M’bégnan Coulibaly1,2*, Attoh-Toure Harvey2,3, Kouassi René Kouao Ahua2, Damus Paquin Kouassi1,4, Salifou Yéo1, Yoboue Véronique5, Tiembré Issaka2,3
1Regional branch of the National Institute of Public Hygiene of Bouaké,
2Interuniversity Doctoral Program in Public Health, Félix Houphouët Boigny University of Cocody, Abidjan, Côted'Ivoire
3National Institute of Public Hygiene, Côte d'Ivoire, BPV 14 Abidjan (Treichville), Côte d’Ivoire
4Department of Public Health and related disciplines, Alassane Ouattara University of Bouaké, Bouaké, Côte d’Ivoire
5Laboratory of Matter, Environmental and Solar Energy Sciences (LASMES) Félix Houphouët-Boigny University, Abidjan, Côte d'Ivoire.
*Corresponding Author: M’bégnan Coulibaly, Regional branch of the National Institute of Public Hygiene of Bouaké- Côte d’Ivoire.
Abstract
Background
The health impact of artisanal smoking on stakeholders makes this activity a risky occupation. Usually carried out in agglomeration, the residents of these sites are exposed to the pollutants resulting from this activity.
Objective
We conducted this survey to determine its health impact on residents.
Methods
We carried out a cross-sectional survey of the type here-elsewhere, targeting the inhabitants of the «Niang on Sud» of Yopougon. This population was divided into two groups: “exposed” consisting of residents in the vicinity of the smoking site and “not exposed”.
The collection of socio-demographic and clinical data was done by interview and the evaluation of respiratory function by spirometry.
The data were captured and analyzed using the SPSS DATA and the Prevalence Report (PR) of the independent variables were expressed and their confidence intervals were estimated at 95 %.
Results
A total of 252 subjects divided by exposure status (50 %) and non-exposed status (50 %) were included in our survey.
Subjects in the exposed group were more likely to have respiratory manifestations with prevalences of 18.18 %, 30 %, 10.74 %, 40.17 %, 21 % and prevalence ratios (PR) of 4.54; 3.72; 4.47; 2.51 and 12.9 respectively for respiratory difficulties, shortness of breath, wheezing in the chest, coughing without expectoration, and coughing with expectoration.
Between our two groups, the prevalence of spirometric abnormalities was not statistically significant (ER=1.16).
Conclusion
Residents in the vicinity of artisanal smoking sites had a higher prevalence of respiratory clinical manifestations; a trend not found for impairment of respiratory function. Cohort studies could be considered to better assess the health impact.
Keywords: smoking, pollution, restrictive syndrome, lung function.
Introduction
In low and middle-income countries, air pollution is responsible for more than 4.5 million premature deaths [1]. In these countries the incomplete combustion of solid fuels used as a source of energy is the main cause of this pollution; mainly indoor pollution [2-5]. The impact of air pollution, especially fine particulate pollution, on human health is dominated by acute broncho-pulmonary diseases in children under 5 years of age and by chronic broncho-pulmonary and cardiovascular diseases in the elderly [6,7].
In addition, some populations, because of their place of residence such as living near a heavy traffic road, are more exposed to air pollution [8]. This is the same for certain professions such as motorcycle, car, and bus taxi drivers but also law enforcement officers in charge of traffic control [9-13]. Women processing fish through an artisanal smoking process, widespread activity in large cities in West Africa, may be considered highly exposed to air pollution [14,15]. Surveys also showed levels of air pollution in the vicinity of smoking sites, particularly personal exposure to fine particulates smaller than 2.5 µm (PM2.5) and a prevalence of asthma in children under 5 years of age of 18.2 % [16-18].
As with the effects of passive smoking, the activity of artisanal smoking of fish could have an impact on the health of the residents of these sites. The purpose of this study was therefore to determine the health impact of the artisanal smoking activity on the inhabitants in the vicinity of the smoking sites.
Materials and Methods
1. Survey Method and Materials
We conducted a cross-sectional survey of type here-elsewhere from November 1, 2018, to May 31, 2019.
