Kafayat Motomori Bakare1, Anyanwu Margaret Nkeiruka2, Ugochukwu Okwudili Matthew3*, Godwin Nse Ebong4, David Oyekunle5, Emmanuel Adebola Adebanjo1
1Public Health (MPH), University of New Haven, USA.
2Kano State College of Nursing and Midwifery, Nigeria.
3Computer Science Department, Hussaini Adamu Federal Polytechnic, Nigeria.
4Data Science Department, University of Salford, United Kingdom.
5Project Management Department, University of Salford, United Kingdom.
*Corresponding Author: Ugochukwu Okwudili Matthew, Computer Science Department, Hussaini Adamu Federal Polytechnic, Nigeria.
Abstract
Analysis of cells, soluble substances, and related tissues and cells that cooperate to extend life continues to awaken researchers' curiosity. Because of its incredible complexity, the immune system is opaque to the outer world. Scholars strive to comprehend the several facets that combine to give a comprehensive picture of the system's operation. Thus, various tactics have been acknowledged for their role in the development of immunotherapy, including genetic approaches, animal models, specialized tools, monoclonal antibodies, and immunological techniques. Much research has been done to understand the effects of immunotherapy on human bodies to develop a range of treatments. Understanding the various aspects of immunotherapy's role in health, sickness management, and aging has been made possible by the discoveries about cell subsets, membrane molecules, and cell functions. A thorough grasp of the systems and elements that provide an enabling platform for welfare management has resulted from a focus on the aspects of immunotherapy. Welfare management falls under the purview of developing various immunotherapies, which have served as the cornerstone for sustainable welfare management. The impact of the application of immunotherapy has been explored in the context of efficient service delivery, ranging from cancer to tumor management. The effect on human bodies has been clarified by developing several immunotherapies, such as adopting infections, adopting them prophylactically, and using monoclonal antibodies and recombinant proteins. Investing in immunotherapy has, therefore, been responsible for the change in how we treat many diseases. The necessity to investigate immunotherapy and its impact on human bodies has led to the development of an enabling platform for examining and adequately managing diseases.
Keywords: Immunotherapy, Tumor Immunology, Cancer Immunotherapy, Cancer Treatment, Enzymatic Glycosylation influences
Introduction
Immunotherapy-related information intelligence has advanced significantly in recent years, and new developments are expected to change how services are provided shortly [1]. Gaining situational awareness has helped people respond appropriately to a variety of situations. The appraisal of the immune system's significant impact on the body has been evident due to its complex structure, which has created a need for new knowledge and abilities in its study and control. The development and assessment of immunotherapy involve multiple dimensions, including monoclonal antibodies that facilitate detecting a wide range of cell subpopulations[2]. Determining the effects on human bodies now mainly depends on the assessment of single-cell function, imaging techniques, omics, and data processing. The foundation for comprehending immunotherapy, then, is the establishment of a collection of strategies in support of immune system management competence. Immunotherapy's effects on human bodies should thus be highlighted in research on the interactions between the immune system and other systems, including the neurological, endocrine, and microbiota [3,4]. The number of patients with cancer is rising every year, making it the second most deadly disease in the world after cardiovascular disorders [5]. Radiation therapy, chemotherapy, targeted medication therapy, and surgery are the main procedures used today to treat tumors.
A postoperative recurrence of the tumor is a common occurrence with conventional surgical resection since it is difficult to eradicate all tumor cells. More damage to patients results from chemotherapy and radiation treatment, which can kill tumor cells in addition to healthy cells [6]. Tumor resistance and recurrence are common adverse effects of targeted medication therapy, even though it can lessen side effects. As tumor biology has advanced, immunotherapy has become a potent clinical tool for treating cancer. Tumor immunotherapy is distinct from traditional treatments in that it primarily targets immune cells by stimulating the body's immune system to eradicate tumor cells. Its benefits include minimal side effects, sound effects, and recurrence avoidance. Considerable progress has been made in cancer treatment within the last ten years. By strengthening their immune systems, patients can now combat cancer thanks to the development of immunotherapies such as immune checkpoint inhibitors, cellular immunotherapy, antibody treatment, and cytokine therapy. Regretfully, as immunotherapy develops further, adverse effects such as off-target effects have surfaced. These may be caused by the intricacy of the therapeutic regimen and the decline in T-cell survival. Specialized T cells within the immune system eliminate specific foreign objects. T cells circulate rather than target every antigen until they encounter their particular antigen. T cells are, therefore, essential for defense against pathogens.
Multistep Glycosylation Proteins Targeting Agents
In cancer therapy based on nanotechnology, various proteins, including modified proteins and antibodies, have been used as targeting agents[7]. It is possible to design these proteins to recognize specific antigens or receptors that are overexpressed in malignant cells. One way to enhance the tumor-targeting capabilities of nanoparticles is to conjugate them with monoclonal antibodies [8]. Two more protein engineering strategies that have enabled the creation of new proteins with high selectivity for targets associated with cancer are phage display and recombinant deoxyribonucleic acid (DNA) technology. Specific antigens or receptors overexpressed on the surface of cancer cells can be identified using recombinant technologies or monoclonal antibodies produced from hybridoma cells. These antibodies' ability to transport therapeutic medications straight to the tumor site is enhanced by conjugating them with nanoparticles. Monoclonal antibodies are beneficial for focusing on particular cancer indicators because of their high specificity. Their clinical application must take into account the limitations they provide, including possible immunogenicity and restricted tissue penetration [9].
