Sarah Esther Díaz-Oliva1 *, Idalmis Aguilera-Matos2, Ángela Elvirez-Gutiérrez3, Nélcido Luis Sánchez-García4
1Institute of Gastroentrology, Department of Pediatric Gastroenterology. Havana Cuba. https://orcid.org/0000-0002-6611-4823
2Institute of Gastroentrology, Department of Pediatric Gastroenterology. Havana Cuba. http://orcid.org/0000-0002-7364-1533
3Institute of Gastroenterology, Imaging Department. Havana Cuba. http//orcid.org/0000-0002-9740-1403
4Institute of Gastroenterology, Department of Digestive Endoscopy. Havana Cuba. https://orcid.org/0000-0002-2061-2085
*Corresponding Author: Sarah Esther Díaz-Oliva, 1Institute of Gastroentrology, Department of Pediatric Gastroenterology. Havana Cuba.
Abstract
Background: Gastrointestinal stromal tumors are defined as tumors of mesenchymal origin, specifically of the interstitial cells of Cajal. In pediatric age it is very rare and a different entity from the adult form.
Objective: Due to the unusualness of this entity, and the difficulties that may exist for its diagnosis and behavior, we present this case in pediatric age.
Case presentation: A 10-year-old schoolchild who started with anemia and high digestive bleeding, usually in the form of a melena, which required blood transfusion. She was admitted several times without reaching an accurate etiology. The performance of abdominal ultrasound, Upper Digestive Endoscopy with Echoendoscope, Computed Tomography of the abdomen and histological study allowed the diagnosis of a gastric stromal gastric tumor, without metastatic lesion. Subtotal gastrectomy was performed, with good evolution of the patient. The presented case corresponds to some of the clinical characteristics described for patients with pediatric gastrointestinal stromal tumor.
Conclusions: Pediatric gastrointestinal stromal tumors are extremely rare; therefore, diagnosis is usually made late. These should be considered as a possible diagnosis in those patients with anemia and gastrointestinal bleeding. The diagnostic approach of this type of tumors is multidisciplinary.
Key words: gastrointestinal stromal tumor; gastric tumor; digestive bleeding; pediatrics.
Introduction
Gastrointestinal stromal tumors (GIST) are defined as gastrointestinal tumors of mesenchymal origin, specifically of the interstitial cells of Cajal. These are located in the myenteric plexuses of the gastrointestinal wall, which are considered pacemakers of the digestive tract. [1,2] In adults, these correspond to the most common subgroup of mesenchymal tumors of the gastrointestinal (GI) tract, formerly classified as neurofibromas, leiomyosarcomas, or leiomyomas. [2,3] GISTs have a reported incidence of 10 to 15 cases per million in However, the incidence of GIST in the pediatric population is elusive in the general population due to its rare nature and these tumors are often misdiagnosed as other acute or chronic abdominal conditions. The annual incidence is 0.02-0.08 cases per million. [2, 4] Approximately 0.4 % of all GIST patients are younger than 20 years. Pediatric GIST is a different entity from the adult form and the clinical-pathological characteristics vary. [1,3]
Before molecular genetic studies, pediatric and adult GIST forms were separated by age of presentation and histologic features. However, with the advent of genetic testing, 85% of pediatric GIST patients have been found to have a tumor that lacks a mutation in the Tirosin kinase inhibitor (KIT) or the platelet-derived growth factor-alpha receptor (PDGFRA), [1,5] while approximately 60 % of GIST tumors in adults present mutations of these, evidenced by positive CD 117 in immunohistochemistry. The alteration of this proto-oncogene favors the promotion of cell growth or resistance to apoptosis. [1,2] Tumors lacking the KIT / PDGFRA mutation are also known as "wild-type" GIST (WT: wild-type). [1,5] The predominant mutation in pediatric GIST / WT is a mutation inactivating the succinate dehydrogenase (SDH) gene complex. All this leads to lower effectiveness in treatment based on Tyrosine Kinase Inhibitors (ITK), unlike adult GISTs, are 95% positive for CD117. [2, 6] Due to the rareness of this entity in the pediatric age and the difficulties that may exist for its diagnosis, we present this rare case of pediatric GIST.