2. Framework and Study Population
Our survey focused on the «Niang on Sud» district of the municipality of Yopougon. This population was divided into two groups: the “exposed” group and the “not exposed” group. The “exposed” group consisted of subjects who resided near the artisanal smoking site of the Lubafrique sector market in the Niangon-Nord district. While the “not exposed” were residents of the same neighbourhood but living far from the smoking site [18]. Yopougon is the largest municipality in Abidjan, the capital of Côte d'Ivoire; even in the entire country [19]. Moreover, the commune of Yopougon is probably the commune of Abidjan if not the country whose level and causes of air pollution are the best known: the levels of indoor and outdoor pollution in fine particulates, emissions of air pollutants from road traffic, the emission of fine particulates at the source of the artisanal smoking site and the concentration of fine particulates near and away from the smoking site of the sector «Lubafrique» neighbourhood «Niangon Sud» of the municipality of Yopougon [7,16,18,20]. The population of this municipality is estimated at 1,071,543 inhabitants with nearly 44 % under 15 years, a life expectancy of 58.7 years and an asthma prevalence among children under 5 of 18.8 % [17].
3. Data collection
The collection of socio-demographic and clinical data was done using a structured, pre-coded questionnaire filled in by interview of all subjects included in the survey (exposed and non-exposed) by a qualified and trained health worker [21]. The main clinical manifestations collected are difficulty breathing, shortness of breath with least effort, wheezing in the chest, dry cough and cough with expectoration.
Respiratory function data were collected by spirometry using WinspiroPRO according to the ATS protocol [22,23]. WinspiroPRO is a software for managing spirometry and oximetry tests carried out with spirometers of the brand MIR (Medical International Research). The measured ventilatory parameters are interpreted according to the size, age and race of the subject being investigated and represented graphically.
A turbine with a single-use cardboard nozzle is mounted on the sensor, half of which is introduced into the mouth of the respondent. The subject to be investigated has the lips hermetically closed on the tip and the nostrils pinched with a nose clip.
The main spirometric parameters measured were:
- Mobilizable pulmonary volumes (in litres) including the maximum expiratory volume during the first second during a forced exhalation (FEV1), the forced vital capacity (FVC) and the slow vital capacity (LVC). The FEV1/VFC report is used to calculate the Tiffeneau ratio.
- Flow rates; this is the calculation of the derivative of volume per unit of time (litre/second) in particular the peak expiratory flow (PEF) and the average expiratory flows at 25 %, 50 %, 75 % and 100 % of the forced vital capacity.
- These different volumes and flows measured or calculated are influenced by age, gender, height, and race. For this purpose, the Global Lung Initiative (GLI) recommends the use of Z-score, indexes independently of these different parameters. This Z-score is calculated by the ratio of the difference between the measured and predicted value with the residual standard deviation (Z-score = (Measured –predicted) /residual standard deviation); with a Z-score at 2.5 % corresponding to a 95 % CI varying between -1.96 and +1.96. Subjects with a Z-score between -1.96 and +1.96 will therefore represent 95 % of normal subjects, and anyone with a score below - 1.96 or above +1.96 will be considered «abnormal» with a risk of judgement error of 2.5 %. This Z-score is an index independent of height, age, gender and ethnic group and indicates how many standard deviations a subject is excluded from its theoretical value [24,25]. These main spirometric parameters make it possible to identify different syndromes [26].
- Restrictive syndrome results in a decrease in CPT of the predicted value or is suspected in a harmonious reduction of FEV1 and FVC below the lower limit of the standard with a normal or abnormally high Tiffeneau ratio (>110 %) of predicted normal value).
- Obstructive syndrome results in a decrease in the FEV1/FVC ratio below the lower limit of the standard (LIN) or 5th percentile of the predicted normal value.
- Mixed syndrome is the combination of a restrictive syndrome and an obstructive syndrome; These different syndromes are classified according to the FEV1 (% predicted) 4 grades of severity; > 80 % Light, 50-79 % Medium, 30-49 % Severe and 30 % Very severe.
4. Data analysis
The socio-demographic, clinical manifestations and spirometry data were captured using the SPSS DATA software and analyzed using the Epi Info software version 7.2.2.6.
The results of the quantitative variables were expressed by means and standard deviations, and qualitative variables were expressed by numbers and percentages.
In bivariate analysis, a Chi2 Mantel-Haenszel test was used to explore the association between qualitative variables and a student test to explore the association between dependent and quantitative variables The associations between the dependent variable and the independent variables were expressed in Prevalence Ratio (PR) and their corresponding confidence intervals (95 % CI) were estimated.
4.1 Administrative and ethical provisions
Our study was carried out within the framework of the ChairePOL Project, based in Benin and with a sub-regional vocation. This project obtained the approval of the ethics committee of Côte d’Ivoire on number 019/MSLS/CNER-KP of 07 March 2016.
The briefing note to be provided to the subjects included in the survey, which explained the purpose, the conduct of the survey, the rights of the respondents but also the constraints related to this survey. After obtaining their informed consent, the subjects included in our study were interviewed. Each subject was assigned a code allowing their anonymity to be observed throughout the study.
