Marketa Koutna1*, Lenka Vasakova2
1KARIM and Geriatric Department, University Hospital, Prague, Czech Republic
2Surgery Department, Hospital Semily, Semily, Czech Republic
*Corresponding Author: Marketa Koutna, 1KARIM and Geriatric Department, University Hospital, Prague, Czech Republic.
Abstract
The term chronic wound (CW) refers to those wounds that does not show a tendency to heal with adequate therapy for 6-9 weeks. Their prevalence has been recently estimated to be 2.21 per 1000 population and, thus, they constitute a significant humanistic and economic burden. As far as their treatment is concerned, an optimal wound dressing should be able to simultaneously i) promote debridement, ii) provide protection against infection, iii) establish a moist environment and iv) boost epidermal migration. State-of-the-art dressings are often based on polymers, among which hyaluronic acid (HA) has increasingly gained interest for its intrinsic characteristic, fulfilling the abovementioned desiderata, and its key role in relevant processes related to wound repair. Nowadays, several HA-based wound dressings are produced and made available for clinical use.
Nevertheless, due to contradictory findings of some clinical investigations, HA effectiveness is still being questioned. Aiming to keep on providing the literature with increasing clinical data, the case series reported in this study focuses on HA efficacy in treating CWs. Specifically, HA-based products including Hyalo4 Start, Hyalo4 Plus, Hyalo4 Foam, and Hyalo4 Regen (Fidia Farmaceutici S.p.A., Abano terme, PD, Italy) were used to treat CWs with different etiologies. Regardless of CW type, we found a significant wound area reduction along with the weeks of HA treatment, supporting the effectiveness of these HA-based products. Moreover, hinting at the safety of these HA-based dressings, no side effects related to the use of HA were observed.
Keywords: Hyaluronic acid; Hyalo4; Chronic wounds; Wound area reduction
Introduction
Human skin represents an extremely efficient barrier which, among other functions, fills the role of protecting us from the external environment. As easy to understand, this first line of defense is not unbreachable, posing the need for fast and reliable reparative mechanisms. To this aim, different molecular and cellular events interact with each other enabling a complex process known as wound healing response [1].
According to a simplified vision of this sophisticated system, wound repair can be divided into four main phases: hemostasis, inflammation, proliferation, and epithelization and dermal remodeling [2]. In normal physiological conditions, this healing process results in complete tissue restoration, both from the anatomical and functional point of view. Conversely, when clinical conditions come into play, the efficiency of the reparative machinery might be compromised at many levels, leading to excessive scarring or, at the other end, hampering the full closure of the injury site [1,2]. Despite some uncertainty regarding the duration of chronicity [3], the term chronic wound (CW) is commonly used to define those wounds that remain unhealed after 3 months [2]. The most common types of CWs are venous ulcers, pressure sores and diabetic ulcers [1,2,4]. Independently from their etiology, CW’s prevalence has been recently estimated to be 2.21 per 1000 population [5], thus constituting a significant humanistic and economic burden [6].
In accordance with the most common types of CWs, primary causes of non-healing wounds include vascular insufficiency, local-pressure effects, and diabetes mellitus. Along with these conditions, several local and systemic factors can alter the molecular and cellular responses involved in wound reparation, eventually concurring with the formation of CWs. Among all, local tissue hypoxia and ischemia, wound infection, advanced age, and compromised nutritional or immunological status are identified as crucial contributors to poor wound healing [1,4].
Current treatment of CWs relies on the TIME concept which refers to a structured approach enabling optimal wound bed preparation. Following the TIME acronym, clinical observations and interventions should focus on four areas: Tissue debridement, Infection/inflammation, Moisture imbalance, and Edge of the wound [7]. Accordingly, an optimal wound dressing would be the one capable of simultaneously i) promote debridement, ii) provide protection against infection, iii) establish a moist environment, and iv) boost epidermal migration. In addition, the ideal candidate should be sterilizable, biocompatible, biodegradable, and prone to functionalization [8].