A customizable method of targeting cancer is provided by engineered proteins produced by recombinant DNA technology. Targeted therapy and diagnostic imaging can be carried out on a flexible platform so that these proteins can be arranged to bind preferentially to cancer-associated indicators. Relative to conventional antibodies, engineered proteins provide the benefit of lower immunogenicity [10]. However, since they can be expensive and hard to produce, additional optimization is required to make the manufacturing process more efficient. Another class of targeting agents is called aptamers, and these are single-stranded DNA or RNA molecules with particular three-dimensional architectures that allow them to attach firmly to biomarkers specific to cancer [11]. Aptamers produced by in vitro selection procedures have a high selectivity, which makes them valuable instruments for targeted drug delivery and imaging. One appealing aspect of them is that they are less immunogenic than antibodies.
Nevertheless, maintaining their stability in biological settings is still a problem that needs to be solved through continued research. A distinct strategy for genetically targeting cancer cells is using small interfering RNAs, or siRNAs[12,13]. SiRNAs can be created chemically or using recombinant technology, and they have the ability to silence genes that are essential for the growth and survival of cancer cells. Because of this precision in gene regulation, there is hope for gene therapy and the suppression of genes linked to cancer.
As helpful as siRNAs are, there are still problems that need to be resolved, including effective delivery and the possibility of off-target effects [14]. In a typical chemical synthetic or recombinant product, peptide ligands attach to particular cell surface receptors to enhance cell penetration and targeted cancer therapy. These adaptable targeting agents have the potential to be used in multi-targeted techniques due to their configurable nature. But for the best clinical usage, issues with their stability in biological contexts and effective delivery methods need to be resolved. Targeting agents that interact with particular signaling pathways implicated in the growth of cancer include a wide array of small chemical compounds [15]. Their ability to have a variety of chemical structures and drug-like qualities makes them advantageous for cancer treatment. Research is still being done to maximize their specificity and selectivity while maintaining stability and delivery to the tumor location. Dual-action targeted therapy is made possible by fusion proteins, which integrate the roles of therapeutic and targeting molecules. These proteins were produced using recombinant DNA technology, which increases therapy efficacy while lowering adverse effects [16]. In clinical applications, their design and fabrication might be intricate, requiring considerable study.
Figure 1: Recent developments on protein glycosylation and its function in tissue regeneration and repair[17].
Concerning Figure 1, the multi-step process of glycosylation, which takes place in both the ER and the Golgi, involves several different enzymes. Depending on whether their coupling to the protein core is through Ser or Asp residues, respectively, the O-linked and N-linked oligosaccharide decorating proteins are categorized [18]. Glycosylation is attaching a carbohydrate, or glycosyl donor, to a hydroxyl or other functional group of another molecule to form a glycoconjugate. In biology, a reaction that is catalyzed by an enzyme is commonly referred to as glycosylation, but a non-enzymatic occurrence may be referred to as glycation.
Experimental Section
According to [19], immunotherapy is a field of study that focuses on improving the body's capacity to recognize and eliminate foreign cells or dangers. The main focus of immunotherapy is the advancement of knowledge in immunology, a field concerned with studying the immune system [20]. The increased understanding of how immune defenses might be used to treat various disorders has spurred interest in the topic. Surgeons and experts have been working hard to figure out how to control the immune system to help attach itself to or eliminate bodily hazards, such as cancer cells. The management approach has included an assessment of the fact sheets on current trends in handling present diseases [21]. The foundation of studying the role in the welfare management of humans has been an introduction to the natural immune system, focusing on its ability to counteract the impacts of cancer cells. The critical assessment areas have been developing comprehensive evaluations of immune checkpoint inhibitors, monoclonal antibodies, adoptive cell transfer, chimeric antigen receptor (CAR) T-cell therapy, and therapeutic vaccines. The fields of study, in conjunction with the body's immune system, have been the focal point of attention for immunotherapy [22].
According to [23], who asserted that the body's primary defense against illnesses like cancer has historically been the immune system, protecting the body from infections, fungus, bacteria, viruses, and cancerous cells, the immune system is a complex network of organs, tissues, and cells. It is essential to maintaining the body's wellness. Defense depends on the body's ability to discriminate between its cells and alien ones. As such, the primary function of the immune system is to distinguish between foreign substances that are safe and those that cause illness. Immune system cells need to be evaluated to control the body's health appropriately because they are essential to the battle against infections [24].
The production of antigens that recognize and target antigens is crucial for several types of lymphocytes, including B-cells, T-cells, and NK cells. B cells are also present in the lymphatic system, including the bone marrow. In addition to helping B cells produce antibodies to combat the invasive pathogen, T lymphocytes perform other activities. As NK cells primarily target aberrant cells and eliminate viruses, their significance is commensurately highlighted by the immune systems evaluation [25]. Standard immune systems function by sending lymphocytes throughout the body to destroy anything seen to be intrusive. By using receptors to analyze antigens on cell surfaces, immune cells use this process to look for alien cells. Upon identification of the antigen by the immune system, the synthesis of antibodies to combat foreign cells is evident, and T-cell activation to eliminate the aberrant cells is triggered [26].
Cancer and Immune System
To fully comprehend immunotherapy, its function in cancer treatment must be thoroughly examined. According to [27], the immune system can stop many malignancies from happening since it can eliminate aberrant cells before they develop into cancer. A word used to characterize the methods by which the immune system scans the body for precancerous abnormalities that may already be present is immunosurveillance by way of the immunological system. It's clear to monitor the cancer-causing proteins, particularly when it comes to cell surfaces. Immunosurveillance is essential in ensuring that malignant cells are eliminated before they reach a critical mass and start to grow into the disease. However, cancer development may not always be avoided by a robust immune system. Even in a functioning immune system, some cells can proliferate and divide. There is cause for concern because immunoediting allows malignancies to avoid the immune system and increase quickly [28]. It is necessary to evaluate immunoediting to understand the aspects of elimination, balance, and escape. The process through which the immune system finds and eradicates cancer cells from the body is known as elimination. Across most body regions, cell elimination is dominant, and immune system balance can develop. When cancer cells are in equilibrium, their ability to proliferate and resist removal by the immune system is compromised [29]. Therefore, balance means that although the immune system can suppress cancer cells, it cannot completely eradicate them.