Results
A total of 252 subjects were included in our survey, according to their exposed and non-exposed status. In our study population, the average age was 29.57 8.99 years with extremes of 14 and 67 years, an average height of 167.20 8.38 cm with extremes of 149 and 192 cm, an average weight of 65.34 11.97 kg with extremes of 44 and 130 kg and an average BMI of 23,45 4.49 and the extremes of 15.61 and 44.98. The exposed subjects had an average age of 32.42 9.67 years with extremes of 16 and 67 years and were 49 % male with a sex ratio M/F=0.97. The unexposed subjects had an average age of 29.26 8.6 years with extremes of 14 and 58 years and were mostly male (58.7 %) with a sex ratio M/F=1.42. At the same time, the exposed subjects had an average BMI of 23.68 2.42 compared to 23.11 1.87 in the non- exposed subjects. The two study groups were statistically identical by sex, age and BMI (Table I).
Table I: Comparative table of socio-demographic characteristics in the exposed and unexposed groups
|
Parameters |
Expo |
Non Expo |
Odds ratio |
Khi 2 |
I C95 % |
P |
|
Sociodemographic characteristics |
||||||
|
Gender |
||||||
|
Male |
62 |
74 |
Ref |
|||
|
Female |
64 |
52 |
1.47 |
2.30 |
0.89-2.42 |
0.13 |
|
Age range |
||||||
|
≤ 40 |
102 |
115 |
Ref |
|||
|
40 < |
21 |
11 |
2,15 |
3,85 |
0.99-4,68 |
0.05 |
|
BMI |
||||||
|
18,5 – 25 |
67 |
75 |
Ref |
|||
|
< 18,5 |
10 |
11 |
1.02 |
0.001 |
0.39-2.4 |
0.97 |
|
25 - 30 |
27 |
14 |
2.16 |
4.44 |
1.05-4.46 |
0.03 |
|
> 30 |
8 |
11 |
0.8 |
0.17 |
0.31-2.14 |
0.67 |
1. Clinical manifestations
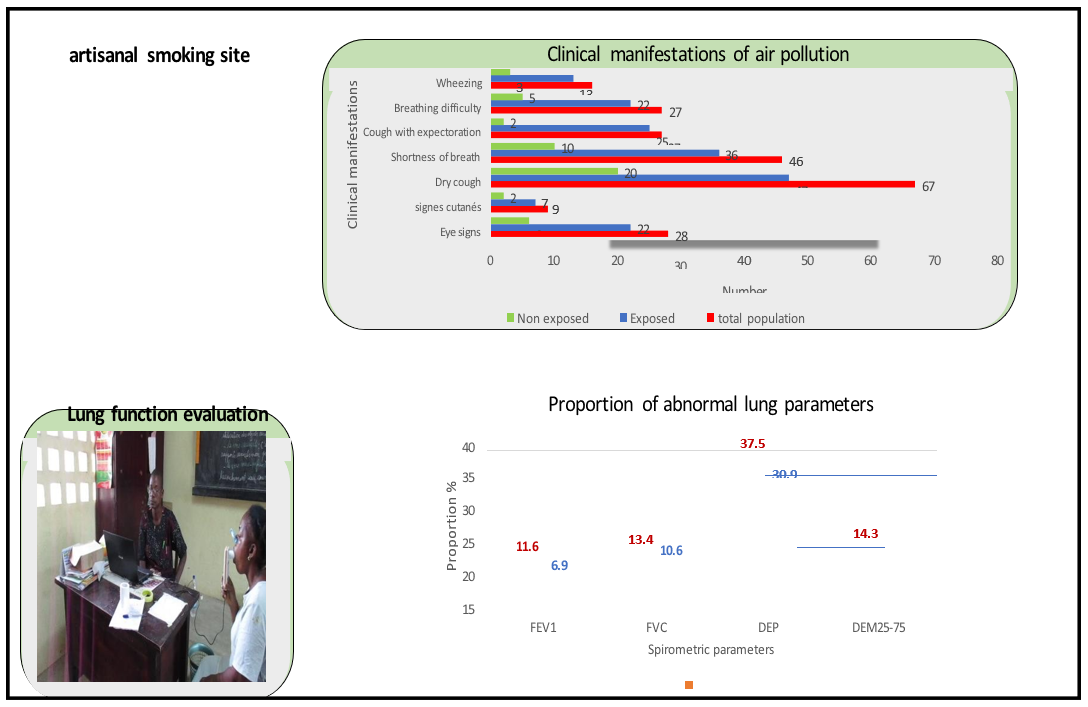
The main clinical manifestations related to air pollution noted during the last twelve months in the subjects included in our survey are:
- Respiratory difficulties in 11 % of all subjects surveyed, 18 % of whom were exposed and 4 % of whom were not exposed with PR=4.54.