Aiming to fulfill these requirements, state-of-the-art dressings are often based on polymers, among which hyaluronic acid (HA) has increasingly gained interest for its intrinsic characteristic – meeting the abovementioned desiderata – and its key role in relevant processes related to wound repair. Indeed, besides being a major constituent of the skin extracellular matrix (ECM), HA takes part at different stages of the wound healing response, spanning from the inflammatory phase to the re-epithelialization phase. Just to mention a few, HA is involved in the prompt generation of fibrin clot, as well as in the debris phagocytosis by stimulating the recruitment of neutrophils cells. Moreover, HA allows the formation of new ECM by promoting dermal fibroblast migration and proliferation [9,10].
For all these reasons, nowadays, several HA-based wound dressings are produced and made available for clinical use, mostly in formulations such as sponges, films, hydrogels, foams, and scaffolds [9,10]. Consequently, a relevant number of studies reporting on CW’s treatments with HA can be found in the literature [11,12]. Nevertheless, due to contradictory findings of some clinical investigations, clear evidence of HA effectiveness is still lacking [13]. Aiming to keep on providing the literature with increasing clinical data, the case series reported in this study focuses on the HA capabilities of improving the healing process of CWs. Specifically, HA-based products including Hyalo4 Start, Hyalo4 Plus, Hyalo4 Foam, and Hyalo4 Regen (Fidia Farmaceutici S.p.A., Abano terme, PD, Italy) were used to treat CWs with different etiologies.
Case Report/Case Presentation
Data from 19 patients affected by CWs of different origins and treated with the hyaluronic acid-based products Hyalo4 Start, Hyalo4 Plus, Hyalo4 Foam, and Hyalo4 Regen (Fidia Farmaceutici S.p.A., Abano terme, PD, Italy) were collected and analyzed in order to confirm the safety and efficacy of such treatments. Patients’ demographic data and medical history are detailed in Table 1.
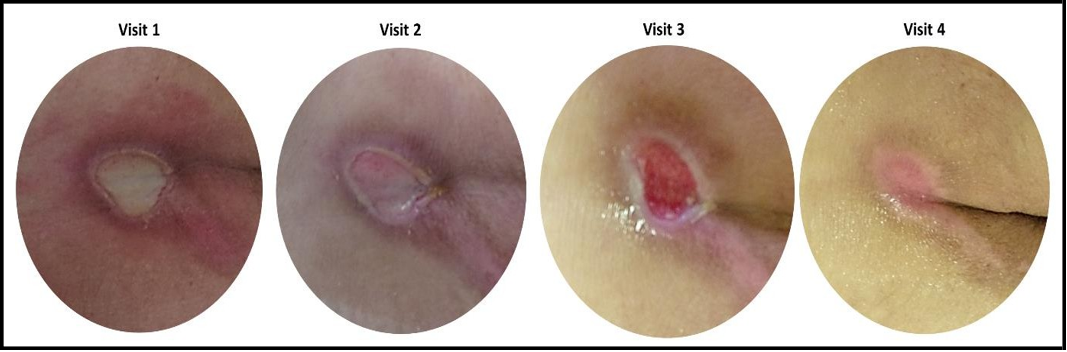
Concerning CW types included in the study, 9 out of 19 (47.37%) patients reported a pressure ulcer (Figure 1), whereas the remaining 10 patients (52.63%) were affected by wounds with diverse etiologies such as venous ulcers, arterial ulcers, surgical wounds, and trauma/laceration-related wounds. Fourteen out of 19 (73.68%) wounds were classified as “new” (i.e., not previously treated) and most of them (i.e., 12 out of 19, 63.16%) emerged less than 1 year before starting the HA treatment. For a more detailed picture of CWs characteristics included in the study refer to Table 1.