The immune system and cancer cells can interact in ways that allow cancer cells to change their genetic makeup [30]. It falls within the scope of the conduct to permit avoiding immune system detection and destruction. Within the escape step, which comprises acquiring the ability to evade immune recognition and eradication, are the cancer cells. Cancer can grow and progress, which affects bodily well-being. During the escape phase, malignant cells use a variety of strategies to change the body's immune response, which may promote the spread of cancer [31]. To reactivate the immune system and combat the aberrant cells, immunotherapy based on the evaluation of the body is crucial. The Food and Drug Administration (FDA) has approved various forms of immunotherapy, and their statistical results can provide valuable insight into their efficacy. Critics like [32] contend that immunotherapy methods, including therapeutic vaccinations, adoptive cell transfer, immune checkpoint inhibitors, and monoclonal antibodies, are essential for the body's defense. Therapeutic approaches are instrumental in addressing blood cancers as they provide information on the response mechanism.
Immunotherapy approaches and human bodies: Immune checkpoint inhibitors.
According to [33], who gave emphasis, T cell checkpoints are innate proteins that control the cells' response to invading cells. As a result, T-cells are distributed throughout the body to detect indications of infections and illnesses, such as cancer. In addition, [34] confirms that T-cells have trouble using some proteins' receptors near another cell. Attacks are launched immediately if the proteins under inspection show signs of being foreign. To combat the intruder, the checkpoint thus interacts with the T-cells to initiate proliferation. The cells receive signals to stop the multiplication response once the intruder has been destroyed. Extended T-cell activation, however, can result in aberrant responses to body-harmless cells [35]. Autoimmune diseases like Crohn's disease and rheumatoid arthritis might arise due to the possible destruction of healthy cells. To prevent aberrant cell behavior, the immune system must produce enough white blood cells to combat invasive cells and lower their population once the attack has been successfully carried out. According to [36], PD-1 and PD-L1 are the two checkpoint proteins that work in tandem to shut down T-cells following a proliferation response. They are present in T-cells and ensure that the cells do not assault healthy cells that are not supposed to be attacked.
According to [37], the immune system receives a signal to divert from an attack when the PD-1 binds to the PD-L1 protein found in normal cells. As a result, there is a decrease in T-cell production, which permits others to pass away. Only after it establishes a connection with the immune system does PD-1 instruct it to slow. However, by producing PD-L1, cancer cells may be able to evade immune system attacks. Responding T-cells investigate the surfaces of cancerous cells but cannot identify them and do not launch an attack, allowing the cancer cells to proliferate. To treat certain tumors, immunotherapy relies on checkpoint inhibitors, which attach to the PD-1 receptor on T cells and prevent PD-L1 and PD-1 from interacting. This strategy prevents the immune system from decelerating, allowing T-cells to stay activated and attack the cancerous cells [29]. As a result, pembrolizumab and nivolumab, two checkpoint inhibitors, need statistical analysis. The effectiveness of pembrolizumab in treating invasive cells, viruses, or bacteria still requires statistical analysis.
The therapy is likely authorized for patients in both adult and pediatric categories who frequently have refractory Hodgkin lymphoma or who have experienced a relapse following three or more prior treatments. When treating patients with primary mediastinal large B-cell lymphoma or those who have relapsed after two or more lines of therapy, pembrolizumab is used equally in both adult and pediatric settings [38]. Conversely, nivolumab is used to treat lymphoma that either has returned or is still progressing following autologous hematopoietic stem cell transplantation. The chosen treatment strategy is predicated on utilizing three or more lines of systemic therapy, which includes auto-HSCT [39]. With the auto-HSCT process, autologous hematopoietic stem cells are infused to facilitate hematological recovery following high-dose chemotherapy, which would otherwise be intolerable. As a result, immune checkpoint inhibitors are administered intravenously, and the duration of treatment ranges from 30 to 60 minutes [40]. The evaluation of the immunotherapy process is essential due to the variances depending on the type of cancer.
Adoptive cell transfer
Immunotherapy is the process of using the patient's T-cells to help fight cancerous cells. Consequently, the patient's blood cells or the tumor cells already present in the lab are treated with substances that allow them to identify and eliminate the invasive malignant cells [41]. Although many forms of adoptive cell transfer therapies exist, the Chimeric Antigen Receptor (CAR) therapy is the most advanced in clinical development and application. The extraction of the patient's T cells via an apheresis process is a crucial step in CAR T-cell therapy. During the phases, blood is extracted from one of the large veins using an apheresis machine to separate the blood into its component parts [42]. The patient receives their remaining blood back after just T-cells are removed from the blood. Chemotherapy is administered to the patient before the CAR cells being delivered; this helps the body get ready for the new cells [43]. When the CAR T-cells are received, they multiply and realize that they must destroy the cancer cells because their surfaces contain the targeted antigen. As a result, the CAR T-cells stay in the body for months after infusion and help certain cancer patients experience remission. Approval of the CAR T-cells is therefore limited to younger patients and requires a thorough evaluation of its effectiveness based on available data [44].
