Sahar Abd Al-Daim1*, Omar Mohammad Atta2, Abdou Kamal Allayeh1, Hosni A. M. Hussein2, Mervat A. Dawood3, Basma A. Khalifa4, Mohamed A. A. Hussein2
1Environmental Virology Lab 176, Water Pollution Research Department, Environment and Climate Change Institute, National Research Centre, 12622-Dokki, Cairo, Egypt.
2Department of Botany and Microbiology, Faculty of Science, Al-Azhar University, Assiut Branch, Assiut 71524, Egypt.
3Clinical Pathology, Mansoura Research Center for Cord SteSm Cells (MARC-CSC), Faculty of Medicine, Mansoura University, El Mansoura 35516, Egypt.
4Department of Botany and Microbiology, Faculty of Science, Minia University, Minia city 61519, Egypt.
*Corresponding Author: Sahar Abd Al-Daim, Environmental Virology Lab 176, Water Pollution Research Department, Environment and Climate Change Institute, National Research Centre, 12622-Dokki, Cairo, Egypt.
Abstract
The herpes simplex virus (HSV) is causing significant concern worldwide because of its indirect infection and severe subsequent health consequences. For those who are seropositive, expected infectious outcomes include genital herpes or eye disease. Furthermore, to treat HSV, several aspects of the present medications have been modified due to their contagious nature. As of now, an incredibly potent and durable cure has yet to be discovered. The issue might be solved by looking into the antiviral potential of bioactive natural-based chemicals and creating innovative drug screening techniques. Extracellular vesicles of mesenchymal stem cells (MSCs) are effective and safe substitutes for stem cells in MSC-based treatments. They examine the antiviral potential of human Wharton's jelly MSC extracellular vesicles against herpes simplex type 2 (HSV 2).
First, the half-maximal cytotoxic concentration (CC50) of extracellular vesicles against Vero cells and the half-maximal inhibitory concentration (IC50) against Herpes simplex type 2 were evaluated. The highly promising results were next subjected to an inhibition assay to assess their antiviral activity based on S.I. values. The results consistently showed moderate viral inhibition percentages at non-toxic dosages. The hWJ- MSC exosome exhibited IC50 and SI of 6.2 and 8.5 µg/mL, respectively, against (HSV 2), but the hWJ-MSC microvesicles' IC50 and SI were 14.49 and 4.3 µg/mL, respectively. In contrast to the anti-adsorption effect, the virucidal and anti-replication antiviral activities of hWJ-MSC-S were quite noticeable.
By examining the antiviral action mechanisms, hWJ-MSC-Exo & M.V. can inhibit viral infection by primarily interfering with the herpes virus type 2 ability to replicate. Using transmission electron microscopy, we were able to characterize predefined molecules and found that there are differences in their size and shape. We can conclude that mesenchymal stem extracellular vesicles produced from Wharton jelly may be a promising antiviral for the type 2 herpes virus.
Keywords: herpes simplex type 2; Exosomes; Macrovesicles; Mesenchymal Stem Cells; antiviral activity.
1. Introduction
The human herpes simplex virus type 2 (HSV-2) is one of the most common viruses in humans, causing severe genital ulceration and is linked to HIV transmission [26]. HSV-2 is a large, double-stranded DNA (dsDNA) that is classified as a member of the subfamily Alpha Herpesviridae [35]. Intimate contact is the most prevalent mode of transmission; HSV-2 is primarily transmitted sexually. HSVs were primarily associated with orolabial ulceration in the past, but more recently, changes in the epidemiology of HSV-2 have been described, including an increase in genital infections [24]. There is a dearth of knowledge regarding the factors that influence virulence, particularly the significance of the genetic makeup of certain isolates and the absence of effective therapeutic agents, necessitating further investigation. Emphasise the need for proper treatment to limit the spread of the virus and slow the progression of the disease. Consequently, the majority of studies on HSV-2 have focused on treatment resistance, vaccine development, seroprevalence, and antiviral development [3,6,11,14,44].
In addition to bone marrow, mesenchymal stem cells (MSCs) can be obtained from various adult tissues, including adipose tissue, amniotic fluid, dental pulp, placenta, umbilical cord blood, Wharton's jelly, as well as organs such as the brain, kidney, liver, lung, spleen, pancreas, and thymus [40]. MSCs have great potential as therapeutic agents for various human diseases [23,43]. This promise has not yet been fully realized because of the adverse reactions and effects of MCS-based therapy (MSCT), which include carcinogenesis, immunological rejection, and infection [17,20,41].
Human Wharton-jelly-derived mesenchymal stem cells (hWJ-MSCs) have recently attracted increased interest after in vivo transplantation due to their improved proliferation rate, immune-privileged properties, and decreased carcinogenic profile [18]. Despite the fact that hWJ-MSCs possess embryonic stem cell characteristics, they do not raise any ethical concerns. It is also possible to use the readily available umbilical cord (U.C.) to separate it. These advantages distinguish hWJ-MSCs from BMSCs and adipose tissue stem cells (ASCs) when comparing hWJ-MSCs to BMSCs and ASCs [4,13]. Stem cells can communicate with neighbouring tissue cells through a group of biologically active external secretions called the secretum, of which exosomes and large vesicles are the most important [25,29]. The capacity of MSCs to secrete vesicles (MSC-EVs) with critical biological functions has been reported in earlier research [7]. Numerous E.V.s with varying sizes, shapes, and contents are secreted by cells; these E.V.s interact with target cells to alter their phenotypes and functions [34]. To protect bioactive molecules from exposure and degradation in the extracellular environment and to enable their specialized transportation and targeting to target cells, extracellular vesicles are a crucial component of paracrine signalling [2].
Bioactive substances that control the phenotype, function, survival, and homing of immune cells, such as enzymes, cytokines, chemokines, immunomodulatory and growth factors, messenger RNA (mRNA), and microRNAs (miRNAs), are abundant in MSC- EVs. [16]. MSC-derived EVs, which are cell-free products, eliminate any safety concerns about the long-term survival of engrafted MSCs, such as their uncontrolled differentiation, malignant transformation, or rejection due to the activation of allogeneic immune responses in MHC-mismatched recipients [16]. Macrovesicles (100–1000 nm) and Exos (30–200nm) are nano-sized extracellular vehicles (E.V.s) derived from MSCs that are released into the extracellular milieu where they exert paracrine and endocrine effects [16]. The purpose of this study was to assess the antiviral potential of human Wharton- jelly-MSCs exosomes and macrovesicles (hWJ-MSCs- Exo) and (hWJ-MSCs- M.V.) against herpes simplex type -2 (HSV-2).