In order to assess HA capabilities of improving wound healing, after a baseline evaluation corresponding to Visit 1, CW area was measured at 3 different timepoints (i.e., Visit 2, Visit 3, and Visit 4). Throughout the text, data will be presented as mean ± SEM (n = 19). On average, the elapsed time between Visit 1 and Visit 2 was 22 ± 5 days, between Visit 2 and Visit 3 was 21 ± 4 days, and a mean of 20 ± 4 days passed between Visit 3 and Visit 4. As far as frequency of administration is concerned, patients were asked to apply the aforementioned HA-based products 3 times a week.
Figure 1: Representative pictures of pressure ulcer taken at Visit 1,Visit 2, Visit 3, and Visit 4.
Table 1: Patient details and ulcer characteristics.
|
|
Sex |
Age |
Nutrition status |
Mobility status |
Smoking |
Alcohol |
Comorbidities |
Wound Type |
Wound Condition |
Wound Duration |
Wound location |
|
Pt 1 |
M |
70 |
Well nourished |
Bad mobility |
No |
No |
Diabetes, Pulmonary |
Pressure ulcer |
New |
<1 year |
Sacrum |
|
Pt 2 |
F |
77 |
Well nourished |
Good mobility |
No |
No |
Venous disease, |
Venous ulcer |
New |
<1 year |
Lower leg |
|
Pt 3 |
M |
82 |
Well nourished |
Good mobility |
No |
No |
Diabetes, Arterial |
Arterial ulcer |
New |
<1 year |
Heel |
|
Pt 4 |
M |
61 |
Well nourished |
Good mobility |
No |
No |
Arterial disease, |
Ischemic ulcer |
New |
> 3 years |
Ankle |
|
Pt 5 |
F |
67 |
Well nourished |
Good mobility |
Yes |
No |
Diabetes, Lymphedema, |
Venous ulcer |
Recurrent |
<1 year |
Lower leg |
|
Pt 6 |
F |
71 |
Malnourished |
Good mobility |
No |
No |
Rheumatoid arthritis, |
Surgical wound |
New |
> 3 years |
Chest |
|
Pt 7 |
F |
81 |
Malnourished |
Bad mobility |
No |
No |
Venous disease, Anemia, Lymphedema |
Venous ulcer |
New |
<1 year |
Lower leg |
|
Pt 8 |
F |
80 |
Malnourished |
Bad mobility |
No |
No |
Hypertension |
Venous ulcer |
New |
<1 year |
Ankle |
|
Pt 9 |
M |
57 |
Malnourished |
Bad mobility |
No |
No |
Anemia |
Pressure ulcer |
New |
<1 year |
Heel |
|
Pt 10 |
M |
39 |
Well nourished |
Bad mobility |
Yes |
No |
Sepsis, Pneumonia |
Pressure ulcer |
New |
<1 year |
Chest |
|
Pt 11 |
F |
62 |
Malnourished |
Bad mobility |
Yes |
Yes |
Anemia |
Pressure ulcer |