Monoclonal Antibodies
The immune system uses the billions of antibodies it produces as one weapon against foreign invaders. [45], stress that a defensive strategy must acknowledge antibodies as crucial proteins that cling to an antigen, such as a virus or bacteria. Therefore, antibodies travel throughout the body until they come into contact with and adhere to a specific antigen that the antibody is capable of binding to, thanks to its receptors. Once attachment is established, the antibody can enlist immune system components to aid in the elimination of the foreign cells. Researchers like [46] admit that the method of treating cancer has improved thanks to the development of laboratory-designed antibodies that target particular antigens. The capacity to identify and target antigens may reduce the damage that often befalls normal cells. Monoclonal antibodies designate cancer cells so the immune system can identify and eliminate them. Antibodies that are monoclonal are crucial in the battle against cancerous cells. Treatments utilizing naked monoclonal antibodies, for example, do not depend on extraneous elements such as the use of radioactive materials or drugs attached to cells. Most monoclonal antibodies bind to antigens on malignant cells. However, some can bind to antigens on non-cancerous cells.
Treatments using monoclonal antibodies that connect to two different proteins at similar periods are known as bispecific antibodies [47]. A bi-specific T-cell antibody known as blinatumomab functions by attaching to the CD19 present in leukemia cells. There is another portion of the blinatumomab that binds to the immune cell protein CD3. The medication effectively targets cancer cells and immune cells by attaching to both proteins, which triggers T-cell activation and attack. Conjugated monoclonal antibodies, on the other hand, are medications that treat aberrant cells by connecting radioactive material or chemotherapy to them [48]. The cells have been modified such that they can administer medication to cancerous cells. Examining the nascent immunotherapies that may provide insight into their effects on human bodies and their ability to treat malignant cells has been equally essential. Novel immune checkpoint targets, macrophage checkpoint antibodies, and tumor-specific T-cell guided treatment are promising research directions. Additionally, evaluating the direct effects on an individual's welfare depends heavily on therapeutic vaccinations. In an effort to create the most efficient means of combining artificial medication with the immune system, researchers work to refine the treatments. It is within the scope of assessment to maintain the appropriate therapy parameters, as this can influence the response to the invading cells.
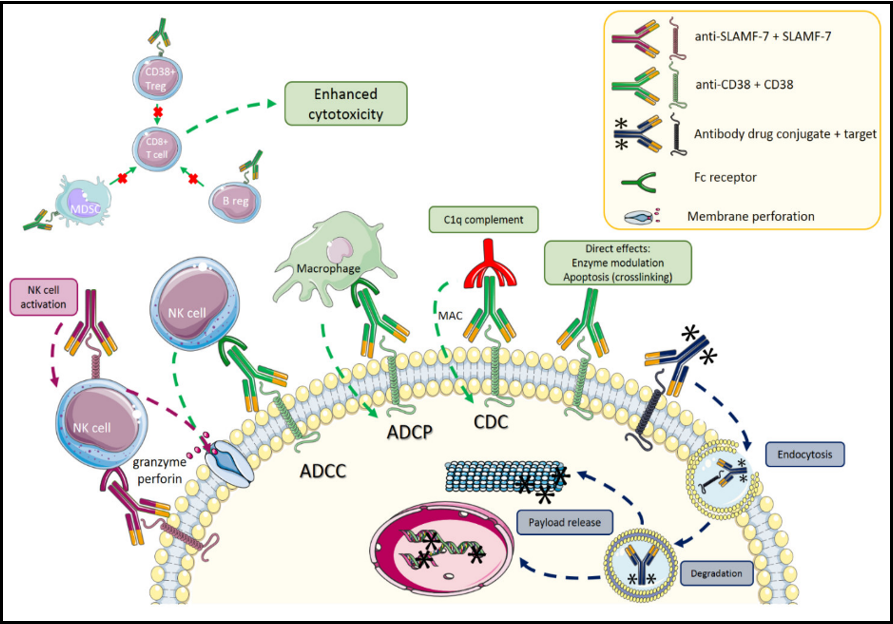
Figure 2: Antibody drug conjugates with monoclonal antibodies in multiple myeloma [49]
With reference to Figure 2, action mechanisms of several unbound antibodies and antibody-drug conjugates. Impacts of antibody drug conjugates (ADC) in dark blue, SLAMF-7 in purple, and CD38 in green. Terminologies: NK stands for natural killer, Treg for regulatory T cells, B reg for regulatory B cells, ADCC for antibody-dependent cellular cytotoxicity, ADCP for antibody-dependent cellular phagocytosis, CDC for complement-dependent cytotoxicity, and MAC for membrane attacking complex.
Statistical Analysis
An extensive analysis of the available data on immunotherapy's efficacy is necessary to develop the best methods. The methods for evaluating the various treatments varied in their efficacy and safety profiles. Furthermore, the foundation for adequate service delivery may be provided by the exponential availability of study designs and patient analytic techniques. The patient's short- and long-term survival depends on evaluating the available facts to guide decisions. The idea behind immunotherapies is to boost the body's defenses against malignant cells by stimulating the immune system [32]. A crucial function is played by focusing on targeting antigens expressed within cancer cells. Now that the four immunotherapy techniques have been identified, it is critical to understand the statistical results of their effectiveness. Targeting antigens expressed within cancer cells, for instance, has become possible thanks to the development of monoclonal antibodies [50]. Finding and modifying the chemicals that control immune systems has been made possible by this finding. Because of this, a sizable portion of the immunotherapy medicines utilized in cancer treatment are represented by antibodies. According to [51], who emphasized the significance of acknowledging monoclonal antibodies as a symbol of several therapeutic modalities necessitating a thorough evaluation of the body's defense mechanisms. The cytotoxic T lymphocyte, linked to the protein CTLA-4 that boosts immunity, can be effectively blocked by the full-length human monoclonal antibody ipilimumab [52].