Presentation of the case
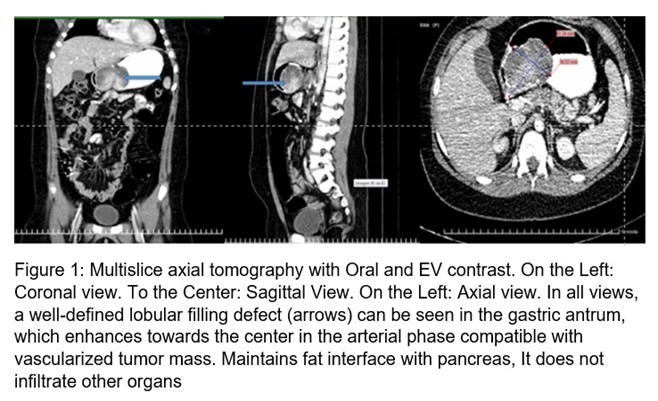
10-year-old female schoolgirl, white, with no personal or family history of interest. In September 2017, the relatives noticed her pale, with decay, and she was taken to the Pediatric Hospital of her province where Hemoglobin (Hb) figures of 60 g / l were found. Her admission is decided, and she is transfused. Later mane was reported. Video Upper Digestive Endoscopy [1] was performed, which reported: Erosive hemorrhagic gastritis, triple eradication therapy of Helicobacter pylori with clarithromycin, metronidazole, and omeprazole was indicated; later omeprazole, sucralfate, and inferno. After 16 weeks, she has admitted again for a melena episode, with a Hb of 70 g / l, UDE was performed, considering the same diagnosis, this time with active bleeding, she presented hematemesis during the procedure. Subsequently, she presented other episodes of melena bleeding and was taken to Pediatric Hospital of the adjoining province and was admitted to the Surgery department with a Hb of 9.9 g / l; A new UDE is performed that reports polypoid appearance formation in the gastric antrum. The patient's admission to the Institute of Gastroenterology is coordinated. Upon arrival, during the physical examination, pale skin and mucosa were observed, without other alterations. In the complementary ones made it was found: Hb: 92 g / l, Serum iron: 2.95 micromol / l. The rest of the values in the hemogram and thermochemistry were in normal parameters. The stool parasitological study was normal, fecal occult blood: positive. Abdominal ultrasound was performed and reported: in gastric body projection, the hypoechoic image of 61.7 mm X 41.6 mm is observed, the image pushes the greater curvature, reaching the serosa; more ecolucent areas compatible with necrosis are seen. Computed tomography with oral and intravenous contrast was performed (Figure 1) in which a full defect in the stomach was visualized, a full defect in the stomach, lobulated, body, and antrum was visualized, regular contours, measuring seven by five cm. The mass in the arterial phase becomes more hyperintense in its central portion, captures contrast. No peripancreatic, periaortic, or perceive lymphadenopathy was seen in the current study. The homogeneous liver without nodular lesion, pancreas, and gallbladder without alterations.
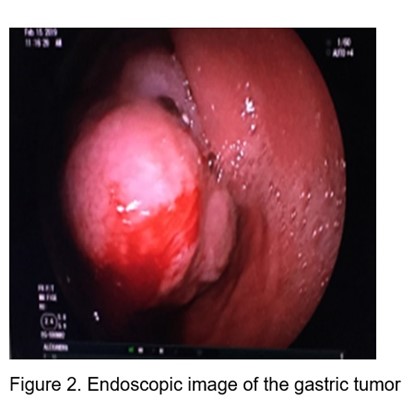
Subsequently Ecoendoscopy is performed: Under endoscopic vision (Figure 2): At the level of the joint between the antrum and the body, towards a lesser curvature and posterior wall, above the angular slit, a polylobulated tumor lesion of approximately 10 cm is observed, with a subepithelial appearance. with ulcerations on its surface, very hard to take a clamp biopsy.