New |
<1 year |
Sacrum |
|
Pt 12 |
F |
72 |
Malnourished |
Bad mobility |
No |
Yes |
Anemia, Paraplegia, Depression, Diarrhea |
Pressure ulcer |
Recurrent |
> 3 years |
Ischial tuberosity |
|
Pt 13 |
F |
77 |
Malnourished |
Bad mobility |
No |
No |
Venous disease, Anemia, Pneumonia |
Pressure ulcer |
New |
<1 year |
Genital vulva |
|
Pt 14 |
M |
45 |
Malnourished |
Bad mobility |
No |
No |
Anemia, Paraplegia, |
Pressure ulcer |
Recurrent |
> 1 year and > 3 years |
Knee |
|
Pt 15 |
M |
46 |
Malnourished |
Bad mobility |
No |
No |
Paraplegia, Incontinence, Urinary system infection |
Pressure ulcer |
Recurrent |
> 3 years |
Ischial tuberosity |
|
Pt 16 |
M |
59 |
Malnourished |
Bad mobility |
No |
No |
Covid, Pneumonia, |
Pressure ulcer |
New |
<1 year |
Chest |
|
Pt 17 |
F |
80 |
Well nourished |
Good mobility |
No |
No |
Venous disease, |
Venous ulcer |
Recurrent |
> 1 year and > 3 years |
Lower leg |
|
Pt 18 |
M |
73 |
Well nourished |
Bad mobility |
No |
No |
Covid, ICHS, FIS, Hypertension, Dementia |
Trauma/Laceration |
New |
<1 year |
Lower leg |
|
Pt 19 |
M |
44 |
Well nourished |
Good mobility |
Yes |
No |
Venous disease |
Venous ulcer |
New |
> 1 year and > 3 years |
Lower leg |
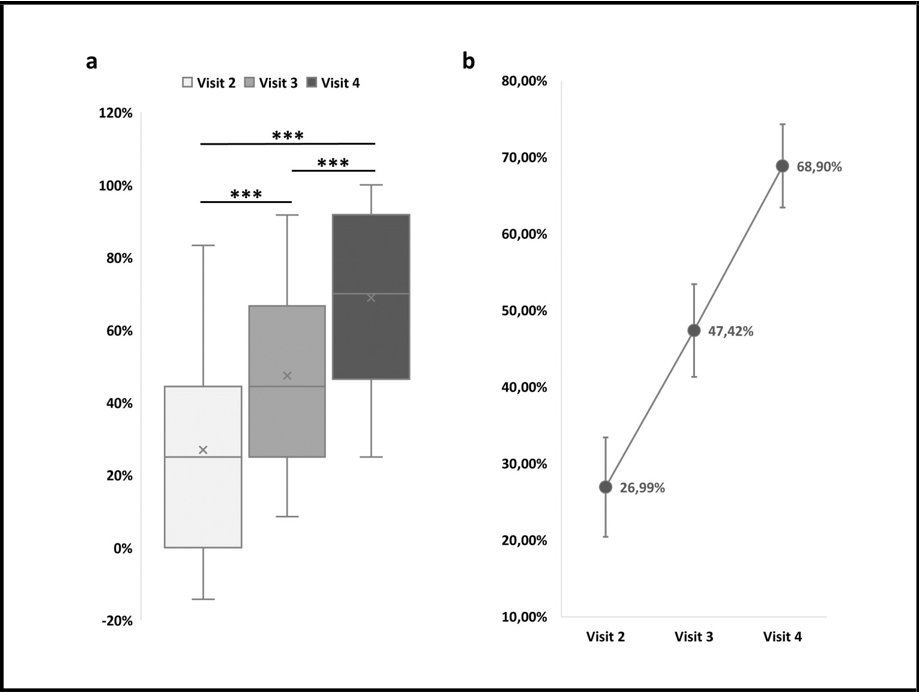
Concerning data analysis, as a first step, the percentage of CW area reduction was calculated with respect to baseline values (i.e., wound area measured at Visit 1). All available data are reported in Table 2 and shown in Figure 2. On average, a CW area reduction of 26.99% ± 6.49% was reported at Visit 2. Such a reduction was almost doubled at Visit 3; indeed, at this timepoint, a 47.42% ± 6.07 decrease of CW area was observed. At Visit 4, and thus after 63 ± 11 days of HA treatment, a CW area reduction of 68.90% ± 5.44% was reached.
In order to verify if the wound area gained a significant reduction after being treated, one-way ANOVA for repeated measures was applied to compare area reduction percentages measured at Visit 2, Visit 3, and Visit 4. At each visit, a significant CW reduction was achieved. Indeed, as shown in Figure 2, all possible pairwise comparisons resulted to be statistically significant (p < 0.001, Bonferroni post-hoc test).
