs was recorded to assess their potential impact on BP and HR. During ABPM, both patients reported no seizures or changes in medication or doses, eliminating their potential influence on BP and HR. Additionally, no changes in diet, daily activities, or other potential factors influencing BP and HR were recorded. During ABPM, both patients did not report acute infection or other associated medical conditions except epilepsy.
The older patient displayed routine physical parameters, with body weight (BW) of 58 kg (50th percentile) and height (BH) of 161 cm (40th percentile). She experienced moderate mental disability, bilateral sensorineural hearing loss, and a slight right hemiparesis, as noted in the neurological examination. She was treated with valproic acid 1250 mg/day, levetiracetam 2 times 500 mg/day, and lamotrigine 250 mg/day during both ABPM studies.
The younger patient also reported normal physical parameters, with BW 22 kg (15th percentile) and BH 119 cm (10th percentile). She displayed mild developmental delay. She was treated with valproic acid 2 times 250 mg/day, Sultiame 15 mg and 25 mg/day (15 mg and 15 mg during the second ABPM study), topiramate 50 and 75 mg/day, and methylprednisolone, the latter in gradually decreasing doses, during both ABPM studies. In addition, she received 10 mg of methylphenidate during the second ABPM.
Two other patients were initially enrolled in this study. However, ABPM before VNS device implantation was terminated in one of them prematurely, after just a few hours, due to technical reasons (the planned procedure was canceled and rescheduled for later, so the patient was discharged from the hospital). Parents of another patient refused VNS device implantation after previous consent.
The VNS parameters had been set to the therapeutic values as planned and tolerated at least one month before the second measurement.
We compared mean BP and HR in both patients, before and post-VNS device implantation (expressed as mean +/- standard deviation) and tested the statistical significance of differences of obtained measurements of BP and HR with a student's t-test.
Institutional Review Board Statement: The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Slovenian National Medical Ethics Committee (No. 179/09/10)
Results
Mean BP in the older patients was 106.1/66.8 mmHg (+/- 14.8/14.7 mmHg) before and 105.0/64.5 mmHg (+/- 10.5/10.6 mmHg) post- VNS device implantation. Mean BP in the younger patients was 97.1/58.1 mmHg (+/- 9.8/9.9 mmHg) before and 101.0/60.8 mmHg (+/-8.5/10.3 mmHg) post-VNS device implantation. The difference in mean BP was statistically non-significant in both patients.
Mean HR in the older patients was 77 beats per minute (bpm) (+/- 13.8 bpm) before and 71 bpm (+/- 11.5 bpm) post-VNS device implantation, a statistically significant difference (p=0.009). Mean HR in the younger patients was 84 bpm (+/- 16.4 bpm) before and 83 bpm (+/- 17.1 bpm) post-VNS device implantation, a statistically non-significant difference. Results are presented in Figure 1.
Figure 1. Mean blood pressure (BP) and mean heart rate (HR) prior to and post the vagus nerve stimulation (VNS) device implantation in both patients.
Legend: bpm – beats per minute; * - statistically significant difference
Discussion
An imbalance of the autonomous nervous system, especially sympathetic overactivity, is a known risk factor for hypertension. There have been several attempts to treat hypertension by negatively influencing the sympathetic part of the autonomous nervous system.[6,7,8] A 6-year-old girl with Turner syndrome, inadequate BP control (despite treatment with maximal doses of calcium antagonist, β-blocker, ACE inhibitor, and clonidine), and an episode of stroke were treated with sympathetic ablation of the renal arteries; BP gradually normalized, and one of the drugs (β-blocker) was discontinued.[7] A later study showed that a respiratory-gated auricular vagal afferent nerve stimulation increased the cardiovagal tone and reduced the sympathetic tone during a paced breathing task, lowering BP,[9], unlike our study where we detected no such effect. In another study, the VNS in salt-sensitive rats attenuated salt-induced hypertension and arrhythmias; intermittent electric stimulation through the right cervical vagus nerve was performed. It induced beneficial changes in the electrophysiological properties of the heart. The results provided evidence for the therapeutic efficacy of VNS in hypertension and hypertension-related heart diseases.
[10] In another study, VNS was able to reduce BP without causing bradypnoea or bradycardia; this could be a side effect of vagal stimulation. [11] In 8 adult patients with advanced heart failure, long-term vagal stimulation proved feasible, safe, and tolerable with promising preliminary efficacy results.[12]
VNS therapy represents a safe and well-tolerated therapeutic method in adults and children with drug-resistant epilepsy; there might be an alternative option – as a possible solution for a subgroup of patients with pharmacoresistant hypertension and chronic conditions. Therefore, fewer drugs would be needed in the long run.