A person's total survival can be statistically improved when they are aware of the combination of dacarbazine and the cell. It is clear that randomized control trials have produced insight into the effectiveness of treatment [53]. Understanding how well the cells are working to combat cancer cells has been made possible by utilizing clinical trials and evaluating the advancements made in the therapeutic process. The best response models may be developed by focusing on several stages of exposure to the enabling cells. Because of this, [54] stress how quickly contemporary therapeutic treatments have evolved to meet the needs of welfare management. The development of immunotherapeutic treatments has been fundamental to the management of healthcare and is gaining significant importance. The emphasis on adoptive transfer of modified T-cells, such as CAR T-cell therapy, which continues to produce substantial response rates and encouraging outcomes, has been especially important. The ability to affect how the body functions and produce favorable results during the course of treatment has been the main focus of the immunotherapy approach. Because of its potential to have transformative effects, CAR T-cell therapy has been acknowledged as the cornerstone of sustainable healthcare management. According to [55], the therapeutic strategy has dramatically improved patient treatment outcomes and has been credited with achieving notable response rates. Treating patients with specific hematological malignancies is arguably the main focus of the immunotherapy strategy. However, due to underlying issues that need a thorough examination, patients either relapse or do not respond to the therapy. In order to come up with the ideal methods for managing health, it is now essential to look at the reaction process from the point of initial remission to the underlying need to investigate methods to reevaluate the efficiency criteria. According to [54], the focus of current research is on examining the in vivo activity, tumor invasion, and trafficking of CAR T-cells, as well as their physiological impact.
The potential of CAR T-cell therapy to revolutionize immunotherapy, particularly in the treatment of cancer, is reviewed statistically by [54]. The successful therapy outcomes and relapse prevention measures produce encouraging outcomes. Developing strategies to get past the current restrictions has been made possible by gaining a significant grasp of the aspects underlying the therapy process. The investigation of CAR T-cell therapy is vital, according to analysts like [56], because it will provide insight into areas for improvement and demonstrate persistence in vivo. Utilizing genetically modified cells, the procedure is based on adopting many therapeutic modalities, including radiation, chemotherapy, and non-cellular immunotherapy. A transformational strategy for treatment is based on offering a promising platform for altering the characteristics of the cell [56]. Following the clinically approved procedures for implementing CAR T-cell therapy might provide insight into the parameters of improvement that may result in success. Treatment potential includes focusing on cell development to produce a more effective CAR T-cell therapy. To understand the possibility of acceptance in immunotherapy, the assessment areas can be analyzed through a statistical presentation of existing findings.
The investigation of ways to reduce the likelihood of relapse and guarantee that the body reacts to the treatment process in an efficient manner is the basis of the therapy. As a result, the implementation process has focused on how surgery, chemotherapy, radiation, and CAR T-cell treatment interact with one another. A vital component of the strategy's implementation has been the understanding that PD-1 and PD-L1 may respond favorably to the treatment. The results of the retrospective adoption are as follows. CAR T-cell therapy targeting PD-1 and PD-L1 has shown promise in its ability to influence the immune system. According to meta-analyses, the co-administration of second-generation CAR T-cells and monoclonal antibodies that target PD-1 has proved essential for immune system regulation. According to [57], the results assessment revealed 95% skewedness and 0.17 potential due to the coefficient congruence assessment. Improved immunological response results have been consistently obtained by upregulating the production of interleukin-2 and interferon-gamma. Using cells as a framework for a more significant response to cancer cells and their impacts on the body has been essential to health management. Examining immune checkpoint inhibitors' efficacy in cancer treatment reveals a novel avenue. Its premise for role analysis in the treatment process is its use as an immunotherapy tool. Thus, to associate the cure models, [58] conduct a meta-analysis using the Cox-TEL approach. There is a mathematical relationship between the construction of an algorithm and the performance efficiency assessment. As such, the methodology relied on identifying suitable treatment-effect estimates from the PH-cure technique. The approach was focused on input-only data presented by the coefficient covariance analysis, with implications for the immune response process being examined [19].
In order to evaluate each respondent's and participant's proper output in the experimental technique, emphasis was placed on the usage of algorithms. As a result, the platform for evaluation included the interaction of the control group, the extra study population, and the assessment of the survival treatment group [56]. Therefore, evaluating the survival curves was crucial to the assessment process, as reported in the report on the random controlled technique. The similarly thorough review of the regression analysis was a vital focus of the immune checkpoint inhibitor research. One emerging treatment crucial to comprehending the spread and elimination of cancer cells is adoptive cell transfer.
Consequently, statistical analysis is based on assessing the adoptive cell transfer's methodological efficacy. To investigate and reduce the spread of invasive cells within the body [58], provide a particular review of adoptive cell transfer. The creation of a statistical evaluation of overall survival, progression, and response length is crucial to the research. Examining the body's reaction and the infusion date is part of the testing process.
A complete evaluation of the cancer that requires systemic therapy is a crucial part of the research, following which the patient may be removed in order to monitor the disease's course and carry out a cancer-specific study of survival at the time of pathologic diagnosis. Investing in a follow-up suggests that the data cut-off function method approach is being applied during the computation process. For the statistical analysis to be conducted, the infusion process must be evaluated and classified using the Kaplan-Meier survival curves as the foundation. The correlation of the p-values is also clearly visible thanks to the dualistic technique, which entails adjusting with multiple platforms for comparison. Meanwhile, [19] conducted a subset analysis into prognostic parameters to gain insight into the potential effects of adoptive cell transfer on the body. For the purpose of developing crucial information regarding the impacts on the immune system, univariate analysis is equally significant. A statistical analysis used to assess the direct influence on the respondents is based on the utilization of multivariate logistic regression.