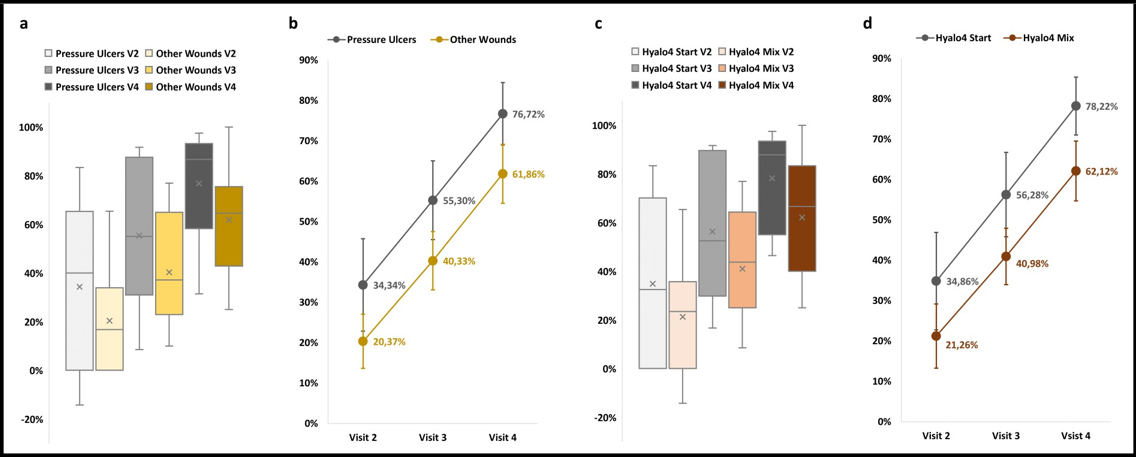
In order to investigate potential differences in terms of HA efficacy in treating different types of wounds, data were pooled according to wound etiology. Specifically, since the majority of patients reported pressure ulcers, data were divided in 2 subgroups: pressure ulcers (n = 9) and other wounds (n = 10). The latter group included: venous ulcers, arterial ulcers, surgical wounds, and trauma/laceration-related wounds. As shown in Figure 3, no significant differences in terms of wound area reduction were observed between the 2 subgroups (p > 0.05, Student t-Test for unpaired data) and, within the two subgroups, the wound area was still significantly reduced at Visit 4 when compared to Visit 2 and Visit 3 (p < 0.05, Student t-Test for paired data).
Aiming to explore other potential diversities in terms of efficacy, data were arranged on the basis of treatment type, namely Hyalo4 Start, Hyalo4 Plus, Hyalo4 Foam, and Hyalo4 Regen (Fidia Farmaceutici S.p.A., Abano terme, PD, Italy) which are produced, respectively, in the form of ointment, cream, foam, and pad. Also in this case, since most of the patients were exclusively treated with Hyalo4 Start, data were divided in 2 subgroups: Hyalo4 Start (n = 8) and mixture of HA formulations (n = 11). The latter group included patients who were treated with more than one type of product. As shown in Figure 3, no significant differences in terms of wound area reduction were observed between the 2 subgroups (p > 0.05, Student t-Test for unpaired data) and, within the two subgroups the wound area was still significantly reduced at Visit 4 when compared to Visit 2 and Visit 3 (p < 0.05, Student t-Test for paired data).
Figure 2: Chronic wound area reduction along with the weeks of HA treatment. a Box plot of wound area reduction measured at Visit 2, Visit 3, and Visit 4 for all cases included in the study. Percentages are calculated with respect to baseline values (i.e., wound area measured at Visit 1).
***p < 0.001, one-way ANOVA for repeated measures, Bonferroni post-hoc test, n = 19. b Average wound area reduction measured at Visit 2, Visit 3, and Visit 4 for all cases included in the study. Percentages are calculated with respect to baseline values (i.e., wound area measured at Visit 1).Data are presented as mean ± SEM (n = 19).