- Shortness of breath at least effort in 18.85 % of all subjects surveyed, of which 30 % were exposed and 8.1 % were not exposed with RP=3.72.
- Wheezing in the chest in 6.50 % of all subjects surveyed, of which 10.7 % were exposed and 2.4 % were not exposed with RP=4.47.
- Cough without expectoration in 26.45 % of all subjects surveyed, of which 40.2 % were exposed and 15 % were not exposed with RP=2.51.
- Cough with expectoration in 11.16 % of all subjects surveyed, 21 % of whom were exposed and 1.6 % of whom were not exposed with PR=12.9.
In our entire study population, males were less likely to have difficulty breathing (PR = 0.36; CI0.05 [0.16-0.80]) and shortness of breath (PR = 0.51; IC0.05 [0.29-0.87]). The incidences of wheezing, cough without sputum, and cough with sputum were not statistically influenced by the gender of subjects in our survey (Table II).
At the same time, subjects over the age of 40 were more likely to have a cough with expectoration than subjects under the age of 40. The onset of breathing difficulties, shortness of breath, wheezing and coughing without expectoration was not influenced by age (Table II). Likewise, subjects exposed to pollutants from the smoking site were more likely to have difficulty breathing (PR=4?54; CI0.05 [1.78- 11.62]), shortness of breath (PR=3.72; CI0.05 [1.93-7.15]), wheezing (PR=4.47; CI0.05 [7.31-15.32]), cough without expectoration (PR=2.51; CI0.05 [1.59-3.97]) and cough with expectoration (PR=12.9; CI0.05 [3.13-53.35]) (Table II).
Table II: Comparative table of the prevalence of clinical manifestations by gender, age, and exposure status.
|
Sociodemographic parameters |
Clinical manifestations |
RP |
Khi2 MH |
CI95 % |
P |
||
|
Yes |
No |
||||||
|
|
Breathing difficulty |
||||||
|
Gender |
Male |
8 |
124 |
0.36 |
7.01 |
Ref 0.16-0.80 |
0.008* |
|
Female |
19 |
95 |
|||||
|
Age |
≥ 40 |
5 |
28 |
1.47 |
0.67 |
0.59-3.60 |
0.6 |
|
˂ 40 |
22 |
191 |
|||||
|
Exposed |
22 |
99 |
4.54 |
12.60 |
Ref 1.78-11.62 |
0.0003* |
|
|
Non exposed |
5 |
120 |
|||||
|
|
|
Shortness of breath |
|
|
|
|
|
|
Gender |
Male |
17 |
114 |
0.51 |
6.35 |
Ref 0.29-0.87 |
0.01* |
|
Female |
29 |
84 |
|||||
|
Age |
≥ 40 |
10 |
36 |
1.87 |
3.26 |
Ref 0.96-3.66 |
0.07 |
|
˂ 40 |
23 |
175 |
|||||
|
Exposed |
36 |
84 |
3.72 |
19.10 |
Ref 1.93-7.15 |
0.0001* |
|
|
Non exposed |
10 |
14 |
|||||
|
|
|
Wheezing |
|
|
|
|
|
|
Gender |
Male |
06 |
125 |
0.52 |
1.70 |
Ref 0.20-1.40 |
0.19 |
|
Female |
10 |
105 |
|||||
|
Age |
≥ 40 |
04 |
29 |
2.15 |
1.97 |
Ref 0.74-6.28 |
0.16 |
|
˂ 40 |
12 |
201 |
|||||
|
Exposed |
13 |
108 |
4.47 |
07.01 |
Ref 7.31-15.32 |
0.008* |
|
|
Non exposed |
03 |
122 |
|||||
|
Dry cough |
|||||||
|
Gender |
Male |
40 |
88 |
1.32 |
1.72 |
Ref 0.87-2.00 |
0.19 |
|
Female |
27 |
87 |
|||||
|
Age |
≥ 40 |
10 |
24 |
1.07 |
0.06 |
Réf 0.61-1.89 |
0.81 |
|
˂ 40 |
57 |
151 |
|||||
|
Exposed |
47 |
70 |
2.51 |
17.56 |
Ref 1.59-3.97 |
0.0003* |
|
|
Non exposed |
20 |
105 |
|||||
|
Cough with expectoration |
|||||||
|
Gender |
Male |
12 |
115 |
0.72 |
0.78 |
Ref 0.35-1.48 |
0.38 |
|
Female |
15 |
100 |
|||||
|
Age |
≥ 40 |
08 |
19 |
2.65 |
7.1 |
Ref 1.33-5.31 |
0.007* |
|
˂ 40 |
24 |
191 |
|||||
|
Exposed |
25 |
94 |
12.9 |
22.83 |
Ref 3.13-53.35 |
0.00001* |
|
|
Non exposed |
2 |
121 |
|||||
2. Spirometry
The spirometry sessions involved 228 subjects, 90.5 of the subjects surveyed and 114 subjects from each study group. But 223 or 98 % who participated in the spirometry sessions were able to succeed including 112 subjects from the group of exposed and 111 subjects from the group not exposed. The majority of the spirometry subjects had a normal body mass index (BMI between 18.5 and 25) and a subnormal body mass index with proportions of 60 % and 24 % respectively in the exposed and 68 % and 13 % in the unexposed.
