Wayne B. Bauerle, MD*, Christopher Roscher, MD, James Gallagher, MD, Allison Estep, MD, Pooja Mody, DO, Maher El Chaar, MD
St. Luke’s University Health Network, Bethlehem, USA
*Corresponding Author: Wayne B. Bauerle, MD, Department of Research and Innovation, St. Luke’s University Health Network, Bethlehem, USA, E-mail: Wayne.Bauerle@sluhn.org
Abstract
Background:
Herpes zoster (HZ) infection is seen in 2.9-19.5 per 1000 people worldwide. The perioperative risk of myocardial infarction (MI) following HZ reactivation is unknown.
Case Presentation:
We present the case of a middle-aged female without prior history of coronary artery disease (CAD) who underwent a paraesophageal hernia repair following two episodes of shingles. The preoperative assessment indicated a “below average” risk (0.1-0.4%) of major adverse cardiac events (MACE). However, the patient experienced an intraoperative MI.
Conclusions:
The case raises awareness that HZ reactivation may be a potential risk factor for perioperative MI. Further research is needed to establish the associated risk along with determining the appropriate perioperative management for patients with HZ reactivation.
Keywords: Perioperative risk, Major Adverse Cardiac Event, Herpes Zoster, Case Report
Introduction
Background
The incidence of herpes zoster (HZ) infection is 2.9-19.5 cases per 1000 people worldwide [1]. Current literature suggests HZ reactivation is associated with an increased risk of stroke, transient ischemic attack (TIA), and myocardial infarction (MI). Risk tends to decrease with time. Zhang et al. showed that the risk of stroke increased at 1 month more than 1-3 months [2]. Infection severity is also associated with increased risk, with the risk of MI approximately 1.5-fold higher in patients hospitalized with HZ infection [3].
Guidelines recommend using evidence-based calculators to estimate the perioperative risk of major adverse cardiac events (MACE) [4]. Risk calculators have the advantage of accurately quantifying perioperative risk. Calculators like the Revised Cardiac Risk Index (RCRI) and National Surgical Quality Improvement Program (NSQIP) were prospectively and externally validated, with c-statistics (a measure of model strength) generally greater than 0.7, indicating strong model performance [5,6].
We present a patient with shingles three weeks before her procedure who was deemed “below average” risk for perioperative MACE yet suffered acute ST-segment elevation myocardial infarction (STEMI) while undergoing robotic paraesophageal hernia repair. The report is presented using EQUATOR guidelines with written HIPAA authorization obtained from the patient.
Case Presentation:
A 56-year-old female with a history of hiatal hernia and obesity presented as a referral to our clinic with recurrent hiatal hernia after hiatal hernia repair and Nissen fundoplication. Symptoms included nausea, vomiting, bloating, heartburn, and epigastric pain.
Past medical history included morbid obesity, hiatal hernia, oral herpes, anxiety, depression, gastroesophageal reflux disease (GERD), hypothyroidism, Sjogren’s disease, and systemic lupus erythematosus. Of note, the patient’s rheumatologic disease was well controlled on hydroxychloroquine, with a most recent erythrocyte sedimentation rate (before HZ reactivation) within normal limits and an anti-double-stranded DNA antibody level only slightly above the range of normal. Prior surgeries included incisional breast biopsy, laparoscopic hiatal hernia repair with Nissen fundoplication, and bilateral reduction mammaplasty. The patient denied tobacco use, occasionally used alcohol, endorsed minimal exercise, and used a self-created diet plan for weight loss. Medications included hydroxychloroquine, pramipexole, and rabeprazole. The patient had no prior history of coronary artery disease (CAD), angina, or family history of significant CAD.
The physical exam was unremarkable, vitals were within normal limits, and body mass index (BMI) was 41.21 kg/m2.
Esophagogastroduodenoscopy (EGD) showed a 6 cm paraesophageal hiatal hernia with possible Nissen disruption on the retroflex view (Figure 1). Antral biopsy was negative for H. pylori, with the mucosa appearing as mild chronic inactive gastritis. The high-resolution manometry study was regular. Computed tomography (CT) imaging confirmed the presence of a paraesophageal hernia with the entire fundus of the stomach in the thoracic cavity.
Figure 1: Image of esophagogastroduodenoscopy retroflex view for the patient presented.
As shown above, the image is suggesting hernia recurrence and Nissen disruption.