Figure 3: Area reduction data divided according to wound etiology and type of treatment. a Box plot of wound area reduction measured at Visit 2, Visit 3, and Visit 4 for all cases included in the study. Data are divided according to wound etiology: pressure ulcers are shown in grey, while all other wound types (venous ulcers, arterial ulcers, surgical wounds, and trauma/laceration-related wounds data were pooled) are depicted in yellow. Mean differences between subgroups were not significantly different. b Average wound area reduction measured at Visit 2, Visit 3, and Visit 4 for all cases included in the study. Data are presented as mean ± SEM and divided according to wound etiology: pressure ulcers are shown in grey (n = 9), while all other wound types are depicted in yellow (n = 10). c Box plot of wound area reduction measured at Visit 2, Visit 3, and Visit 4 for all cases included in the study. Data are divided according to type of treatment: Hyalo4 Start ointment is shown in grey, while mixture of Hyalo4 formulations (cream, foam, and pad data were pooled) are depicted in yellow Mean differences between subgroups were not significantly different. d Average wound area reduction measured at Visit 2, Visit 3, and Visit 4 for all cases included in the study. Data are presented as mean ± SEM and divided according to type of treatment: Hyalo4 Start treatment is shown in grey (n = 8), while mixture of Hyalo4 formulations are depicted in yellow (n = 11).
Table 2: Wound area reduction along with the weeks of HA treatment.
|
|
Chronic wound area (in cm2) |
Area reduction (% with respect to Visit 1) |
|||||
|
|
Visit 1 |
Visit 2 |
Visit 3 |
Visit 4 |
Visit 2 |
Visit 3 |
Visit 4 |
|
Pt 1 |
9 |
5 |
4 |
1.5 |
44.44 |
55.56 |
83.33 |
|
Pt 2 |
19.5 |
6.75 |
4.5 |
1.6 |
65.38 |
76.92 |
91.79 |
|
Pt 3 |
4 |
3.6 |
3 |
1.5 |
10.00 |
25.00 |
62.50 |
|
Pt 4 |
5 |
5 |
4.5 |
1.5 |
0.00 |
10.00 |
70.00 |
|
Pt 5 |
56 |
52 |
42 |
42 |
7.14 |
25.00 |
25.00 |
|
Pt 6 |
14 |
9 |
5 |
8.4 |
35.71 |
64.29 |
40.00 |
|
Pt 7 |
24 |
24 |
20 |
12 |
0.00 |
16.67 |
50.00 |
|
Pt 8 |
1.5 |
1 |
0.5 |
0 |
33.33 |
66.67 |
100.00 |
|
Pt 9 |
17.5 |
20 |
16 |
12 |
-14.29 |
8.57 |
31.43 |
|
Pt 10 |
150 |
25 |
25 |
20 |
83.33 |
83.33 |
86.67 |
|
Pt 11 |
6 |
6 |
0.5 |
0.15 |
0.00 |
91.67 |
97.50 |
|
Pt 12 |
40 |
30 |
18 |
12 |
25.00 |
55.00 |
70.00 |
|
Pt 13 |
4.5 |
2 |
3 |
0.5 |
55.56 |
33.33 |
88.89 |
|
Pt 14 |
24 |
6 |
2 |
1.5 |
75.00 |
91.67 |
93.75 |
|
Pt 15 |
400 |
240 |
200 |
30 |
40.00 |
50.00 |
92.50 |
|
Pt 16 |
28 |
28 |
20 |
15 |
0.00 |
28.57 |
46.43 |
|
Pt 17 |
4.5 |
3.21 |
2.5 |
1.4 |
28.67 |
44.44 |
68.89 |
|
Pt 18 |
16 |
12.25 |
9 |
9 |
23.44 |
43.75 |
43.75 |
|
Pt 19 |
3.6 |
3.6 |
2.5 |
1.2 |
0.00 |
30.56 |
66.67 |
Discussion/Conclusion
Chronic wounds treatment with HA is grounded on its unique properties, possibly supporting the wound healing process at different stages of the TIME management strategy (i.e., Tissue debridement, Infection/inflammation, Moisture imbalance, and Edge of wound) [7]. Indeed, HA is capable of creating an environment which is both moist and suitable for proliferation and migration of fibroblasts, endothelial cells, keratinocytes and angiogenesis [9,10]. Even if on the one hand it has been proposed as an extremely appealing polymer for optimal wound bed preparation [11,12], on the other hand, its effectiveness is still being questioned [13].