In the group of exposed subjects.
- Z-scores average of FEV1 was -0.75 0.20.
- Z-scores average FVC was -0.96 0.44.
- Z-scores average of FEV1/CCF was 1.64 1.12.
- Z-scores average of DEM25-75 was -1.03 0.13. In the group of unexposed subjects.
- Z-scores average of FEV1 was -0.72 0.26.
- Z-scores average FVC was -0.96 0.15.
- Z-scores mean FEV1/FVC was 0.86 1.02.
- Z-scores average of DEM25-75 was -0.94 0.80.
Across our study population, different spirometric abnormalities were found (Table III).
In the exposed group, 11.61 % FEV1, 13.40 % FVC, 14.29 % DEM25-75 were abnormal compared to 07.08 % FEV1, 10.62% FVC, 11.50 % DEM25-75 in the unexposed group.
The multivariate analysis found no statistically significant difference in the incidence of spirometric abnormalities between our two study groups; FEV1 (PR=1.68; CI0.05 [0.72-3.90]), FVC (PR=1.26; CI0.05 [0.62-2.57]), DEM25-75 (PR=1.24; CI0.05 [063-2.46]).
The overall prevalence of spirometry syndromes was 25 % (56/223). Among the syndromes identified with spirometry, the most common were restrictive syndromes with a proportion of 89 % (50/56) and obstructive syndromes or asthma had a proportion of 07.14 % (4/56). The vast majority of restrictive syndromes (96 %) and all obstructive syndromes (100 %) were mild and moderate in severity.
This proportion was 27.68 % (31/112) and 22.52 % (25/111) respectively in the exposed and non-exposed groups. In the exposed, the proportions of the different spirometric syndromes were 87.01 % (27/31), a prevalence of 24 % for restrictive syndromes and 09.67 % (03/31), a prevalence of 2.7 % for obstructive syndromes. In the non-exposed group, restrictive syndromes represented 92.00 % (23/25) of spirometric syndromes, a prevalence of 20.7 % versus 0.8 % (01/25) for obstructive syndromes, a prevalence of 0.1 %.
In addition, the multivariate analysis found no significant difference in the incidence of spirometric abnormalities in our two study groups; restrictive syndromes (PR=1.21; CI0.05 [0.64-2.28]), obstructive syndromes (PR=3.00; CI0.05 [0.31-29.56]) (Table III).
Table III: Comparative table of the prevalence of impaired lung function in the two study groups.
|
|
Spirometric parameters |
RP |
Khi2MH |
IC95% |
P |
|
|
Abnormal |
Normal |
|||||
|
FEV1 |
||||||
|
Exposed |
13 |
99 |
1.68 |
1.50 |
Ref 0.72-03.90 |
0.22 |
|
Non exposed |
08 |
108 |
||||
|
FVC |
||||||
|
Exposed |
15 |
97 |
1.26 |
0.41 |
Ref 0.62-2.57 |
0.52 |
|
Non exposed |
12 |
101 |
||||
|
DEP |
||||||
|
Exposed |
42 |
70 |
1.21 |
1.06 |
Ref 0.84-1.74 |
0.3 |
|
Non exposed |
35 |
78 |
||||
|
DEM25-75 |
||||||
|
Exposed |
16 |
96 |
1.24 |
0.39 |
Ref 0.63-2.46 |
0.53 |
|
Non exposed |
13 |
100 |
||||
|
FEV1 / FVC |
||||||
|
Exposed |
08 |
104 |
1.34 |
0.32 |
Ref 0.48-3.75 |
0.57 |
|
Non exposed |
06 |
107 |
||||
|
|
Restrictive syndrome |
|
|
|
|
|
|
|
Yes |
No |
|
|
|
|
|
Exposed |
27 |
85 |
1.16 |
0.37 |
Ref 0.71-1.90 |
0.54 |
|
Non exposed |
23 |
88 |
||||
Discussion
The purpose of this survey was to determine the health impact of the artisanal smoking activity on people living near these sites
1. Clinical symptoms
Across our study population, lung symptomatology prevalence ranges from 6.5 % for wheezing in the chest, 11 % for breathing difficulties,
11.16 % for fatty cough with expectoration, 18.85 % for shortness of breath at the least effort and 26 % for dry coughs. Acute respiratory infections that are manifested by induced signs are, after malaria, the second reason for consultation in Côte d'Ivoire in children under 5 years 164.5 as well as in the general population 59.1 [27]. After headache and fever, lung signs (cough, runny nose, wheezing) are the most frequently reported clinical events in people working in risky professions such as salespeople on the major highways and bus drivers in Dakar [8,13]. These manifestations alter the quality of daily life, and, in addition, they are indicators or revealing of acute or chronic pathologies; allergic, infectious and tumour [27].