Result
The bulk of research on immunotherapy concentrates on clinical trials to identify implementation strategies and evaluate the impact on human bodies. The monoclonal antibodies thus demonstrate that it is a significant cancer therapeutic modality [59]. It is important to comprehend the relevance of the many mechanisms revealed by the longitudinal approach. Thus, the research findings indicate that the methodology plays a pivotal role in determining the effectiveness results within the management procedure. Research indicates that promising engineering strategies are used in the answers [59]. When tumor-targeted monoclonal antibodies are combined with other approaches, the framework for evaluating the signaling pathways and antibody targets can be established. To evaluate the effectiveness and potential resistance of the treatment protocols, biomarkers for monoclonal antibodies are required. In treating cancer patients, treatment paradigms involving monoclonal antibodies may provide a potentially curative platform.
In contrast, immune checkpoint inhibitors emphasize the need for a thorough review of randomized clinical trials to determine their potential therapeutic implications [60]. An extensive analysis both before and after the Cox-TEL adjustments that are required in the clinical trials is indicated by the report on the survival endpoints. Findings from the algorithms show that there is a discernible 10% increase in the probability of both short- and long-term survival. Moreover, the survival data make it evident that treatment outcomes vary over time.
The analysis of the responses and identification of the changing areas provide the whole picture of the survival results. It is imperative to advance a consistent analysis and convey an understanding of the survival advantages in the primary analysis. The examination of a pool of respondents about treatment response should indicate the necessity of using participatory frameworks when making decisions regarding the well-being of the participants. Positive coefficient congruence, as shown by primary analysis, indicates that immune checkpoint inhibitors may have an impact on immunotherapy [59]. Conversely, the results of CAR T-cell treatment indicate that increasing its use is the best course of action. It's critical to keep situational awareness of the production process because the therapeutic approach depends on the in vivo proliferation and response rate measurement. The results emphasize the necessity of expanding treatment modalities through innovative approaches. The cornerstone for the trial outcomes should be the generation of insight into the T-cell phenotype and the evaluation of the markers [61].
Nonetheless, knowledge of the fatigue markers ought to affect choices made during the study. The evaluation of the immediate and long-term response outcomes is essential to the assessment. Adoptive T-cell treatment and CAR T-cell therapy interaction fall under the purview of the evaluation process. Based on the statistical analysis, it is very promising to emphasize the combination of adoptive care and CAR T-cell therapy [62]. The foundation for future integration into the wellness evaluation may be provided by requiring several administrations.
Discussion
Numerous immunotherapy modalities demonstrate the therapeutic potential for integration, particularly in the context of cancer treatment. The potential for significant improvements, particularly in the evaluation of clinical outcomes—may have served as the driving force behind its adoption. The immune system can be manipulated using a combination of randomized and statistical approaches. Consequently, the host immunological milieu is critical, and the clinical trials fall under the purview of evaluating the consequences of the methods of therapy. The processes used in clinical trials and the routine evaluation of patients both before and after assessments of the patient present significant barriers to the use of immunotherapy.
Conclusion
Understanding how the human body reacts to invasive species and malignant cells can be gained through immunotherapy. For the treatment of cancer, the various methods are effective and safe choices. They do, nevertheless, provide light on the difficulties that can affect the process of implementation. The cell-centric techniques have the potential to provide beneficial results. Implementing localized evaluation and using participatory methods should be the main focus of first-line therapy techniques. Accessibility should be predicated on understanding the immune system's influence on the body and the recognition of treatment techniques as the foundation for patient management in the future. Owing to their implementation in clinical trials, immunotherapy approaches do not exhibit significant disputes. It is possible to get beneficial results by prioritizing their evaluation as the foundation for the welfare assessment.
Conflict of interest: There is no conflict in this manuscript.
References
- Vladimirov N, Perlman O (2023) Molecular MRI-Based Monitoring of Cancer Immunotherapy Treatment Response. International Journal of Molecular Sciences. 24(4): 3151.
- Gorodilova AV, Kitaeva KV, Filin IY, Mayasin YP, Kharisova CB, et al. (2023) The Potential of Dendritic Cell Subsets in the Development of Personalized Immunotherapy for Cancer Treatment. Current Issues in Molecular Biology. 45(10): 8053-8070.
- Campbell C, Kandalgaonkar MR, Golonka RM, Yeoh BS, Vijay- Kumar M, et al. (2023) Crosstalk between gut microbiota and host immunity: Impact on inflammation and immunotherapy. Biomedicines. 11(2): 294.
- Dora D, Bokhari SMZ, Aloss K, Takacs P, Desnoix JZ, et al. (2023) Implication of the Gut Microbiome and Microbial-Derived Metabolites in Immune-Related Adverse Events: Emergence of Novel Biomarkers for Cancer Immunotherapy. International Journal of Molecular Sciences. 24(3): 2769.
- Mehta LS, Watson KE, Barac A, Beckie TM, Bittner V, et al. (2018) Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation. 137(8): e30-e66.
- Kwon S, Jung S, Baek SH (2023) Combination Therapy of Radiation and Hyperthermia, Focusing on the Synergistic Anti- Cancer Effects and Research Trends. Antioxidants. 12(4): 924.
- Tiwari H, Rai N, Singh S, Gupta P, Verma A, et al. (2023) Recent advances in nanomaterials-based targeted drug delivery for preclinical cancer diagnosis and therapeutics. Bioengineering. 10: 760.