2. Materials and Method
2.1. Cells and Viruses
In vitro examination of the antiviral effect of hWJ-MSC- Exo & L.V. on Vero (African green monkey kidney) cells. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Grand Island, NY, USA) containing 10% fetal bovine serum (FBS) (Grand Island, NY, USA) and 1% antibiotic-antimycotic (A.A.) mixture (GIBCO, Waltham, MA, USA) at 37 ºC in a humidified atmosphere containing 5% CO2. Egyptian company Nawah-Scientific supplied human Herpes simplex virus type-2 and Vero cells.
2.2. Collection and Processing of Human Umbilical Cords (hUCs)
In our prior research, we detailed the method and protocol used [18]. In summary, we obtained hUC samples (n=20) from the obstetrics and gynecology department at Al-Azhar University Hospital in Assiut. This study was approved by the ethics committees of the medical and scientific faculties at Al-Azhar University in Assiut (APPROVAL NUMBER/ID: 202015). Twenty healthy mothers were recruited after a full-term pregnancy, and hUCs were collected and transported to the lab in a sterile environment using phosphate-buffered saline (PBS) containing 100 U/mL penicillin and 100µ g/mL streptomycin.
After sterilizing the external surface of the U.C. with 70% ethanol, the cord was washed twice with phosphate-buffered saline (PBS) and serum-free DMEM (GIBCO, USA) to remove any remaining blood in the lab. The U.C. was severed longitudinally with sterile surgical scissors, and the Wharton's jelly (W.J.) was pulled from the cord with a scalpel. After the blood vessels from the cord were surgically removed (two arteries and one vein), the remaining cord tissue (C.T.) was collected. The W.J. and C.T. were each chopped into pieces measuring 1-2 mm, then incubated with the enzymatic mixture for 30 minutes at 37 ºC in a 5% CO2 incubator.
2.3. Culturing of hUC-Derived Mesenchymal Stem Cells (hUC-MSCs)
Our previous report detailed the cultivation of human umbilical cord Mesenchymal Stem Cells (hUC-MSCs) [18]. Specifically, partially digested W.J. and C.T. pieces were each plated on a six-well plate of DMEM/F12 (GIBCO, Waltham, MA, USA) supplemented with 10% FBS and 1% A.A. solution. Plates were incubated at 37°C in an incubator with 5% CO2 and a temperature of 37 °C. After seven days in the culture medium, the hUC fragments were removed, and a new culture medium was added. Before passage, cells were grown to 80% confluence. If isolated cells can be cultured and maintained in good health until the fifth passage (P5) without contamination, the isolation is considered successful. Stem cells were cryopreserved in 10% DMSO-supplemented FBS.
2.4. Flow Cytometry Characterization of hUC-MSCs
hUC-MSC cells were detached using 0.25% Trypsin-EDTA and collected by centrifugation at 1000 x g for 10 min. Then, 106 hWJ- MSCs in 100 µL volume were stained with 10 µL of Pyridinium- chlorophyll-protein (Per-CP)-conjugated anti-CD105/Endoglin (Mouse IgG1; Clone 166707, R&D Systems, McKinley Place, MN, USA), Carboxy fluorescein (CFS) conjugated-CD73 (Mouse IgG2B; Clone 606112, R&D Systems, McKinley Place, MN, USA), allophycocyanin (APC) conjugated anti-CD90/Thy1 (Mouse IgG2A; Clone Thy-1A1, R&D Systems, McKinley Place, MN, USA), phycoerythrin (P.E.) conjugated anti-CD45 (Mouse IgG1; Clone 2D1, R&D Systems, McKinley Place, MN, USA), and PE-CD34 (Mouse IgG1; Clone QBEnd10, R&D Systems, McKinley Place, MN, USA) monoclonal antibodies for 30 min. The viability of cells was monitored using 7-amino actinomycin D (7-AAD) staining. A total of 50,000 events were acquired and analyzed using the FACS Cantor flow cytometer (Becton Dickinson Biosciences, Franklin Lakes, NJ, USA) and Kaluza analysis software 1.5a (Beckman Coulter, Brea, CA, USA).
2.5. Collection of hWJ-MSC-S
hWJ-MSCs were grown to 80% confluence in a complete growth medium before being transferred to a serum-free medium. After 48 hours, Conditioned medium (CM) was harvested, centrifuged at 1000 rpm for 10 minutes to remove cell residues, and stored at -80ºC for future use.
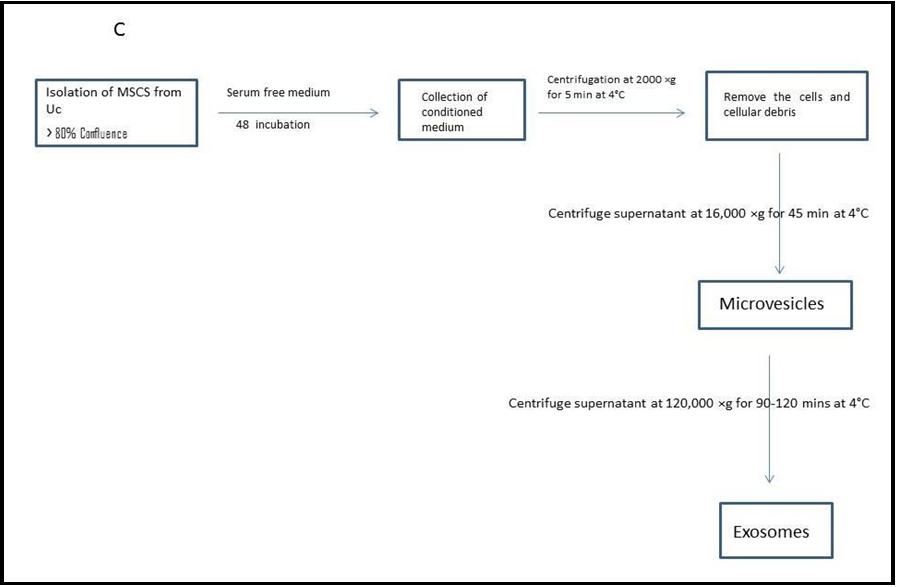
2.6. Isolation of extracellular vesicles
To obtain microvesicles and exosomes from secrtome, multiple steps of modified differential centrifugation were required. A low-speed spin (3000×g for 10 minutes) eliminated dead cells and apoptotic debris, while a spin at a higher speed (90,000×g for 45 minutes) eliminated microvesicles. The high-speed spin (150,000×g) precipitated exosomes in 90 minutes. (SORVALL MTX 150 micro- ultracentrifuge) It is stored (at -80°C for further characterization and analysis) [1,31].