Aiming to increase the amount of available clinical data, in this case series, we have reported on the efficacy of different types of HA- based products to treat non-healing wounds with diverse etiologies. Notably, we found a significant wound area reduction along with the weeks of HA treatment. Specifically, after approximately 2 months of HA treatment, we observed a CW area reduction of 68.90% ± 5.44%. Moreover, it is worth noticing that such treatments seem to be characterized also by a robust safety profile since no side effects related to the use of HA were recorded over the 2-months treatment period.
Our results corroborate the findings of a recent study by De Francesco and colleagues where, as in our case, CWs of different origins were treated with one of the HA-based products used in our study (i.e., Hyalo4 Start) [14]. Indeed, in line with our observations collected at Visit 2 (27% of wound area reduction obtained after ~3 weeks of treatment), they reported on a CW area reduction of the 26% after 2 weeks of treatment. In accordance with our statistical evaluations, such a reduction, as well as the ones collected during the subsequent visits, resulted to be statistically significant. In addition, they also report on a total recovery of the 87% of the patients included in the study (n = 70) after 8 weeks of treatment.
Therefore, according to what has been observed by De Francesco and in other studies present in literature, to achieve complete wound healing with HA-based products a time span of 8 weeks seems to be required [14,15]. Even in this case, taking into account the small dimension of our dataset, it can be speculated that our results are in line with the healing times reported in the literature, providing evidence of the effectiveness of these devices over a period of approximately 2 months of use.
More interestingly, such healing times can be compared to mean wound durations described in the literature. A recent study reported on a dataset including 100 patients affected by different types of CWs which were treated differently according to the standard of care for each specific etiology. The mean wound duration observed by the authors of this study was about 200 days [16]. Notably, such a wound duration is almost 4 times longer than the time to heal associated to the treatment with HA-based products (i.e., 56 days reported in references 14 and 15).
In our case report, we have also investigated potential differences in terms of HA efficacy when aiming to treat different types of wounds or when HA is used in different formulations. In both cases, we found no significantly different capabilities of HA in terms of achieved wound reduction. Nevertheless, it might be worth noticing that the best recorded performances were observed when focusing only on pressure ulcers treated with the product Hyalo4 Start (data not shown). In this specific case, a mean reduction of 82.25% ± 6.84% (n= 7) was reached after ~2 months of HA-treatment. Since pressure sores represent one of the most common and hard-to-treat type of CWs [1,2,4], such a potential clinically-relevant outcome should be further investigated in future studies focused on the treatment of pressure ulcers with HA and involving large patient populations.
Indeed, although it can be regarded as an intrinsic characteristic of case reports, the small dimension and heterogeneity of our dataset certainly represent the main limitations of our study. Nevertheless, keep on providing the literature with valuable clinical data is particularly relevant for: i) future drafting of valuable systematic reviews and meta-analysis which will help to better understand if HA- based dressings are more effective in treating specific types of wounds or if some HA formulations should be privileged; ii) encouraging the future conduction of large controlled trials focusing only on specific CWs types as the only way to undoubtably demonstrate the potentially superior performances of HA in terms of balance between effectiveness and safety.
To conclude, within the limitations of our study, the findings reported in our case series seem to support the safe and effective deployment of HA-based products for CWs treatment. Indeed, we achieved a significant wound area reduction at each evaluated timepoint (i.e., after ~3, ~6, and ~9 weeks of treatment) and no adverse events associated to the use of the devices were observed. Since the amount of clinical data related to the use of HA-based products as wound healing enhancers is still too limited to draw strong conclusions, these findings should be taken as a starting point for the future design of appropriate clinical studies, methodically investigating the potential of this therapeutic approach.