- Kumari M, Acharya A, Krishnamurthy PT (2023) Antibody- conjugated nanoparticles for target-specific drug delivery of chemotherapeutics. Beilstein Journal of Nanotechnology. 14: 912- 926.
- Chehelgerdi M, Chehelgerdi M, Allela OQB, Pecho RDC, Jayasankar N, et al. (2023) Progressing nanotechnology to improve targeted cancer treatment: overcoming hurdles in its clinical implementation. Molecular cancer. 22: 169.
- Kandari D, Bhatnagar R (2023) Antibody engineering and its therapeutic applications. International Reviews of Immunology. 42(2): 156-183.
- Yoo H, Jo H, Oh SS (2020) Detection and beyond: Challenges and advances in aptamer-based biosensors. Materials Advances. 1: 2663-2687.
- Ranasinghe P, Addison ML, Dear JW, Webb DJ (2023) Small interfering RNA: Discovery, pharmacology and clinical development—An introductory review. British Journal of Pharmacology. 180: 2697-2720.
- Huang C, Li M, Chen C, Yao Q (2008) Small interfering RNA therapy in cancer: mechanism, potential targets, and clinical applications," Expert opinion on therapeutic targets. 12: 637-645.
- Khan S, Rehman U, Parveen N, Kumar S, Baboota S, et al. (2023) siRNA therapeutics: insights, challenges, remedies and future prospects. Expert Opinion on Drug Delivery. 20(9): 1167-1187.
- Dimri M, Satyanarayana A (2020) Molecular signaling pathways and therapeutic targets in hepatocellular carcinoma. Cancers. 12: 491.
- Glick BR, Patten CL (2022) Molecular biotechnology: principles and applications of recombinant DNA: John Wiley & Sons.
- Yue Z, Yu Y, Gao B, Wang D, Sun H, et al. (2023) Advances in protein glycosylation and its role in tissue repair and regeneration," Glycoconjugate Journal. 40(3): 355-373.
- Krasnova L, Wong CH (2016) Exploring human glycosylation for better therapies. Molecular Aspects of Medicine. 51: 125-143.
- Zahavi D, Weiner L (2020) Monoclonal antibodies in cancer therapy. Antibodies. 9(3): 34.
- Colciago RR, Fischetti I, Giandini C, La Rocca E, Rancati T T, et al. (2023) Overview of the synergistic use of radiotherapy and immunotherapy in cancer treatment: current challenges and scopes of improvement. Expert Review of Anticancer Therapy. 23: 135- 145.
- Shusharina N, Yukhnenko D, Botman S, Sapunov V, Savinov V, et al. (2023) Modern Methods of Diagnostics and Treatment of Neurodegenerative Diseases and Depression. Diagnostics. 13(3): 573.
- Swallow J (2023) Enrolling the body as active agent in cancer treatment: Tracing immunotherapy metaphors and materialities. Social Studies of Science. 27: 3063127231199217.
- Mattiola I, Diefenbach A (2023) Regulation of innate immune system function by the microbiome: Consequences for tumor immunity and cancer immunotherapy. Seminars in Immunology. 66: 101724.
- Ashique S, Kumar S, Hussain A, Mishra N, Garg A, et al. (2023) A narrative review on the role of magnesium in immune regulation, inflammation, infectious diseases, and cancer. Journal of Health, Population and Nutrition. 42(1): 117.
- Fan YN, Zhao G, Zhang Y, Ye QN, Sun YQ, et al. (2023) Progress in nanoparticle-based regulation of immune cells. Medical Review. 3(2): 152-179.
- Kyrysyuk O, Wucherpfennig KW (2023) Designing cancer immunotherapies that engage T cells and NK cells. Annual review of immunology. 41: 17-38.
- He Y, Huang J, Li Q, Xia W, Zhang C, et al. (2022) Gut Microbiota and Tumor Immune Escape: A New Perspective for Improving Tumor Immunotherapy. Cancers. 14(21): 5317.
- Aragon-Sanabria V, Kim GB, Dong C (2018) From cancer immunoediting to new strategies in cancer immunotherapy: the roles of immune cells and mechanics in oncology. Biomechanics in Oncology. 1092: 113-138.
- Farhana A, Alsrhani A, Khan YS, Rasheed Z (2023) Cancer bioenergetics and tumor microenvironments—Enhancing chemotherapeutics and targeting resistant niches through nanosystems. Cancers. 15(15): 3836.
- Le TT, Oudin MJ (2023) Understanding and modeling nerve– cancer interactions. Disease Models & Mechanisms. 16(1): dmm049729.
- Zhu Y-H, Zheng J-H, Jia Q-Y, Duan Z-H, Yao H-F, et al. (2023) Immunosuppression, immune escape, and immunotherapy in pancreatic cancer: focused on the tumor microenvironment. Cellular Oncology. 46: 17-48.
- Jha AM, Veerakumar R, Puhazhendhi T, Kesavan R, Naveenraj NS (2023) Immunotherapy's Promising Potential for Cancer Treatment: Oncology-Immunotherapy for Cancer. International Journal of Trends in OncoScience. 1(3): 25-35.
- Dutta S, Ganguly A, Chatterjee K, Spada S, Mukherjee S (2023) Targets of Immune Escape Mechanisms in Cancer: Basis for Development and Evolution of Cancer Immune Checkpoint Inhibitors. Biology. 12(2): 218.
- Toor SM, Saleh R, Sasidharan Nair V, Taha RZ, Elkord E (2021) T‐ cell responses and therapies against SARS‐CoV‐2 infection. Immunology. 162: 30-43.