2.7. Characterization by Transmission Electron Microscopy
For transmission electron microscopy, isolated exosome and microvesicle pellets were treated overnight at 4°C with 2% glutaraldehyde in 0.1 M sodium cacodylate buffer. The fixed exosome pellet was rinsed in 0.15 M sodium cacodylate buffer (pH 7.4), followed by a post-fixation rinse in 2% osmium tetroxide at 4°C. It was then stained with osmium tetroxide and uranyl acetate. The dyed pellet was cut into pieces that were then looked at with a JEM- 100CX II 80 kV transmission electron microscope. The pictures were taken with a digital camera.
2.8. Determination of Viral Inhibitory Concentration 50 (IC50) and Cytotoxicity (CC50) Assay
The CPE-inhibition assay was used to identify potential antivirals against Herpes simplex virus type 2 in humans. The dose-response assay was designed to determine the range of antiviral efficacy, i.e., the 50% inhibitory concentration (IC50) and the range of cytotoxicity (CC50). This test is essential for determining antiviral effectiveness in cell culture systems. Using the recently reported cytopathic (CPE) inhibition effect, antiviral activity and cytotoxicity assays were evaluated using the Crystal violet method. A day before infection, vero cell cells were seeded at a density of 2x10000 cells/well in a 96-well culture plate. The following day, the culture medium was removed, and the cells were washed with phosphate-buffered saline. The infectivity of HSV-2 was determined utilizing the crystal violet method, which monitored CPE and allowed for the calculation of the percentage of viable cells. Mammalian cells received 0.1 mL of diluted virus suspension of human herpes virus type 2 containing CCID50 (1.0 * 10000) of virus stock. This dose was determined to produce the desired CPEs 3 days after infection. At the onset of the disease, 0.01 mL of medium containing the desired compound concentration was added to the cells for compound treatments. The antiviral activity of each test sample was determined using a concentration range of 0.1-100 g/ml. The virus and cell controls. Plates of culture were incubated at 37°C and 5% CO2 for 96 hours. The progression of the cytopathic effect was observed using light microscopy. The cell monolayers were fixed and stained with a 0.03% crystal violet solution in 2% ethanol and 10% formalin following a PBS wash. The optical density of individual wells was measured spectrophotometrically at 570/630 nm after washing and drying.
Antiviral activity = (mean optical density of cell controls minus mean optical density of virus controls) / (optical density of test minus mean optical density of virus controls) ×100%. Based on these findings, the 50% CPE inhibitory dose (IC50) was determined. Before this assay, the cytotoxicity was evaluated; 2x104 cells/well were seeded in a 96- well culture plate. The following day, the culture medium containing serially diluted samples was added to the cells, which were then incubated for 72 hours before the culture medium was removed and the cells were washed with PBS. The subsequent steps were performed the same manner described previously for the antiviral activity test. Using GraphPad PRISM software (Graph-Pad Software, San Diego, USA), the 50% cytotoxic concentrations (CC50) and 50% inhibitory concentrations (IC50) were calculated [1].
2.9. Determination of the Mode of Action
As previously described, the mode of antiviral activity of test compounds was determined by evaluating their virucidal, anti- adsorption, and anti-replication effects in Vero cells [12,42]. To evaluate virucidal activity, 200 µL (1000 PFU) of the virus was combined with various non-toxic concentrations of hWJ-MSC-S and incubated at RT for 1 h before infecting the cell monolayer for 1 h at 37ºC. To determine the anti-adsorption effect, the cell monolayer was pre-treated for 2 h at 4 ºC with varying concentrations of hWJ- MSC-S before infection for 1 h at 37°C with 200 µL (1000 PFU) of the virus. To determine the anti-replication effect, the cell monolayer was infected with 200 µL (1000 PFU) of the virus for 1 h at 37°C, followed by 1 h of treatment with hWJ-MSC-S. In all protocols, the supernatant was aspirated, cells were washed, and DMEM medium supplemented with 2% agarose, 1% A.A. mixture, and 4% BSA was used to incubate the cells at 37°C. After three days, the overlay medium was discarded, and the cells were fixed for one hour in 10% formalin solution and stained with 0.1% crystal violet working solution. The optical density of individual wells was measured spectrophotometrically at 570/630 nm after washing and drying. The percentage of antiviral activity of the test compounds was calculated using the following equation: Antiviral activity = (mean optical density of cell controls minus mean optical density of virus controls) / (optical density of test minus mean optical density of virus controls) 100%. Based on these findings, the 50% CPE inhibitory dose (IC50) was determined.
Results
3.1. hUC-MSC Isolation and Characterization
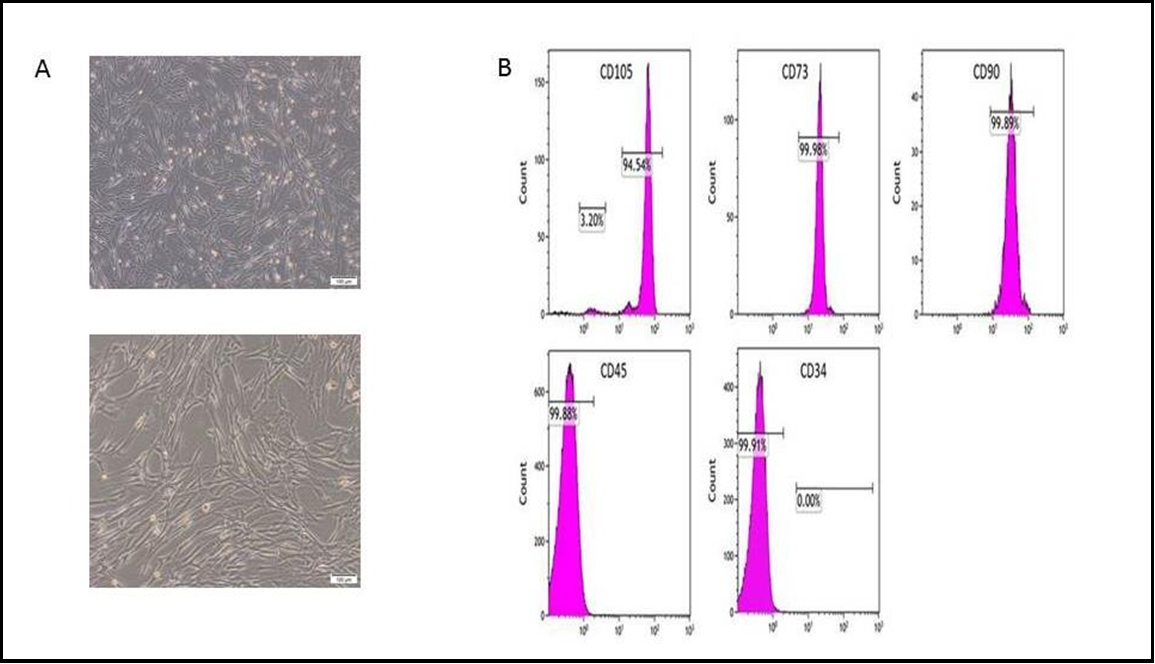
The isolation of hUC-MSCs was achieved in 75% (15 out of 20) of the obtained umbilical cords. Cells were successfully isolated via 80% (12 out of 15) of the collected WJ samples and 40% (6 out of 15) of the collected CT samples. The isolation rate was notably higher in WJ compared to CT (Figure 1A). The isolated cells exhibited typical spindle-shaped stem cells with adherent properties, as shown in (Figure 1B). Furthermore, there were no discernible differences in cell morphology between stem cells derived from WJ and CT. The flow cytometry analysis conducted on the isolated UC-MSCs revealed positive expression of MSC markers CD73, CD105, and CD90, which is consistent with previous findings. Conversely, the hematopoietic stem cell markers CD45 and CD34 were found to be negative (Figure 1C), further supporting previous research [18].
