Ting Ting Feng*, Su Lone Lim, Chun Peng Goh, Nivedh Dinesh, Shiong Wen Low, Ira Sun Siyang
Division of Neurosurgery, Department of General Surgery, Ng Teng Fong General Hospital, Singapore
*Corresponding author: Dr. Ting Ting Feng, Division of Neurosurgery, Department of General Surgery, Ng Teng Fong General Hospital Tower B Level 2, 1 Jurong East Street 21, Singapore.
Abstract
We present a case of a patient who developed Hypoxic Ischemic Encephalopathy is a neurological complication due to severe Coronavirus disease in 2019 (COVID-19).COVID-19 is a pandemic disease caused by a novel coronavirus - severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2). Since its emergence in December 2019, it has rapidly spread to the rest of the world, with more than 25million reported cases globally to date. Patients with COVID-19 often present with fever, cough, and dyspnea, among other non-specific symptoms. AlthoughCOVID-19 primarily affects the respiratory system, systemic complications are common, especially in severe disease. Recently there has been increasing clinical evidence of neurological manifestations and complications associated with COVID-19. Although rare, Hypoxic Ischemic Encephalopathy is one of the possible neurological complications of COVID-19, especially in severe disease.
Keywords: COVID-19; hypoxic ischemic encephalopathy; neurological complications
Introduction
In December 2019, a cluster of pneumonia cases of unknown cause emerged in Wuhan city, China. Subsequent analysis of the respiratory samples of these patients identified a novel coronavirus - severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the disease caused by this virus was named coronavirus disease 2019 (COVID-19) [1]. Since its emergence, there has been a rapid spread of the virus to many other countries, leading to a global pandemic. As of Aug 30th, 2020, there has been more than 25 million reported cases and more than 847,500 deaths worldwide. Common clinical manifestations of COVID-19 include fever, cough, dyspnea, myalgia and fatigue, among other non-specific symptoms [2-4]. While the majority of COVID-19 cases result in mild respiratory disease, some patients can rapidly progress to acute respiratory distress syndrome (ARDS), and/or develop systemic complications such as acute renal failure, heart failure, liver failure, coagulopathy or septic shock [3-5]. With more clinical data as the disease emerges, it was reported that COVID-19 can potentially damage the nervous system as well and cause neurological manifestations [6-9]. Here we report a case of a patient with COVID-19 who developed severe Hypoxic Ischemic Encephalopathy (HIE) and eventually died from it, with clinical and radiological findings.Case report
A 58-year-old, previously healthy woman presented to the emergency department with 3 days’ history of worsening shortness of breath. She also reported cough, rhinorrhea, and fever of the same duration. She recently travelled to Turkey and returned 5 days before she presented it to the hospital. One of the family members living with her was recently diagnosed to be COVID-19 positive. She appeared ill. Her body temperature was 39.9 °C, heart rate 106 beats per minute, respiratory rate 17 breaths per minute, and blood pressure 156/100 mmHg. Her oxygen saturation was 95 % on room air.
The typical symptoms and the positive contact and travel history raised a high index of suspicion for COVID-19. The patient was admitted to an isolation ward and had a full workup done, including routine laboratory tests, a nasopharyngeal swab for COVID-19 RNA PCR, and a chest x-ray. Her initial full blood count showed a white blood cell count of 6.24 with lymphopenia (lymphocytes absolute was 0.93). The presence of SARS-CoV-2 RNA was detected in her nasopharyngeal swab. Her initial chest x-ray on admission was normal, but subsequent serial x-rays showed worsening bilateral patchy ground-glass consolidations in the next few days (figure 1), in keeping with COVID-19 pneumonia.