One of the main causes of lung diseases is air pollution especially fine particulates, which appears to be an important factor of respiratory complications, especially for developing countries that use biomass and coal fuels for heating and cooking [28].
Long-term exposure to ambient air pollution was associated with a number of pulmonary clinical manifestations, such as wheezing, coughing, or mucus [29]. This could confirm the occurrence of the morbid effects of artisanal smoking activity on residents of smoking sites in our survey. In comparison to subjects not exposed to pollutants from the smoking site, the subjects exposed were statistically more at risk of presenting pulmonary symptomatology.
2. Spirometric data
The main parameters assessed at spirometry are mobilizable lung volumes and expiratory flows. These parameters are strongly influenced by ethnicity, age, height, and weight [25,30].
Various spirometric abnormalities were identified in the main measured parameters (FEV1, FVC and DEM25-75). The decrease in DEM25-75 with a prevalence of 12.89 % across our study population was the most common spirometric abnormality. This drop-in DEM25-75 reflects an altered flow rate in small bronchi; a precursor sign of chronic obstructive pulmonary disease (COPD) [31]. These alterations are relatively greater in the exposed group (14.29 % versus 11.50 %), but the prevalence of the decrease in DEM25-75 in both groups was not statistically significant.
Our study population was relatively young overall but had a high prevalence of precursor signs of COPD and was living permanently in an environment with PM2.5 concentrations above WHO standards. All this reflects a potential great risk for our surveyed groups, also of the population of the municipality of Yopougon as a whole to develop COPD at a later age. Moreover, this potential risk is corroborated by the diagnosis of the obstructive ventilatory disorder in 1.8 % of our total study population of about 30 years of average age; 02.7 % in exposed versus 0.1 % in non-exposed. Likewise, symptoms suggestive of bronchial syndromes such as cough with expectoration and shortness of breath at least effort reported.
Our overall study population has a restrictive syndrome prevalence of 22.4 %. The restrictive syndrome reflects among other things an impairment of pulmonary parenchyma of which one of the main etiologies is of infectious origin [26]. This could explain the high prevalence of restrictive syndromes across our study population as well as acute respiratory infections (ARI) in the Ivorian population and particularly among children under 5 years of age [27]. This is also confirmed by the fact that air pollution especially fine particulate pollution is recognized as a factor promoting the occurrence and also a factor aggravating lung infections [32].
The prevalence of restrictive syndromes appears to be higher in the exposed group compared to the non-exposed group, but this difference is not statistically found.
The most vulnerable to air pollution are children under five (5) years of age, the elderly and those who carry tares but in general low socio-economic populations. Our study population was mostly young adults; living with those most vulnerable to air pollution, the clinical manifestations obtained may be greater in more vulnerable subjects in terms of frequency and virulence as well as spirometric abnormalities especially obstructive ventilatory disorders in the elderly.
Limitations
Our survey has two main limitations. The first is related to the type of survey carried out; the cross-sectional survey allows us to assess the situation in the form of an image at a given moment. A longitudinal survey would have been more appropriate. The second limitation relates to the fact that clinical data were collected by interview. Our data may therefore be subject to respondent bias (memory bias) and confusion bias. However, these limitations do not affect the power of the observations. Indeed, in addition to the clinical manifestations, we have collected spirometric data; especially since the data were collected by qualified personnel and for the interpretation of the results of spirometry, we have made use of the lower limit of the standard (LIN) the predicted normal values and the Z-score index; totally independent of the social and anthropometric parameters.
Conclusion
The purpose of this study was to determine the health impact of air pollution-induced by the activity of artisanal smoking on the residents. The analysis of our results in a high prevalence of pulmonary clinical manifestations and spirometric syndromes in the whole of our study population but more marked in the inhabitants near the sites of artisanal smoking.