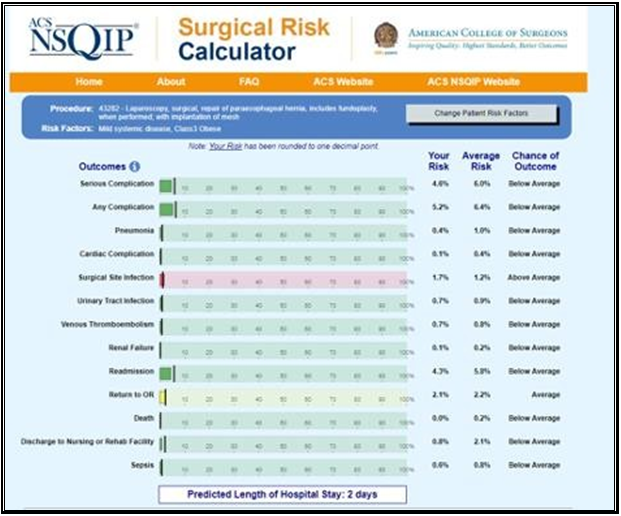
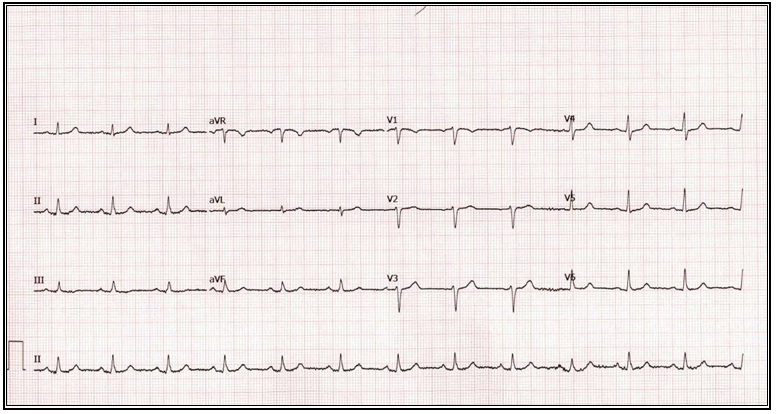
Given the findings, we recommended a Nissen takedown, repair of the hiatal hernia, and subtotal gastrectomy with Roux-en-Y reconstruction. The patient was enrolled in our program, evaluated by dietician, social worker and referred to cardiology for risk stratification. The cardiologist noted she was a life-long non-smoker, had no family history of CAD, and denied symptoms of angina or dyspnea on exertion. Vital signs and physical exam were unremarkable. An echocardiogram showed an estimated ejection fraction of 55% without regional wall motion abnormalities. The valvular function was normal. Based on these findings, the cardiologist deemed the patient as low risk. This assessment was affirmed by the patient’s NSQIP perioperative risk score, which was estimated at 0.1% (Figure 2). The patient’s preoperative electrocardiogram (ECG) is shown in Figure 3.
Figure 2: Image of NSQIP surgical risk calculator results for the patient presented.
When referring to risk of cardiac complications, the estimated risk for the patient was 0.1% where average risk was 0.4% meaning the patient was “below average” risk.
Figure 3: Image of the Patient’s Pre-operative Electrocardiogram
Before meeting the team for preoperative follow-up, the patient presented to the emergency department twice. The initial visit was two weeks before her procedure. She presented with a left-sided neck rash which was deemed to be shingles. She was started on valacyclovir and Silvadene cream. A week later, she represented with left-sided facial and neck pain. She was started on another course of antiviral medication. She completed the course of antiviral therapy, and her symptoms improved. Consideration was given to delaying surgery; however, given the patient’s improving symptoms and completion of her antiviral regimen, the decision was made to proceed with surgery.
On the day of surgery, the history and physical were reviewed with no changes noted. The patient’s shingles had almost resolved. She was taken to the operating room, and anaesthesia was induced with standard American Society of Anaesthesiology (ASA) monitors and average induction doses of medications. The anaesthesiologist noted a brief period of hypotension after induction, which was treated with phenylephrine. The patient’s intraoperative vital trends are shown in Figure 4. Intraoperative notes from approximately 30 minutes after induction noted transient ST-segment elevations in lead III. The blood pressure was stabilized, and ST segments improved. This episode was thought to be due to the reverse Trendelenburg position. Robotic-assisted Nissen takedown, subtotal gastrectomy with Roux-en-Y reconstruction, and repair of the paraesophageal hernia were completed with only 20 ml of blood loss. The total operative time was 3 hours and 29 minutes.