Figure.1 A: 90 % confluence, and magnification was of 10x; B: Flow cytometry shows how hWJ-MSCs are characterised; C: A diagram shows the collection of WJ-MSCs Exv.
3.2 Antiviral Activity and Cytotoxicity of hWJ-MSC-S
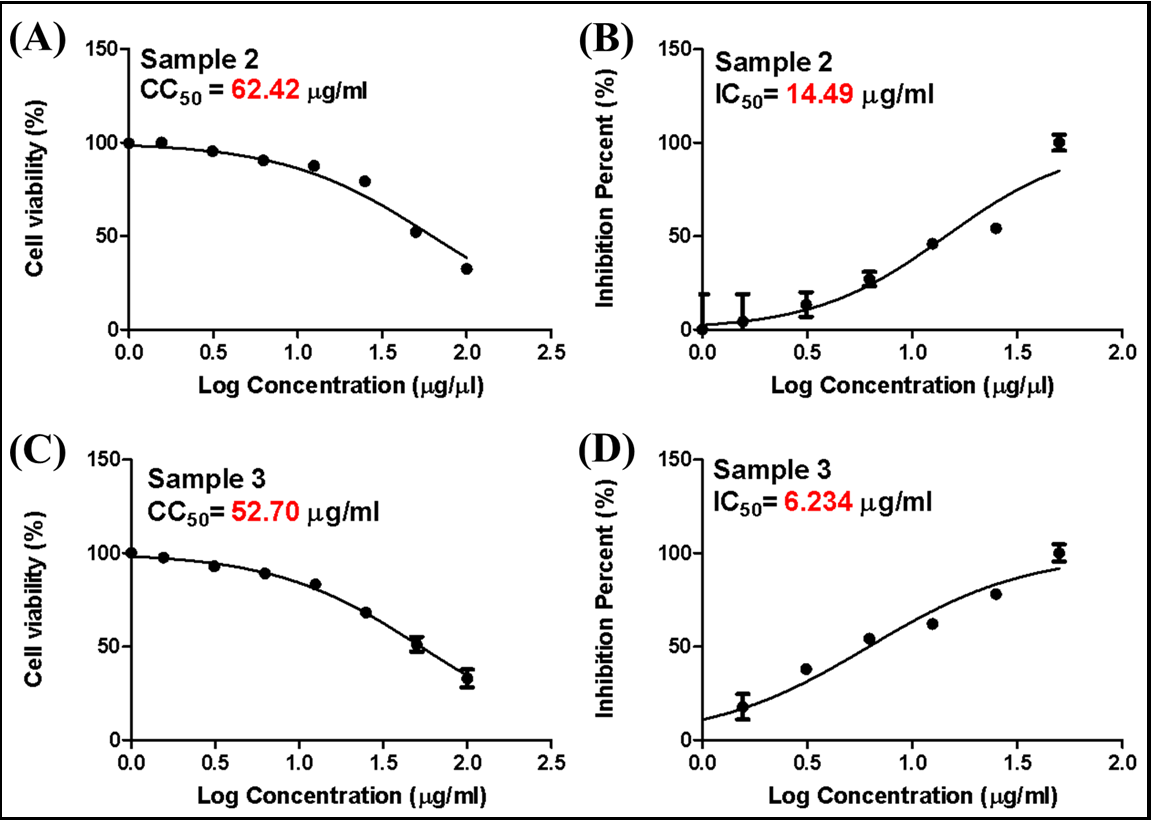
The study assessed the antiviral activity and cytotoxicity of hWJ- MSC-Extracellular Vesicles against HSV-2. Specifically, the focus of the study was on microvesicles and exosomes. The evaluation of these samples was conducted using the Vero cell line. It was determined the half-maximal inhibitory concentration (IC50), half-maximal cytotoxicity concentration (CC50), and selective index (SI) of hWJ- MSC-MV and hWJ-MSC-EX. To determine CC50, Vero cells were treated with varying concentrations of each fraction, and cytotoxicity levels were determined using the MTT assay. (Figure 2A&C) CC50 value of hWJ-MSC-MV and hWJ-MSC-EX were 62.42 µg/ml and 52.70 respectively against HSV-2), HSV-2 was individually treated with a variety of non-toxic concentrations of HWMSC-MV and hWJ- MSC-EX before being allowed to infect Vero cells in order to assess the antiviral effect of each sample. The antiviral activity of hWJ- MSC-extracellular vesicles was evaluated using a cytopathic effect assay. The IC50 values of hWJ-MSC-MV and hWJ-MSC-EX against HSV-2 were 14.49 and 6.234 µg/ml, respectively, as shown in (Figure 2B&D). In addition, the SI (CC50/IC50) of hWJ-MSC-MV and hWJ-MSC-EX against HSV-2 were 4.3 and 8.5, respectively (Table 1). The secreted factors of hWJ-MSCs effectively mitigated the cytopathic effect caused by HSV-2. There was a difference in the ability of hWJ-MSC-MV and hWJ-MSC-EX to inhibit the virus. The effectiveness of hWJ-MSC-MV and hWJ-MSC-EX in preventing the spread of HSV-2 was significantly reduced.