- Lu X (2017) Dissecting the Genetic Etiology of Lupus at ETS1 Locus. University of Cincinnati.
- van Gulijk M, van Krimpen A, Schetters S, Eterman M, van Elsas M, et al. (2023) PD-L1 checkpoint blockade promotes regulatory T cell activity that underlies therapy resistance. Science Immunology. 8(83): eabn6173.
- Banday AH, Abdalla M (2023) Immune Checkpoint Inhibitors: Recent clinical advances and future prospects. Current Medicinal Chemistry. 30(28): 3215-3237.
- Camus V, Bigenwald C, Ribrag V, Lazarovici J, Ja rdin F, et al. (2021) Pembrolizumab in the treatment of refractory primary mediastinal large B-cell lymphoma: safety and efficacy. Expert Review of Anticancer Therapy. 21(9): 941-956.
- Goklemez S, Hasni S, Hakim FT, Muraro PA, Pirsl F, et al. (2022) Long-term follow-up after lymphodepleting autologous haematopoietic cell transplantation for treatment-resistant systemic lupus erythematosus. Rheumatology. 61: 3317-3328.
- Sheng J, Srivastava S, Sanghavi K, Lu Z, Schmidt BJ, et al., (2017) Clinical pharmacology considerations for the development of immune checkpoint inhibitors. The Journal of Clinical Pharmacology. 57: S26-S42.
- Liao K, Zhang X, Liu J, Teng F, He Y, et al. (2023) The role of platelets in the regulation of tumor growth and metastasis: the mechanisms and targeted therapy. MedComm. 4(5): e350.
- Gniadek T (2021) Production of components by apheresis. Transfusion Medicine. 90-110.
- Yakoub-Agha I, Chabannon C, Bader P, Basak GW, Bonig H, et al. (2020) Management of adults and children undergoing chimeric antigen receptor T-cell therapy: best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE). haematologica. 105(2): 297-316.
- Hartmann J, Schüßler-Lenz M, Bondanza A, Buchholz CJ (2017) Clinical development of CAR T cells—challenges and opportunities in translating innovative treatment concepts. EMBO molecular medicine. 9(9): 1183-1197.
- Sompayrac M (2022) How the immune system works: John Wiley & Sons.
- Ghai T, Das A, Patel R (2022) The Investigation of the Effect of Antibody Recruiting Molecules on Various Antigenic Markers (Cancer, Bacteria, Viruses): A Literature Review. Undergraduate Research in Natural and Clinical Science and Technology Journal. 6(1): 1-7.
- Guo X, Wu Y, Xue Y, Xie N, Shen G (2023) Revolutionizing cancer immunotherapy: unleashing the potential of bispecific antibodies for targeted treatment. Frontiers in Immunology. 14: 1291836.
- Etrych T, Braunova A, Zogala D, Lambert L, Renesova N, Klener P (2022) Targeted drug delivery and theranostic strategies in malignant lymphomas. Cancers. 14(3): 626.
- Radocha J, van de Donk NWCJ, Weisel K (2021) Monoclonal antibodies and antibody drug conjugates in multiple myeloma. Cancers. 13: 1571.
- Muluh TA, Chen Z, Li Y, Xiong K, Jin J, et al. (2021) Enhancing cancer immunotherapy treatment goals by using nanoparticle delivery system. International Journal of Nanomedicine. 16: 2389- 2404.
- Huppert LA, Green MD, Kim L, Chow C, Leyfman Y, et al. (2022) Tissue-specific Tregs in cancer metastasis: opportunities for precision immunotherapy. Cellular & molecular immunology. 19(1): 33-45.
- Fessler J, Matson V, Gajewski TF (2019) Exploring the emerging role of the microbiome in cancer immunotherapy. Journal for immunotherapy of cancer. 7: 108.
- Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA (2018) The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer cell. 33(4): 570-580.
- Abdoli Shadbad M, Hemmat N, Khaze Shahgoli V, Derakhshani A, Baradaran F, et al. (2022) A systematic review on PD-1 blockade and PD-1 gene-editing of CAR-T cells for glioma therapy: from deciphering to personalized medicine. Frontiers in Immunology. 12: 788211.
- Safarzadeh Kozani P, Naseri A, Mirarefin SMJ, Salem F, Nikbakht M, et al. (2022) Nanobody-based CAR-T cells for cancer immunotherapy. Biomarker Research. 10: 24.
- Scott AM, Allison JP, Wolchok JD (2012) Monoclonal antibodies in cancer therapy. Cancer immunity. 12: 14.
- Hoti K, Atee M, Chivers P, Vahia I, Hughes J (2023) Technology- guided assessment of vocalisations and their diagnostic value as pain indicators for people living with dementia. Age and Ageing. 52(6): afad088.
- Seitter SJ, Sherry RM, Yang JC, Robbins PF, Shindorf ML, et al. (2021) Impact of prior treatment on the efficacy of adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma. Clinical Cancer Research. 27(19): 5289-5298.
- Blache U, Popp G, Dünkel A, Koehl U, Fricke S (2022) Potential solutions for manufacture of CAR T cells in cancer immunotherapy. nature communications. 13(1): 5225.
- Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, et al. (2019) Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance," Nature reviews Clinical oncology. 16: 563-580.
- Mehta PH, Fiorenza S, Koldej RM, Jaworowski A, Ritchie DS, et al. (2021) T cell fitness and autologous CAR T cell therapy in haematologic malignancy. Frontiers in Immunology. 12: 780442.
- Gonzalez Castro LN, Dietrich J (2023) Evaluation of the Functional Performance of KLK2 Chimeric Antigen Receptor (CAR) T Cell Designs. Drexel University. 8(3): 259-265.