It remains an efficacious adjunctive treatment in patients with drug-resistant epilepsy who are ineligible candidates for curative surgery. VNS therapy has been widely accepted since its approval in the USA in 1997. [13,14] With VNS, the brain (stem) receives intermittent electric stimulation through the left vagus nerve; every 5 min for a period of 30 s with an average amplitude of up to 2.0 mA. The electrical stimuli are delivered by a compact device implanted at the thoracic level. The hypothesized mode of action of VNS is the decreased excitatory/increased inhibitory neurotransmission; this is induced by the stimulation of the ascendant fibers of the vagus nerve to the nucleus tractus solitarii and the medullary reticular formation.[15] VNS is implanted on the left vagus nerve, as the right innervates the sinoatrial node; its stimulation might result in bradycardia. [15] It is recognized that the effectiveness of VNS therapy improves with the duration of vagus nerve stimulation. [14,16]
In addition to the treatment of drug-resistant epilepsy in patients who are not appropriate candidates for surgery, VNS has also received approval for the treatment of major depression, obesity, and episodic cluster headache from the Food and Drug Administration. Some studies have been conducted to study heart function and BP changes and found subtle or no significant differences in heart rate variability and blood pressure in epileptic patients, [17] similar to our study. A study that evaluated the effects of VNS on heart rate variability (HRV) in children with epilepsy found a remarkable improvement after 6 months of VNS treatment, yet no further changes were observed at 12 months compared to levels at 6 months in all parameters, still significantly lower than those of controls. Longer duration of epilepsy and specific localizations of epileptic focus were found to further contribute to diminished basal HRV levels. According to the authors, impaired cardiovascular autonomic regulation may be associated with the epileptic process and with some additional factors' contribution.[18] Individuals with refractory epilepsy are prone to dysfunction of the autonomic nervous system, and reduced HRV is its marker, according to other authors. The latter proved a striking reduction in vagal tone during slow-wave sleep and reduced modulation capacity in patients with refractory epilepsy compared to individuals in the control group. Implantation of VNS induced a shift in sympathovagal balance towards sympathetic predominance and an improvement in autonomic modulation. [19] Clancy et al. investigated a non-invasive method of VNS through electrical stimulation of the auricular branch of the vagus nerve, measured the autonomic effects, and showed significantly increased HRV and reduced sympathetic nerve outflow in healthy participants, which is desirable in conditions characterized by enhanced sympathetic nerve activity, such as heart failure and other diseases. [20] Parasympathetic activity is often reduced in hypertension and can elicit anti-inflammatory mechanisms. Chapleau et al. hypothesized that chronic VNS may alleviate cardiovascular end-organ damage in stroke-prone spontaneously hypertensive rats. They implanted VNS devices into them and proved that chronic VNS prevented hypertension-induced endothelial dysfunction and aortic stiffening in an animal model of severe hypertension and speculated that anti-inflammatory mechanisms might contribute to these effects. [21]
Conclusion
The vagus nerve stimulation, theoretically, is a promising idea; however, it did not result in a significant reduction of BP in our patients. However, it showed a significant decrease in HR in one of them. The study limitations are the small number of patients and the presence of normal BP and HR at the baseline. The reduction could be significant in patients with elevated BP or HR. But this is not an approved method for the treatment of hypertension. Therefore, such a study would be ethically disputed at this moment.
We know many studies evaluating the effect of VNS on BP and HR. However, to the best of our knowledge, our study is the first one done in epilepsy patients treated with a VNS device in real-life settings which makes it unique.
Further studies in VNS-treated epilepsy patients experiencing hypertension might help evaluate this intriguing therapeutic option in an improved manner.
Conflict of Interest: None Declared
Acknowledgements: We would like to thank Editage (www.editage.com (http://www.editage.com) for their editing of the manuscript and writing support.
Authors' Contributions
Conceptualization, M.K.; Methodology, M.K. and Z.R.P.; Software, M.K.; Validation, M.K. and Z.R.P.; Formal Analysis, M.K.; Investigation, M.K. and Z.R.P.; Data Curation, M.K. and Z.R.P.; Writing – Original Draft Preparation, M.K.; Writing – Review & Editing, M.K. and Z.R.P.; Visualization, M.K.; Supervision, M.K. and Z.R.P.