Figure 4: Image of the Patient’s Intraoperative Vital Trends
The patient was transported to the post-anesthesia care unit (PACU). However, postoperative ECG demonstrated ST-elevations in the inferolateral leads concerning STEMI (Figure 5). Cardiac enzyme elevations supported the diagnosis of MI, and a follow-up echocardiogram revealed inferior wall hypokinesis. Cardiac catheterization was deferred as perioperative heparinization would raise the risk of bleeding-related surgical complications. Instead, the patient was medically optimized with beta-blockers, a statin, and an angiotensin-converting enzyme (ACE) inhibitor. Antiplatelet therapy was added the night of surgery. The patient was discharged on postoperative day 4. Outpatient nuclear stress testing confirmed the presence of a sizeable inferolateral perfusion defect, and cardiac catheterization demonstrated a 100% proximal left circumflex stenosis, consistent with recent inferolateral STEMI. Diffuse 30% stenosis of the right coronary artery and 40-60% stenosis of the mid-left anterior descending artery was additionally noted, but these lesions were considered not to be “high grade” by the interventional cardiologist.
Figure 5: Image of the Patient’s Post-operative Electrocardiogram
Discussion
Preoperative cardiac risk stratification for this patient allows for evidence-based quantification of the perioperative risk of (MACE) according to the 2014 American College of Cardiology (ACC) and American Heart Association (AHA) Risk Stratification Guidelines. Standard risk-stratification calculators utilized to estimate perioperative risk have the benefit of being derived from large data sets and are either prospectively or externally validated to increase generalizability. Their accuracy comes from the c-statistic for each of these calculators which is reported to be between 0.7 and 0.8 [5,6]. The RCRI is a six-point index in which a score greater than or equal to 2 indicates a greater than 1 % risk of MACE [7]. This patient’s score of 1 (high-risk surgery) suggests the patient is at low risk for perioperative MACE. The NSQIP and Gupta Risk calculators are additional calculators derived from the NSQIP database, which incorporate both patient and surgical factors to calculate risk [8,9]. The patient’s perioperative risk of MACE was estimated to be between one (NSQIP) and four (Gupta) events per thousand cases, supportive of the assessment of the low likelihood of perioperative MACE (Figure 2, Figure 6). The fact that this patient suffered intraoperative STEMI may be explained by the fact that risk calculators may have underestimated her preoperative risk or that her risk may have been accurately estimated, but that she suffered the unfortunate 1-4 events per thousand cases suggested by preoperative risk assessment. However, an alternative and provocative hypothesis is that the patient’s recent HZ reactivation may have represented an additional unappreciated risk for perioperative MI. Viral migration from the neuron into the vasculature has previously been demonstrated, and the subsequent inflammatory response leading to vessel occlusion and ischemia provides a precise pathophysiologic mechanism for HZ-induced vascular events [10]. We speculate that the patient’s recent HZ reactivation combined with the expected intraoperative surgical stress response represented a “two-hit” mechanism to increase this patient’s risk of perioperative MI.
Figure 6: Image of Gupta risk results for the patient presented.
The patient’s risk of myocardial infarction or cardiac arrest when entering the patient’s clinical information was deemed to be 0.2 %.
Several recent systematic reviews have linked HZ reactivation to an increased risk of vascular events such as stroke or MI. Erskine et al. included 12 articles, with 9/12 being retrospective cohort studies and 3/12 being self-controlled case series [11]. Results showed that patients with HZ reactivation were 20-40% more likely to experience a cerebrovascular event and cardiovascular event at 3 months of HZ onset, and 10-30% were more likely to experience a cerebrovascular and cardiovascular event within 1 year of HZ onset [11]. Zhang et al. reviewed 11 studies, most of which were cohort studies (8/11), with the remaining being case controls, and again found that the risk of stroke (RR 1.9, CI 1.47-2.51) and MI (RR 1.18, 1.07-1.30) was increased after HZ reactivation (2). Several limitations must be considered: (1) reviews were based on lower-quality retrospective evidence; (2) variance in disease coding among the studies could lead to higher rates of false positives, and (3) the probability of unaccounted for MI’s is likely higher than anticipated [2,11].