Our study population was theoretically less vulnerable than children under 5 years of age, pregnant women, people with chronic diseases, and older people, particularly those who would reside near artisanal smoking sites. Mitigation measures should therefore be considered. Moreover, cohort studies could also be considered for all residents in the vicinity of artisanal smoking sites in order to better assess the health impact.
Acknowledgements
The authors thank N'BGOCHO Sosthene, ATTOUMOU Marcelin, Dr. Konan and the staff of the Saint Sauveur Clinic of Yopougon- Lubafrique for their decisive role in data collection. This survey was funded by the Ecosanté Chair in Urban Air Pollution and Non- Communicable Diseases (ChairePol) and the International Development Research Centre (IDRC) Grant No. 107347-001. Our deep gratitude to the coordination of ChairePol.
Author Contributions
Conception and design conceptualization: M’bégnan Coulibaly, Attoh-Toure Harvey.
Analysis and interpretation of data: M’bégnan Coulibaly, Attoh- Toure Harvey and Kouassi, René Kouao Ahua.
Drafting the article: M’Bégnan Coulibaly; Attoh-Toure Harvey and Kouassi René Kouao Ahua
Revising: Salifou Yéo.
Final approval: Yoboue Véronique and Tiembré Issaka.
Conflict of Interest: The authors state that there is no conflict of interest regarding the publication of this manuscript.
Funding: This survey was funded by the Ecosanté Chair in Urban Air Pollution and Non-Communicable Diseases (ChairePol) and the International Development Research Centre (IDRC) Grant No. 107347-001
References
- Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, et al. (2018) The Lancet Commission on pollution and health. Lancet. 391(10119): 462–512.
- World Health Organization WHO. WHO Guidelines for Indoor Air Quality: Household Fuel Combustion. 2014.
- Bautista LE, Correa A, Baumgartner J, Breysse P, Matanoski GM (2009) Indoor charcoal smoke and acute respiratory infections in young children in the Dominican Republic. American journal of epidemiology. 169(5): 572-580.
- Ezzati M, Lopez AD, Rodgers AA, Murray CJL (2004) Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. 1er vol. Geneva World Health Organization.
- Khalequzzaman M, Kamijima M, Sakai K, Hoque BA, Nakajima T (2010) Indoor air pollution and the health of children in biomass-and fossil-fuel users of Bangladesh: situation in two different seasons. Environmental health and preventive medicine. 15(4): 236-43.
- Lelieveld J, Haines A, Pozzer A (2018) Age-dependent health risk from ambient air pollution: a modelling and data analysis of childhood mortality in middle-income and low-income countries. Lancet Planet. Health. 2(7): e292-e300.
- Kouao AKR, N’datchoh ET, Yoboue V, Silue S, Attoh H, et al. (2019) Exposure to indoor and outdoor air pollution among children under five years old in urban area. Global J. Environ. Sci. Manage. 5(2).
- Ndong A, Verdin A, Cazier F, Garcon G, Thomas J, et al. (2019) Individual exposure level following indoor and outdoor air pollution exposure in Dakar (Senegal). Environmental Pollution. 248: 397-407.
- Sylla FK, Faye A, Fall M, Lo M, Diokhané A, et al. (2017) Near Road Exposure to Air Pollution and Allergic Rhinitis: A Cross- Sectional Study among Vendors in Dakar, Senegal. Occupational Diseases and Environmental Medicine. 5(4): 106-120.
- Limasset JC, Diebold F, Hubert G (1993) Exposition des conducteurs de bus urbains aux polluants de la circulation automobile. Science of The Total Environment. 134: 39-49.
- Zagury E, Le Moullec Y, Momas I (2000) Exposure of Paris taxi drivers to automobile air pollutants within their vehicles. Occup Environ Med. 57(6): 406-10.
- Maitre A, Soulat JM, Masclet P, Stoklov M, Marquès M, et al. (2002) Exposure to carcinogenic air pollutants among policemen working close to traffic in an urban area. Scand J Work Environ Health. 28(6): 402-10.
- Sylla FK, Faye A, Diaw M, Fall M, Tal-Dia A (2018) Traffic Air Pollution and Respiratory Health:A Cross-Sectional Study among Bus Drivers in Dakar (Senegal). Open Journal of Epidemiology. 8(1): 1-13.
- Agodokpessi G, Ade G, Hinson V, Ade S, Okoumassou C-X, et al. (2011) Prévalence des troubles respiratoires chez les femmes exerçant sur un site artisanal de fumage de poisson à Cotonou au Bénin. Mali Médical. 26(4): 34-38.