References
- Rao G (2016) Diagnosis, Epidemiology, and Management of Hypertension in Children. Pediatrics. 138(2): e20153616.
- Bernstein D. Systemic Hypertension. In: Behrman RE, Kliegman RM. Jenson HB, eds. (2007) Nelson Textbook of pediatrics. 18th ed. Philadelphia: WB Saunders Company. 1592- 1598.
- Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. (2016) 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertension. 34(10): 1887-1920
- Lurbe E, Sorof JM, Daniels SR (2004) Clinical and research aspects of ambulatory blood pressure monitoring in children. J Pediatr. 144: 7-16.
- Stas S, Appesh L, Sowers J (2006) Metabolic safety of antihypertensive drugs: myth versus reality. Curr Hypertens Rep. 8(5): 403–408.
- Schlaich MP, Krum H, Sobotka PA (2010) Renal sympathetic nerve ablation: the new frontier in the treatment of hypertension. Curr Hypertens Rep. 12: 39–46.
- Bonanni A, Pasetti F, Ghiggeri GM, Gandolfo C (2015) Case Report: Renal denervation for severe hypertension in a small child with Turner syndrome: miniaturisation of the procedure and results. BMJ Case Rep. 2015: bcr2014208777.
- Ng FL, Saxena M, Mahfoud F, Pathak A, Lobo MD (2016) Device-based therapy for hypertension. Curr Hypertens Rep. 18: 61.
- Sclocco R, Garcia RG, Gabriel A, Kettner NW, Napadow V, et al. (2017) Respiratory-gated auricular vagal afferent nerve stimulation (RAVANS) effects on autonomic outflow in hypertension. Conf Proc IEEE Eng Med Biol Soc. 2017: 3130- 3133.
- Annoni EM, Xie X, Lee SW, Libbus I, KenKnight BH, et al. (2015) Intermittent electrical stimulation of the right cervical vagus nerve in salt-sensitive hypertensive rats: effects on blood pressure, arrhythmias, and ventricular electrophysiology. Physiol Rep. 11(3): e-12476.
- Plachta DT, Gierthmuehlen M, Cota O, Espinosa N, Boeser F, et al. (2014) Blood pressure control with selective vagal nerve stimulation and minimal side effects. J Neural Eng. 11(3): 036011.
- Schwartz PJ, De Ferrari GM, Sanzo A, Landolina M, Rordorf R, et al. (2008) Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur J Heart Fail. 10: 884-891.
- Schachter CS, Clifford BS (1998) Vagus nerve stimulation. Epilepsia. 39(7): 677-686.
- DeGiorgio CM, Schachter SC, Handforth A, Salinsky M, Thompson J, et al. (2000) Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia. 41(9): 1195-1200.
- Ben-Menachem E, Hamberger A, Hedner T, Hammond EJ, Uthman BM, et al. (1995) Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res. 20(3): 221–227.
- Ulate-Campos A, Cean-Cabrera L, Petanas-Argemi J, García- Fructuoso G, Aparicio J, et al. (2015) Vagus nerve stimulator implantation for epilepsy in a paediatric hospital: outcomes and effect on quality of life. Neurologia. 30(8): 465-471.
- Garamendi-Ruiz I, Gómez-Esteban JC (2019) Cardiovascular autonomic effects of vagus nerve stimulation. Clin Auton Res. 29(2): 183-194.
- Hirfanoglu T, Serdaroglu A, Cetin I, Kurt G, Capraz IY, et al. (2018) Effects of vagus nerve stimulation on heart rate variability in children with epilepsy. Epilepsy Behav. 81: 33-40.
- Jansen K, Vandeput S, Milosevic M, Ceulemans B, Van Huffel S, et al. (2011) Autonomic effects of refractory epilepsy on heart rate variability in children: influence of intermittent vagus nerve stimulation. Dev Med Child Neurol. 53(12): 1143-1149.
- Clancy JA, Mary DA, Witte KK, Greenwood JP, Deuchars SA, et al. (2014) Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul. 7(6): 871-877.
- Chapleau MW, Rotella DL, Reho JJ, Rahmouni K, Stauss HM (2016) Chronic vagal nerve stimulation prevents high-salt diet- induced endothelial dysfunction and aortic stiffening in stroke- prone spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 311(1): H276-285.