Alternative explanations of the patient’s perioperative MI must be considered. For example, the simplest explanation may be that the patient had undiagnosed coronary artery disease and suffered a perioperative MI. This is supported to an extent by the postoperative findings of CAD on cardiac catheterization. However, the severity of CAD was judged only to be “mild-to-moderate” by the cardiologist (the left circumflex total occlusion representing recent thrombosis), and evidence-based risk stratification suggested that the risk of MACE was around 1-5 events/thousand cases. SLE itself has been associated with an increased risk of cardiovascular events, including perioperative MI; however, in the case of the latter, a single retrospective report suggested that risk was only slightly elevated (2.4% vs. 2.0%) and that this association was driven mainly by significant differences in event rates in a younger patient cohort [12,13]. Despite these associations, this patient’s SLE was well controlled on medical therapy, with a normal recent ESR and essentially normal anti-double-stranded DNA antibody level, implying that inflammatory risks related to SLE were less likely to be causative.
Conclusions
We present a surgical patient who was judged to be “below average risk” for MACE but, with recent HZ reactivation, suffered perioperative STEMI. We propose that HZ reactivation may have represented a novel risk factor for her perioperative MI. A growing body of literature suggests an association between HZ reactivation and vascular events, with viral migration into arteries leading to inflammation and thrombosis. This is the first report of a possible association between recent HZ reactivation and perioperative MI. Given the presence of other risk factors for MACE, we recognize the speculative nature of the proposed association. Despite this, we believe that our report may serve to foster further research to determine the potential strength and magnitude of the association between HZ and perioperative MI, as well as the optimal interval to delay surgery after HZ reactivation.
List of Abbreviations:
HZ – Herpes Zoster, MI – Myocardial Infarction, CAD – Coronary Artery Disease, MACE – Major Adverse Cardiac Events, TIA – Transient Ischemic Attack, RCRI – Revised Cardiac Risk Index, NSQIP – National Surgical Quality Improvement Program, STEMI – ST-Segment Elevation Myocardial Infarction, GERD – Gastroesophageal Reflux Disease, BMI – Body Mass Index, EGD – Esophagogastroduodenoscopy, CT – Computed Tomography, ASA – American Society of Anaesthesiology, PACU – Post-Anaesthesia Care Unit, ECG – Electrocardiogram, ACE – Angiotensin-Converting Enzyme, ACC – American College of Cardiology, AHA– American Heart Association.
References
- van Oorschot D, Vroling H, Bunge E, Diaz-Decaro J, Curran D, et al. (2021) A systematic literature review of herpes zoster incidence worldwide. Hum Vaccin Immunother. 17(6): 1714- 1732.
- Zhang Y, Luo G, Huang Y, Yu Q, Wang L, et al. (2017) Risk of Stroke/Transient Ischemic Attack or Myocardial Infarction with Herpes Zoster: A Systematic Review and Meta-Analysis. J Stroke Cerebrovasc Dis. 26(8): 1807-1816.
- Seo HM, Cha MJ, Han JH, Han K, Park SH, et al. (2018) Reciprocal relationship between herpes zoster and cardiovascular diseases: A nationwide population-based case-control study in Korea. J Dermatol. 45(11): 1312-8.
- Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, et al. (2014) 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 130(24): 2215-45.
- Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, et al. (2010) Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 21(1): 128-38.
- Glance LG, Faden E, Dutton RP, Lustik SJ, Li Y, et al. (2018) Impact of the Choice of Risk Model for Identifying Low-risk Patients Using the 2014 American College of Cardiology/American Heart Association Perioperative Guidelines. Anaesthesiology. 129(5): 889-900.
- Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, et al. (1999) Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 100(10): 1043-9.
- ACS NSQIP Surgical Risk Calculator American College of Surgeons.
- Gupta P. Gupta Perioperative Risk for Myocardial Infarction or Cardiac Arrest (MICA).
- Nagel MA, Traktinskiy I, Azarkh Y, Kleinschmidt-DeMasters B, Hedley-Whyte T, et al. (2011) Varicella zoster virus vasculopathy: analysis of virus-infected arteries. Neurology. 77(4): 364-70.
- Erskine N, Tran H, Levin L, Ulbricht C, Fingeroth J, et al. (2017) A systematic review and meta-analysis on herpes zoster and the risk of cardiac and cerebrovascular events. PLoS One. 12(7): e0181565.
- Li H, Tong Q, Guo L, Yu S, Li Y, et al. (2018) Risk of Coronary Artery Disease in Patients With Systemic Lupus Erythematosus: A Systematic Review and Meta-analysis. Am J Med Sci. 356(5): 451-463.
- Smilowitz NR, Katz G, Buyon JP, Clancy RM, Berger JS (2018) Systemic lupus erythematosus and the risk of perioperative major adverse cardiovascular events. J Thromb Thrombolysis. 45(1): 13-7.