Figure 2. Antiviral activity and cytotoxicity of hWJ-MSC- extracellular vesicls, (A and C) Cytotoxicity concentration (CC50) of hWJ-MSC-S on Vero cells against HSV-2. Different concentrations of hWJ-MSC-S were applied to cells for 24 hours. The cytotoxicity levels were measured using an MTT assay. (B and D) Half-maximal inhibitory concentration (IC50) of hWJ-MSC-S against HSV-2 infection in Vero cells.
The virus was incubated for one hour with varying concentrations of hWJ-MSC-extracellular vesicles before infection of Vero-E6 cells. In order to diminish the virus-induced cytopathic effect (CPE) by 50% relative to the virus control, the IC50 was determined to be the concentration of hWJ-MSC-extracellular vesicles necessary to achieve this reduction. Nonlinear regression analysis was employed to determine the IC50 and CC50 values through the plotting of log inhibitor versus normalized response (with a variable slope). The findings are presented as the means ± standard deviations of three separate experiments conducted in triplicate each. A p-value less than 0.01 signifies a statistically significant correlation between the extracellular vesicles containing hWJ-MSC and the antiviral activities against HSV-2.
Table 1. Antiviral activity and cytotoxicity of hWJ-MSC- extracellular vesicles.
|
Samples |
cell |
CC50 (µg/ml) |
IC50 (µg/ml) |
SI (CC50, IC50) |
|
hWJ-MSC-MV |
Vero |
62.42 |
14.49 |
4.3 |
|
hWJ-MSC-EX |
52.70 |
6.234 |
8.5 |
3.3. Determination of the Mode of Action of hWJ-MSC- extracellular vesicles
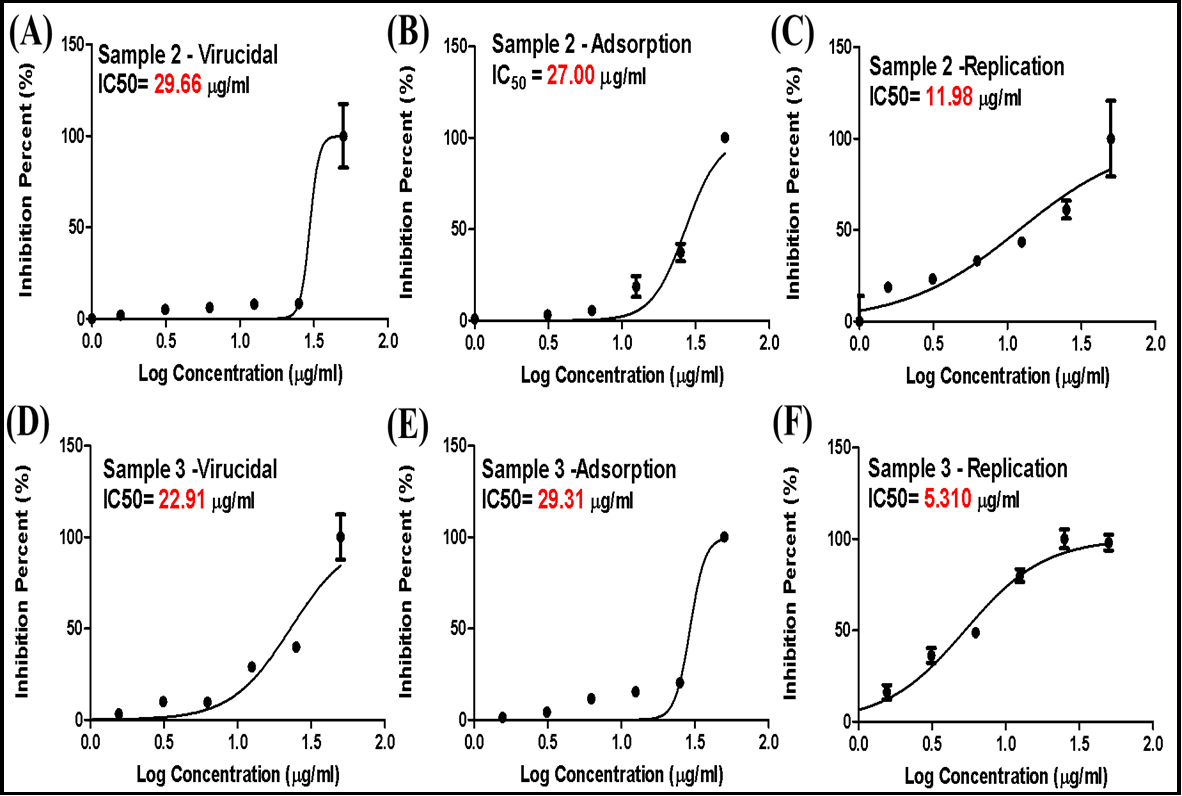
The mode of action of hWJ-MSC- extracellular vesicles was elucidated by investigating its antiviral activity against HSV-2 infection in Vero cells. This investigation employed three distinct antiviral protocols: (i) virucidal, (ii) inhibition of viral adsorption, and (iii) inhibition of viral replication, as depicted in Figure 3. The inhibitory effect of hWJ-MSC- extracellular vesicles on HSV-2 infection was observed across all antiviral protocols. However, there was a notable variation in the degree of inhibition observed among different antiviral assays and concentrations of hWJ-MSC- extracellular vesicles (Figure 3). While all antiviral protocols indicated that hWJ-MSC-extracellular vesicles inhibited HSV-2 infection, the percentage of inhibition varied considerably between antiviral assays and concentrations of hWJ-MSC-extracellular vesicles (Figure 3).
In virucidal, anti-replication, and anti-adsorption protocols, as depicted in Figure 3. The virucidal, anti-replication, and anti- adsorption IC50 values of hWJ-MSC-MV against HSV-2 were 29.66, 11.98, and 27.0 µg/ml, respectively. Moreover, the SI (CC50/IC50) of hWJ-MSC-S against HSV-2 was 2.1, 5.2, and 2.3 µg/ml, respectively, in virucidal, anti-replication, and anti-adsorption protocols. whereas the virucidal, anti-replication, and anti-adsorption IC50 values of hWJ-MSC-EX against HSV-2 were 22.9, 5.3, and 29.3 g/ml, respectively. Moreover, the SI (CC50/IC50) of hWJ-MSC-S against HSV-2 was 2.3, 9.93, and 1.8 µg/ml, respectively. These findings suggested that hWJ-MSC-extracellular vesicles inhibited HSV-2 infection directly by inactivating the virion and indirectly by preventing viral replication.