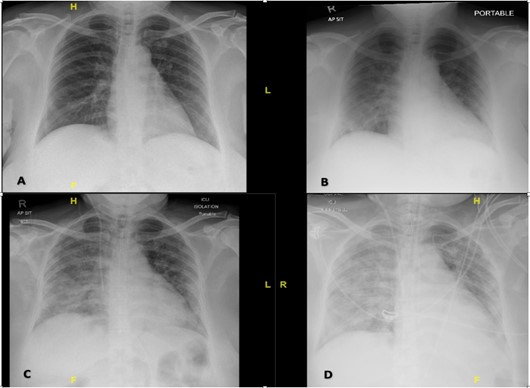
Figure 1: Chest X-rays showing interval development of bilateral patchy ground glass opacities characteristic of COVID-19 infection. X-rays were taken on Day 1 (A), day 5 (B), day 7 (C) and day 8 (D) of admission, respectively.
The patient was initially managed with high flow nasal cannula oxygen therapy in the ward, but she developed type 1 respiratory failure on day 8 of admission and required endotracheal intubation and admission to ICU. The therapies she received for COVID-19 pneumonia include hydroxychloroquine, multiple courses of antibiotics in the course of her disease, steroids, nitric oxide, and nebulized heparin. The monoclonal antibody was considered when she developed possible cytokine release syndrome but was not administered after extensive discussion weighing the risks and benefits. To optimize her pulmonary function, various treatment strategies were used, including steroids, sedation, paralytics, and prone ventilation, etc. Despite these efforts, the pO2 on her arterial blood gas was still suboptimal most of the time. She failed extubating twice and remained on high ventilation requirements since her admission to ICU. In addition to ARDS, she developed multiple complications including ventilator-associated pneumonia, septic shock, acute renal failure, acute liver failure, and cytokine release syndrome.
On the evening of day 34 of admission, her pupils were found to be fixed and dilated during routine ICU assessment. Her pupils were both 4mm and reactive to light when last checked 2 hours ago. Her GCS (Glasgow Coma Scale) has been E1VTM1 since she was last re- intubated 9 days ago (GCS was E3 - 4M6 before that), and she hasbeen kept on sedation since then. Urgent CT brain was performed and showed diffuse cerebral edema with near complete effacement of the basal cisterns, Sylvian fissures, and cerebral sulci. The ventricles were slit like. There is a decrease in cortical grey matter attenuation with a loss of normal grey-white differentiation. The scan did not show any evidence of acute territorial infarct or intracranial hemorrhage. A CT angiogram of Circle of Willis was performed, which showed abrupt truncation of contrast flow at the level of the terminal internal carotid arteries bilaterally. There was progressive non-opacification of bilateral vertebral arteries, with no opacification seen in the intracranial vessels at all, including on the delayed phase images (Figure 2).
Neurosurgery was consulted urgently in view of the scan findings. On examination, the patient’s GCS was E1VTM1; her pupils were both 7mm and nonreactive to light stimulation. There was no corneal reflex. The cough and gag reflexes were both absent. The oculo-cephalic reflex was absent.
In view of the clinical and radiological findings, a diagnosis of severe hypoxic-ischemic encephalopathy was made, and the likely cause is prolonged hypoxemia from ARDS due to COVID-19 infection. A formal brain death testing was performed the next day, and the patient was certified brain dead.
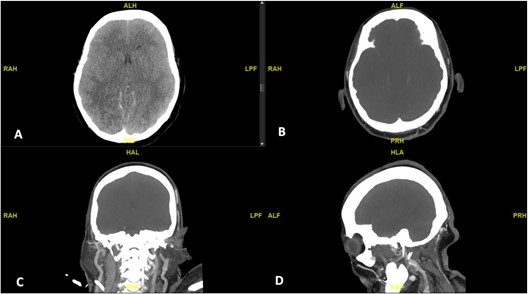
Figure 2: CT brain (A) showing diffuse cerebral edema with near complete effacement of ventricles, cisterns and sulci; there is decrease in cortical grey matter attenuation with a loss of normal grey-white differentiation. CT Angiogram Circle of Willis (B to D) showing abrupt truncation of contrast flow at the level of the terminal internal carotid arteries bilaterally, with no opacification seen in the intracranial vessels.