Statement of Ethics
All Case reports are retrospective and anonymous. Informed consent was obtained from the patients to publish the cases. Patients were treated according to the best clinical practice and no additional or experimental exams or treatments were performed. All procedures performed were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required. Thus, the ethics approval and consent was not required.
Conflict of Interest Statement: The authors have no conflicts of interest to declare.
Funding Sources: None.
Author Contributions: All authors have contributed equally to this work.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author upon reasonable request.
References
- Eming SA, Martin P, Tomic-Canic M (2014) Wound repair and regeneration: Mechanisms, signaling, and translation. Sci Transl Med. 6(265): 265sr6.
- Wilkinson HN, Hardman MJ (2020) Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 10(9): 200223.
- Kyaw BM, Järbrink K, Martinengo L, Car J, Harding K, et al. (2018) Need for Improved Definition of «Chronic Wounds» in Clinical Studies. Acta Derm Venereol. 98(1): 157–158.
- Mustoe TA, O’Shaughnessy K, Kloeters O (2006) Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis. Plast Reconstr Surg. 117(7 Suppl): 35S-41S.
- Martinengo L, Olsson M, Bajpai R, Soljak M, Upton Z, et al. (2019) Prevalence of chronic wounds in the general population: systematic review and meta-analysis of observational studies. Ann Epidemiol. 29: 8–15.
- Olsson M, Järbrink K, Divakar U, Bajpai R, Upton Z, et al. (2019) The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc. 27(1): 114–125.
- Leaper DJ, Schultz G, Carville K, Fletcher J, Swanson T, et al. (2012) Extending the TIME concept: what have we learned in the past 10 years?(*). Int Wound J. 9 Suppl 2(Suppl 2): 1–19.
- Dhivya S, Padma VV, Santhini E (2015) Wound dressings - a review. BioMedicine. 5(4): 22.
- Cortes H, Caballero-Florán IH, Mendoza-Muñoz N, Córdova- Villanueva EN, Escutia-Guadarrama L, et al. (2020) Hyaluronic acid in wound dressings. Cell Mol Biol (Noisy-le-grand). 66(4): 191–198.
- Graça MFP, Miguel SP, Cabral CSD, Correia IJ (2020) Hyaluronic acid-Based wound dressings: A review. Carbohydr Polym. 241: 116364.
- Voigt J, Driver VR (2012) Hyaluronic acid derivatives and their healing effect on burns, epithelial surgical wounds, and chronic wounds: a systematic review and meta-analysis of randomized controlled trials. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc. 20(3): 317–31.
- De Francesco F, Riccio M, Jimi S (2022) Contribution of Topical Agents such as Hyaluronic Acid and Silver Sulfadiazine to Wound Healing and Management of Bacterial Biofilm. Medicina (Kaunas). 58(6): 835.
- Shaharudin A, Aziz Z (2016) Effectiveness of hyaluronic acid and its derivatives on chronic wounds: a systematic review. J Wound Care. 25(10): 585–592.
- De Francesco F, De Francesco M, Riccio M (2022) Hyaluronic Acid/Collagenase Ointment in the Treatment of Chronic Hard- to-Heal Wounds: An Observational and Retrospective Study. J Clin Med. 11(3): 537.
- Onesti MG, Fioramonti P, Fino P, Sorvillo V, Carella S, et al. (2016) Effect of enzymatic debridement with two different collagenases versus mechanical debridement on chronic hard- to-heal wounds. Int Wound J. 13(6): 1111–1115.
- Borda LJ, Jaller JA, Kallis PJ, MacQuhae FE, Herskovitz I, et al. (2018) Patients’ prediction of their wound healing time. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc. 26(3): 297–9.