Figure 3. Antiviral mechanism of hWJ-MSC-MV. (A, B and C) Virucidal, anti-adsorption, and anti-replication activities of hWJ-MSC-MV against HSV-2. (D, E and F) Virucidal, anti-adsorption, and anti-replication activities of hWJ-MSC-EX against HSV-2.
2.7. Characterization by Transmission Electron microscopy
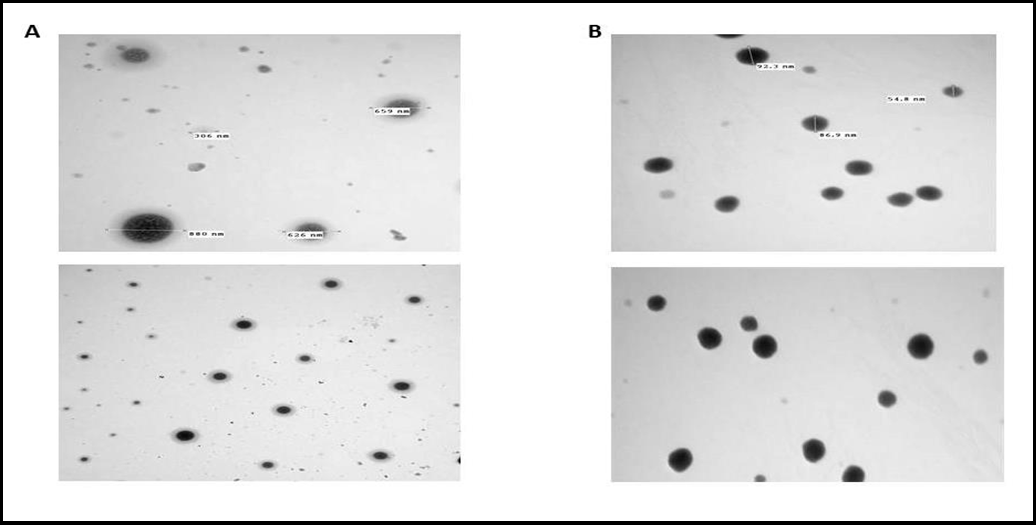
Two different extracellular vesicles, exosome, and microvesicles producing human cell types, were used. We used exosomes and microvesicles isolated from a human umbilical cord stem cell. (Figure 4 A) shows the results of TEM. Exosomes had a spherical shape with a diameter of about 50–100 nm. The microvesicles have a diameter of 300-900 nm. Note the high difference between each vesicle in size and shape (Figure 4 B).
Figure 4 A: Transmission electron microscopy (TEM) characterization of hWJ-MSC-MV and B: Transmission electron microscopy (TEM) characterization of hWJ-MSC-EX.
Discussion
Herpesviruses are highly infectious and lead to high mortality and morbidity rates in infected, immunocompromised people, particularly with human immunodeficiency virus (HIV) infection, are among the most common causes of diseases in humans. Moreover, herpesvirus infections can be extremely severe.
They potentially lead to life-threatening diseases and chronic, persistent, and active infections [9,39]. In addition, the therapeutic options currently available for curing HSV have undergone modifications due to their highly complex infectious nature [37]. Yet, there is no cure that is both permanent and extraordinarily effective. Although contemporary antiviral therapies have the capacity to mitigate symptoms and restrict viral transmission to a specific extent, they are unable to completely eradicate the inherent HSV infection or latency outcomes [27]. At present, there is no approved vaccine against HSV infection; nevertheless, numerous vaccination candidates are undergoing research in preparation for the development of such a vaccine [19]. HSV-2 is the focus of most clinical efforts because of its more infectious consequences [38]. Therefore, the advancement of efficacious antiviral therapeutic strategies for herpes infections represents a pivotal stride in the management of herpes-related diseases.
Cellular therapy with mesenchymal stem cells (MSCs) could be offered a new therapeutic approach due to their broad pharmacological effects, including anti-inflammatory immunomodulatory, regenerative, pro-angiogenic, and anti-fibrotic properties. Stem cell exosomes and microvesicles are gaining popularity as viable substitutes for stem cells in order to minimize their associated side effects. Leveraging extracellular vesicles derived from stem cells presents many benefits, encompassing enhanced safety and effectiveness, adaptability in storage and manipulation, and substantially heightened immune tolerance, among others. Therefore, we aimed in this study to analyze the antiviral activity of two samples from hWJ-MSC-S against herpes simplex type 2. A frequently used metric to suggest a treatment is the selectivity index, which is calculated as the ratio of the 50% cytotoxic concentration (CC50) to the 50% antiviral concentration (IC50). An extract or pure substance has been shown to be effective in vitro at blocking viral propagation while having no cytotoxic or cytostatic side effects. Different SI values have been suggested by studies to support a chemical or herbal extract for preclinical or in vitro research. Thus, we continued our investigations to define the potential mode of anti-herpes simplex type 2 to investigate specific steps of the mod of action at which the virus is inhibited. From the obtained antiviral result, we can observe that both tested samples are good candidates against herpes simplex type 2 in the replication step, which means samples inhibit the virus replication cycle inside cytoplasm by preventing polymerases or inhibiting capsid formation.
The isolation of hUC-MSCs was achieved in 75% (15 out of 20) of the obtained umbilical cords. Cells were successfully isolated via 80% (12 out of 15) of the collected WJ samples and from 40% (6 out of 15) of the collected CT samples. This was confirmed by the high growth and proliferation rates of WJ-MSC, which also demonstrated their therapeutic potential [18]. The hWJ-MSCs were characterized by the presence of CD105, CD146, and CD90 and the absence of CD45 and CD34 molecular markers. MSCs were defined by their multi- differentiation potency and expression of cell surface markers (CD105, CD73, and CD90) and lack of expression of (CD45, CD34, and CD14) [30,36]. The isolated WJ-MSC showed potential and corresponded well with the standard positive and negative phenotypic and molecular profile (CD44+, CD90+, CD 105+, and CD34-) [33].
The treatment with MSCs was improved disease-associated parameters in acute respiratory distress syndrome (ARDS) [10], as well as bronchopulmonary dysplasia, chronic obstructive pulmonary disease, pulmonary hypertension, and idiopathic pulmonary fibrosis (Mathew, 2020). Paracrine factors were considered as a new approach in regenerative medicine and may be represented as a novel and feasible clinical application [8].