Discussion
Complications associated with COVID-19 commonly involves the respiratory, cardiovascular, and hematological systems. However, as clinicians gain a better understanding of the disease, there have been more reports of neurological complications of COVID-19 recently, which can be associated with significant morbidity and mortality [6,7]. Common neurological manifestations of COVID-19 include dizziness, headache, impaired level of consciousness, loss of smell/taste, among other symptoms. Significant complications of COVID-19 include cerebrovascular disease, encephalopathy, encephalitis, epilepsy, and Guillain Barre Syndrome.
It is proposed that COVID-19 results in neurological damage likely by two mechanisms: hypoxic brain injury and immune-mediated damage to the central nervous system [8,9]. Severe pneumonia and ARDS have known causes of hypoxia and hypoxemia, which may lead to brain damage through cerebral vasodilation, hypercarbia, anaerobic metabolism with an accumulation of toxic products. These can further decompensate into cerebral edema, ischemia, and ultimately neurological damage [10,11]. On the other hand, systemic inflammatory response from COVID-19 infection results in increases level of inflammatory cytokines, activation of various immune cells, leading to vascular leakage, activation of the complement system, coagulation cascade, producing a massive pro-inflammatory and pro- coagulant state. This pro-inflammatory and pro-coagulant state will eventually result in end-organ damage and multi-organ failure [12]. Acute cerebrovascular disease, or ischemic stroke, remains the most common and serious neurological complication of COVID-19. The etiology is multifactorial and mediated by a global inflammatory response and hyper-coagulable state as described earlier, resulting in higher risk of cerebral vessel occlusion [13].
The patient in our case report is a previously healthy 58-year-old woman, who suffered from severe ARDS secondary to COVID-19 pneumonia. She did not have previous history of neurological disease or cardiovascular disease. However, her Body Mass Index is 35, which may be a possible contributing factor to her severe disease [14]. It is worth noting in the last week before she died, her sepsis, renal function and liver function were all showing improvements. However, her respiratory function remained poor, and ventilating her posed significant challenges. Despite various treatment strategies to optimize her lung function, she remained ventilator-dependent, with persistent hypoxemia on her arterial blood gas. With this clinical history and the radiological findings on her CT brain and CT angiogram, we concluded that the prolonged period of hypoxemia likely caused a hypoxic brain injury, which eventually progressed into HIE and caused her demise. In this case, HIE is a secondary neurological complication, from prolonged hypoxemia.
HIE is a significant cause of mortality and severe neurologic disability. Regardless of the cause of injury, the underlying physiological process that results in HIE is diminished blood flow (ischemia) and reduced blood oxygenation (hypoxemia). With prolonged hypoxemia, cardiac hypoxia occurs and leads to decreased cardiac output, and ultimately to brain ischemia [15]. Imaging findings in HIE are highly variable and early imaging findings can be subtle and often overlooked. On the other hand, many of the treatment strategies for HIE have a limited window of effectiveness, making early detection of injury critically important. Therefore, it is important for clinicians to be aware of HIE as a possible complication of COVID-19, secondary to hypoxia from severe respiratory disease. Common CT findings of HIE include diffuse edema, effacement of the CSF-containing spaces, decreased cortical gray matter attenuation with loss of normal gray-white differentiation and decreased bilateral basal ganglia attenuation [15,16]. In our case, all these findings were present in her CT brain, suggestive of severe HIE. The additional CT angiogram further confirmed the absence of any intracranial blood flow and provided radiological evidence of brain death.