- Chabi NW, Konfo CTR, Emonde PDM, Chichi MTC, Chabi Sika KJK, et al. (2014) Performance d’un dispositif amélioré de fumage (four chorkor) sur la qualité du poisson fumé dans la commune d’Aplahoué (Sud Est du Bénin). International Journal of Innovation and Applied Studies. 9(3): 1383–1391.
- Djossou J, Léon JF, Akpo AB, Liousse C, Yoboué V, et al. (2018) Mass concentration, optical depth and carbon composition of particulate matter in the major southern west African cities of Cotonou (Benin) and Abidjan (Côte d’Ivoire). Atmos Chem Phys. 18: 6275-6291.
- René KAK, Kouadio K, Siele S, Harvey AT, M’begnan C, et al. (2019) Prevalence of Asthma in Children Under 5 Years old Exposed to air Pollution in Abidjan, (Côte D’ivoire). Int J Recent Sci Res. 10(07): 33353-33358.
- Coulibaly M, Attoh-Toure H, Kouao AKR, Kouassi PD, Yoboue V, et al. (2021) Traditional Smoking and Personal Exposure to Particulate Matter (PM2.5) in Urban Area in Abidjan (Côte D’Ivoire). Journal of Environmental Science and Public Health. 5(2): 226-239.
- Institut National de la Statistique (INS). Enquête sur le niveau de la vie des ménages en Côte d’Ivoire. ENV.
- Doumbia M, Toure ND, Silue S, Yoboue V, Diedhiou A, et al. (2018) Emissions from the road traffic of west Africa’s cities: assessment of vehicle fleet and fuel consumption. Energies. 11(9): 2300.
- Schilmann A, Riojas-Rodriguez H, Ramirez-Sedeno K, Berrueta VM, Pérez-Padilla R, et al. (2015) Children's Respiratory Health After an Efficient Biomass Stove (Patsari) Intervention. Ecohealth. 12(1): 68-76.
- Breysse PN, Diette GB, Matsui EC, Butz AM, Hansel NN, et al. (2010) Indoor Air Pollution and Asthma in Children. Am Thorac Soc. 7(2): 102-6.
- Redlich CA, Tarlo SM, Hankinson JL, Townsend MC, Eschenbacher WL, et al. (2013) Official American ThoracicSociety Technical Standards: Spirometry in the Occupational Setting. American Journal of Respiratory and Critical Care Medicine. 189(8): 983–93.
- Nozha BS, Dorra B, Houda S, Yacine O, Nadia M, et al. (2017) Le Z-score : Nouvel outil dans l’interprétation des données spirométriques. La Tunisie Medicale. 95: 767-71.
- Kamanga BM, Kayembe JMN, Nkiama CE, Kayembe PK, Kikontwe LK, et al. (2019) Valeurs spirométries de référence dans la population bantoue de Kinshasa de 20 à 70 ans. The Pan African Medical Journal. 33: 295.
- Pierre-Olivier B, Nicolas P, Konrad EB, Jean-Marc F, Jean-Paul J, et al. (2018) Exploration de la fonction pulmonaire par spirométrie. SWISS MEDICAL FORUM – FORUM MÉDICAL SUISSE. 18(2627): 555–562.
- Direction de l’informatique et de l’information sanitaire (DIIS) Rapport annuel sur la situation sanitaire (RASS) 2018 Edition 2019 Abidjan ; 2019.
- Won HK, Lee JH, An J, Sohn KH, Kang MG, et al. (2020) Impact of Chronic Cough on Health-Related Quality of Life in the Korean Adult General Population: The Korean National Health and Nutrition Examination Survey 2010-2016. Allergy Asthma Immunol Res. 12(6): 964-979.
- Kurt OK, Zhang J, Pinkerton KE (2016) Pulmonary Health Effects of Air Pollution. Curr Opin Pulm Med. 22(2): 138–143.
- Guénard H, Rouatbi S (2004) Aspects physiologiques du vieillissement respiratoire. Rev Mal Respir. 21(5): 13-24.
- Fischberg S. Motamed S. Janssens JP (2009) Pratique et interprétation de la spirométrie au cabinet du médecin de premier recours. Rev Med Suisse. 5(218): 1882-1889.
- Horne BD, Joy EA, Hofmann MG, Gesteland PH, Cannon JB, et al. (2018) Short-Term Elevation of Fine Particulate Matter Air Pollution and Acute Lower Respiratory Infection. American Journal of Respiratory and Critical Care Medicine. 198(6): 759- 66.
Graphical Abstract