The cytotoxic and antiviral activities of hWJ-MSC-MV against HSV- 2 were evaluated using the Vero cell line, and the CC50, IC50, and SI (CC50/IC50) values (µg/ml) were 62.42, 14.94, and 4.3, respectively. In the case of hWJ-MSC-EX, the CC50, IC50, and SI (CC50/IC50) values (µg/ml) were 52.70, 6.23, and 8.5 in a dose-dependent manner, which proved the safety and efficacy of stem cells secreted factor [15,21,45].
The virucidal, anti-replication, and anti-adsorption protocols of hWJ- MSC-MV and hWJ-MSC-EX against HSV-2 in Vero cells were assessed. The virucidal, anti-replication, and anti-adsorption IC50 values of hWJ-MSC-MV against HSV-2 were 29.66, 11.98, and 27.0 µg/ml, respectively. Moreover, the SI (CC50/IC50) of hWJ-MSC-S against HSV-2 was 2.1, 5.2, and 2.3 µg/ml, respectively, in virucidal, anti-replication, and anti-adsorption protocols (Table 2). whereas the virucidal, anti-replication, and anti-adsorption IC50 values of hWJ- MSC-EX against HSV-2 were 22.9, 5.3, and 29.3 µg/ml, respectively. Moreover, the SI (CC50/IC50) of hWJ-MSC-S against HSV-2 was 2.3, 9.93, and 1.8 g/ml, respectively.
Moreover, the veridical, anti-replication, and anti-adsorption mechanisms of hWJ-MSC-p against SARS-CoV-2 in Vero-E6 cells were assessed. At a concentration (1000 µg/ml), hWJ-MSC-p inhibited herpes type-2 infection by > 70 %, 50 %, and 80 % in virucidal, anti-replication, and anti-adsorption mechanisms, respectively. This is due to the presence of a diverse array of molecules in hWJ-MSC-p, including paracrine molecules (including cytokines, growth factors, microRNAs (miRNAs), and exosomes and microvesicles)) that regulate their impact on various effector cells associated with innate and adaptive immunity [5,22,32]. MSC- derived extracellular vesicles (MSC-EVs) moderately suppressed HSV2 replication after treatment.
Conclusions
In this study, we have shown that hWJ-MSC-S extracellular vesicles exhibit antiviral efficacy against the medically significant HSV-2. In vitro, hWJ-MSC-EV quietly reduced HSV-2 infection. This work offers insight into novel hWJ-MSC-S-based antiviral treatments against infection. The transmission electron microscopy data demonstrated that the microvesicles and the exosome differ in size and that the two had different effects on the virus under investigation.
Abbreviations
AA: Antibiotic-Antimycotic
CC50: Cytotoxic Concentration 50
CPE: Cytopathic Effect
DMEM: Dulbecco's modified Eagle's medium
FBS: Foetal Bovine Serum
HIV: Human Immunodeficiency Virus
HSV-2: Herpes Simplex Type 2
hWJ-MSCs: Human Wharton-Jelly-Derived Mesenchymal Stem Cells
IC50: Inhibitory Concentration
miRNAs: microRNAs
mRNA: Messenger RNA
MSCs: Mesenchymal Stem Cells
OD: Optical Density
PBS: Phosphate-Buffered Saline
PFU: Plaque Forming Unit
SI: Selective Index
TCID50: Tissue Culture Infective Dose 50
Vero cells: African green monkey kidney
Data Availability
No data was used to support this study.
Conflicts of Interest
Regarding the publication of this paper, the authors declare that there is no conflict of interest.
References
- AbouAitah K, Allayh AK, Wojnarowicz J, Shaker YM, Swiderska-Sroda A, et al. (2021) Nanoformulation composed of ellagic acid and functionalized zinc oxide nanoparticles inactivates DNA and RNA viruses. Pharmaceutics. 13(12): 2174.
- Alvites R, Branquinho M, Sousa AC, Lopes B, Sousa P, et al. (2022) Mesenchymal stem/stromal cells and their paracrine activity—immunomodulation mechanisms and how to influence the therapeutic potential. Pharmaceutics. 14(2): 381.
- Awasthi S, Shaw C, Friedman H (2014) Improving immunogenicity and efficacy of vaccines for genital herpes containing herpes simplex virus glycoprotein D. Expert Review of Vaccines. 13(12): 1475-88.
- Barrett AN, Fong CY, Subramanian A, Liu W, Feng Y,et al. (2019) Human Wharton's jelly mesenchymal stem cells show unique gene expression compared with bone marrow mesenchymal stem cells using single-cell RNA-sequencing. Stem Cells and Development. 28(3): 196-211.
- Bazzoni R, Takam Kamga P, Tanasi I, Krampera M (2020) Extracellular vesicle-dependent communication between mesenchymal stromal cells and immune effector cells. Frontiers in cell and developmental biology. 8: 596079.
- Bradley H, Markowitz LE, Gibson T, McQuillan GM (2014) Seroprevalence of herpes simplex virus types 1 and 2—United States, 1999–2010. The Journal of infectious diseases. 209(3): 325-33.
- Brennan MÁ, Layrolle P, Mooney DJ (2020) Biomaterials functionalized with MSC secreted extracellular vesicles and soluble factors for tissue regeneration. Advanced functional materials. 30(37): 1909125.
- Burdon TJ, Paul A, Noiseux N, Prakash S, Shum-Tim D (2011) Bone marrow stem cell derived paracrine factors for regenerative medicine: current perspectives and therapeutic potential. Bone marrow research. 2011: 207326.
- Chatterjee A, Roy D, Mukherjee S, Ghosh H, Maiti A, et al. (2022) A comparative analysis depicting the disease characteristics and phylogenetic signature of human cytomegalovirus infection in Human Immunodeficiency Virus 1 seropositive patients with end- organ retinitis and gastro-enteric diseases. Scientific Reports. 12(1): 7617.
- Cruz FF, Rocco PRM (2019) Cell therapy for acute respiratory distress syndrome patients: the START study. Journal of Thoracic Disease. 11(Suppl 9): S1329-S1332.
- Delaney S, Gardella C, Saracino M, Magaret A, Wald A (2014) Seroprevalence of herpes simplex virus type 1 and 2 among pregnant women, 1989-2010. Jama. 312(7): 746-8.
- Elebeedy D, Elkhatib WF, Kandeil A, Ghanem A, Kutkat O, et al. (2021) Anti-SARS-CoV-2 activities of tanshinone IIA, carnosic acid, rosmarinic acid, salvianolic acid, baicalein, and glycyrrhetinic acid between computational and in vitro insights. RSC advances. 11(47): 29267-29286.
- Fong CY, Chak LL, Biswas A, Tan JH, Gauthaman K, et al. (2011) Human Wharton’s jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Reviews and Reports. 7(1): 1- 16.
- Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA , et al. (2006) Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta- analysis of longitudinal studies. Aids. 20(1): 73-83.
- Galland S, Stamenkovic I (2020) Mesenchymal stromal cells in cancer: a review of their immunomodulatory functions and dual effects on tumor progression. The Journal of pathology. 250(5): 555-572.
- Harrell CR, Fellabaum C, Jovicic N, Djonov V, Arsenijevic N, et al. (2019) Molecular mechanisms responsible for therapeutic potential of mesenchymal stem cell-derived secretome. Cells. 8(5): 467.
- Hatzistergos KE, Blum A, Ince T, Grichnik JM, Hare JM (2011) What is the oncologic risk of stem cell treatment for heart disease. Circ Res. 108(11): 1300-3.
- Hussein MAA, Hussein HAM, Thabet AA, Selim KM, Dawood MA, et al. (2022) Human Wharton’s Jelly Mesenchymal Stem Cells Secretome Inhibits Human SARS-CoV-2 and Avian Infectious Bronchitis Coronaviruses. Cells. 11(9): 1408.
- Jaishankar D, Shukla D (2016) Genital herpes: insights into sexually transmitted infectious disease. Microbial Cell. 3(9): 438- 450.
- Jin X, Lin T, Xu Y (2016) Stem cell therapy and immunological rejection in animal models. Current molecular pharmacology. 9(4): 284-288.
- Kint J, Dickhout A, Kutter J, Maier HJ, Britton P, et al. (2015) Infectious bronchitis coronavirus inhibits STAT1 signaling and requires accessory proteins for resistance to type I interferon activity. J Virol. 89(23): 12047-57.
- Koch M, Lemke A, Lange C (2015) Extracellular vesicles from MSC modulate the immune response to renal allografts in a MHC disparate rat model. Stem cells international. 2015: 486141.
- Kuehnle I, Goodell MA (2002) The therapeutic potential of stem cells from adults. Bmj. 325(7360): 372-6.
- Lau J, Balasubramaniam R (2023) Oral Herpes Simplex Virus Infections. Sexually Transmissible Oral Diseases. 159.
- Lin L, Du L (2018) The role of secreted factors in stem cells- mediated immune regulation. Cellular immunology. 326: 24-32.
- Looker KJ, Welton NJ, Sabin KM, Dalal S, Vickerman P, et al. (2020) Global and regional estimates of the contribution of herpes simplex virus type 2 infection to HIV incidence: a population attributable fraction analysis using published epidemiological data. The Lancet Infectious Diseases. 20(2): 240-249.
- Malik S, Sah R, Ahsan O, Muhammad K, Waheed Y (2023) Insights into the novel therapeutics and vaccines against herpes simplex virus. Vaccines. 11(2): 325.
- Mathew R (2020) Signaling pathways involved in the development of bronchopulmonary dysplasia and pulmonary hypertension. Children. 7(8): 100.
- Meldolesi J (2018) Exosomes and ectosomes in intercellular communication. Current Biology. 28(8): R435-R444.
- Mennan C, Wright K, Bhattacharjee A, Balain B, Richardson J, et al. (2013) Isolation and characterisation of mesenchymal stem cells from different regions of the human umbilical cord. Biomed Res Int. 2013:916136.
- Momen-Heravi F, Balaj L, Alian S, Trachtenberg AJ, Hochberg FH, et al. (2012) Impact of biofluid viscosity on size and sedimentation efficiency of the isolated microvesicles. Front Physiol. 3: 162.
- Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, et al. (2017) Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 196(10): 1275-1286.
- Musiał-Wysocka A, Kot M, Sułkowski M, Badyra B, Majka M (2019) Molecular and functional verification of Wharton’s jelly Mesenchymal stem cells (WJ-MSCs) Pluripotency. Int J Mol Sci. 20(8): 1807.
- Nawaz M, Fatima F, Vallabhaneni KC, Penfornis P, Valadi H, et al. (2016) Extracellular vesicles: evolving factors in stem cell biology. Stem cells international. 2016. 1073140.
- Parija SC (2023). Herpesviruses. In Textbook of Microbiology and Immunology. Springer. 751-773.
- Pérez-Silos V, Camacho-Morales A, Fuentes-Mera L (2016) Mesenchymal stem cells subpopulations: application for orthopedic regenerative medicine. Stem Cells Int. 2016: 3187491.
- Schalkwijk HH, Snoeck R, Andrei G (2022) Acyclovir resistance in herpes simplex viruses: Prevalence and therapeutic alternatives. Biochem Pharmacol. 206: 115322.
- Spicknall IH, Looker KJ, Gottlieb SL, Chesson HW, Schiffer JT, et al. (2019) Review of mathematical models of HSV-2 vaccination: Implications for vaccine development. Vaccine. 37(50): 7396-7407.
- Stewart JA, Reef SE, Pellett PE, Corey L, Whitley RJ (1995) Herpesvirus infections in persons infected with human immunodeficiency virus. Clin Infect Dis. 21(Supplement_1): S114-S120.
- Teixeira FG, Carvalho MM, Sousa N, Salgado AJ (2013) Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration? Cellular and Molecular Life Sciences. 70(20): 3871-82.
- Tullis GE, Spears K, Kirk MD (2014) Immunological barriers to stem cell therapy in the central nervous system. Stem cells international. 2014: 507905.
- Yang G, Paintsil E, Dutschman GE, Grill SP, Wang CJ, et al. (2009) Impact of novel human immunodeficiency virus type 1 reverse transcriptase mutations P119S and T165A on 4′- ethynylthymidine analog resistance profile. Antimicrobial agents and chemotherapy. 53(11): 4640-6.
- Zakrzewski W, Dobrzynski M, Rybak Z, Szymonowicz M, Wiglusz RJ (2020) Selected nanomaterials’ application enhanced with the use of stem cells in acceleration of alveolar bone regeneration during augmentation process. Nanomaterials. 10(6): 1216.
- Zhang P, Xie L, Balliet JW, Casimiro DR, Yao F (2014) A herpes simplex virus 2 (HSV-2) glycoprotein D-expressing nonreplicating dominant-negative HSV-2 virus vaccine is superior to a gD2 subunit vaccine against HSV-2 genital infection in guinea pigs. PLoS One. 9(6): e101373.
- Zheng B, He ML, Wong KL, Lum CT, Poon LL, et al. (2004) Potent inhibition of SARS-associated coronavirus (SCOV) infection and replication by type I interferons (IFN-alpha/beta) but not by type II interferon (IFN-gamma). J Interferon Cytokine Res. 24(7): 388-90.