Although there are other entities that can present similar findings on CT scan, such as septic encephalopathy, meningitis, severe traumatic brain injury; these are unlikely in this patient for the following reasons. During the period when she was most septic (second to the third week of admission, at least 10 days before her demise), her GCS had been consistently E3 - 4M6, with no altered mental status or delirium observed. She did not suffer any trauma or head injury either. Cerebral venous sinus thrombosis is a possible differential diagnosis, but severely raised intracranial pressure in cerebral venous thrombosis is usually associated with intraparenchymal hemorrhage secondary to increased venous and capillary pressure, causing these vessels to rupture. Hence, with the relevant clinical history and imaging findings (absence of hemorrhage), HIE is the most likely diagnosis. In her last days of life, she was kept sedated to optimize her ventilation and to reduce the work of breathing. The agents used include propofol infusion (50 – 150 mg/Hr), Fentanyl (50 – 150 mcg/Hr), with additional midazolam, dexmedetomidine, and/or paralytic agent (rocuronium) as required. Her GCS and pupil’s size/reactivity were monitored and recorded every 1 – 2 hours according to ICU protocol. Her persistently low GCS in the last days were attributed to sedation. A CT brain was considered and discussed earlier, but it was not done due to the logistic challenges as the patient was on prone ventilation, with ongoing nitric oxide and nebulized heparin. No other neurological evaluation was performed prior to this. The authors feel that this is something that is worth review and discussion, to raise clinicians’ awareness of possible neurological complications associated with COVID-19 so that we can detect them early and treat them promptly.
Conclusion
Neurological involvement in patients with COVID-19 is not uncommon and can result in significant morbidity and mortality, if not detected and intervened early. Patients with severe disease are at higher risk of developing such complications. In addition, those patients are often intubated, sedated, and sometimes paralyzed to facilitate easier ventilation, making neurological monitoring more difficult. In our case, as the patient was GCS 3 and sedated, the only clinical monitoring of the neurological system was her pupil size and reactivity. Hence, we would like to use this case to remind our readers that a high index of suspicion should be maintained, for early detection of potentially fulminant neurological complications. We strongly suggest that frequent GCS and pupil monitoring should be included in the standard of care for all patients with severe COVID- 19 disease. Sedation and paralytic agents should be used with caution, with their dosage and duration frequently reviewed. If there are any concerning signs or symptoms, imaging of the brain should be performed. Although there were not many reported cases of death from HIE in COVID-19 patients, it is important for clinicians to be aware of HIE as one of the possible neurological complication secondary to hypoxia, so that early diagnosis can be made and early treatment can be instituted.
Source(s) of Financial Support: Nil.
Conflicts of Interest: Nil.
References
- World Health Organization (2019) Naming the coronavirus disease (COVID-19) and the virus that causes it.
- Gandhi RT, Lynch JB, Rio CD (2020) Mild or moderate COVID- 19. N Engl J Med.
- Huang C, Wang Y, Li X, Ren L, Zhao J, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223): 497-506.
- Chen N, Zhou M, Dong X, Qu J, Gong F, et al. (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395(10223): 507-513.
- Berlin DA, Gulick RM, Martinez FJ (2020) Severe Covid-19. N Engl J Med.
- Mao L, Jin H, Wang M, Hu Y, Chen S, et al. (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 77(6): 1-9.
- Helms J, Kremer S, Merdji H et al. (2020) Neurologic Features in Severe SARS-CoV-2 Infection. N Engl J Med 382(23): 2268- 2270.
- Ahmad I, Rathore FA (2020) Neurological manifestations and complications of COVID-19: A literature review. J Clin Neurosci 77: 8-12.
- Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, et al. (2020) Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun 87: 18-22.
- Tu H, Tu S, Gao S, Shao A, Sheng J (2020) The epidemiological and clinical features of COVID-19 and lessons from this global infectious public health event. J Infect 81(1): 1-9.
- Sekhon MS, Ainslie PN, Griesdale DE (2017) Clinical pathophysiology of hypoxic ischemic brain injury after cardia carrest: a “two-hit” model. Crit Care 21(90): 30-33.
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, et al. (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395(10229): 1033-1034.
- Bridwell R, Long B, Gottlieb M (2020) Neurologic complications of COVID-19. Am J Emerg Med 38(7): 1549.e3-1549.e7.
- Samuels JD (2020) Obesity and severe COVID-19 disease: a strong association Obesity (Silver Spring).
- Huang BY, Castillo M (2008) Hypoxic-ischemic brain injury: imaging findings from birth to adulthood. RadioGraphics 28(2): 417-439.
- Kjos BO, Brant-Zawadzki M, Young RG (1983) Early CT findings of global central nervous system hypoperfusion. AJR Am J Roentgenol 141(6): 1227-1232.