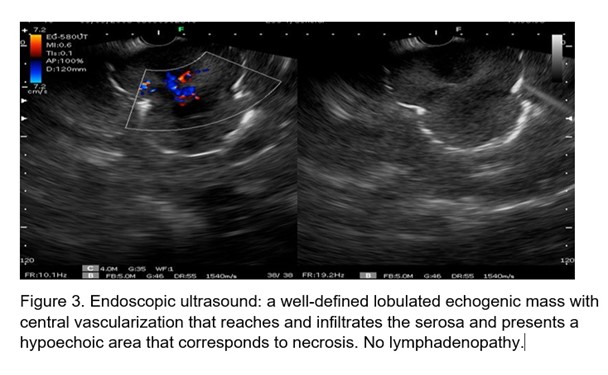
Ultrasound the view was performed (Figure 3) and an echogenic, lobulated mass was visualized, with hypoechoic areas inside, as signs of necrosis. It measures 4.8 cm by 7 cm. Vascularized in color Doppler, which reaches the serosa, the rest of the layers are not displayed at that level. Ultrasound-guided fine-needle biopsy is performed.
Histological study of gastric lesion: Fragments of gastric mucosa and ulcer-necrotic tissue with bacterial colonies. Areas are observed that due to their morphology are suggestive of gastric GIST. Immunohistochemistry (IHC) techniques for diagnosis are indicated. It was not possible due to the low sample and abundant necrotic tissue.
Subsequently, the patient was referred to Pediatric Hospital for surgical treatment. Subtotal gastrectomy was performed. Reception of the tumor of approximately 8 cm in its largest diameter was performed. The girl recovered satisfactorily from the surgery. She currently receives multidisciplinary medical care including Oncology, Surgery, and Gastroenterology specialists.
Histological study of the surgical specimen: GIST-type spindle cell tumor of the gastrointestinal stroma, intermediate in size, eight by six by five cm, with a pedicle of two by one cm. Mitosis Index: Three for 50 High Magnification Fields (CGA) CD117: positive. CD 34: positive. DOC 1: Not available. K 167: positive in ten 10 % of tumor nuclei.
Discussion
We report the first pediatric case of GIST diagnosed at the Institute of Gastroenterology in Cuba. In the databases and revised bibliography of Cuba, we only found one report of a pediatric patient in 2017. A 15-year-old male patient was described, and the tumor location was also gastric. [7] As in the patient presented, it is described that pediatric cases are frequently treated in an erroneous way, consequently, a long time of evolution passes before the definitive diagnosis. [2]
Pediatric GIST behaves very differently clinically from the adult variant. The age of onset is much younger with a median in the second decade of life, (5) while the average age of diagnosis in adults is 63 years, with a predilection in men or no gender predominance in other studies. (1,3,8) In pediatrics, the female sex is predominantly affected (70 %). [1,2,8] Commonly, these tumors arise from the stomach (60 -80 %), [9] as in the reported patient. Less frequently these are located in the small intestine (25 -35 %). Other rare sites are the colon, esophagus, and appendix. Rarely, these may also involve extraintestinal sites, such as omentum, retroperitoneum, and mesentery. [10]
Another difference in the pediatric population is that tumors tend to have an indolent course over a prolonged period. [1] Anemia (acute or chronic) has been clinically documented in children in 86 % of cases. This finding is associated with gastrointestinal bleeding (33 %) with melena or hematemesis, paleness, fatigue, and syncope. This corresponds to the case described. Other symptoms are abdominal pain (16 %), vomiting (11 %), and a palpable tumor (10 %). In addition, it can be a cause of intussusception, intestinal or biliary obstruction, depending on the location. [2]
GIST appear sporadically, only 10 % are associated with genetic syndromes such as familial GIST with KIT germline mutation, neurofibromatosis type 1, Carney's triad (gastric GIST, paraganglioma, and pulmonary chondromas), and Carney-Stratakis syndrome (gastric GIST dyad and paraganglioma). [2,11]
The diagnosis of GIST in the pediatric population can be a clinical challenge. Imaging remains an important modality for diagnosing and determining anatomic relationships, however, concerns about radiation exposure in this population should be considered when choosing an imaging modality. It is recommended, according to availability, ultrasound with contrast enhancement, Magnetic Resonance Imaging, Computed Tomography, which is less recommended in children due to radiation exposure, and positron emission tomography.[1] The gastric tumor mass of the patient as evidenced by abdominal ultrasound. It was not possible to perform a contrasted ultrasound; However, the size of the tumor and professional experience made it possible to diagnose the lesion. Due to the frequency of pediatric cases debuting with anemia or digestive bleeding, UDE is indicated, and echoendoscopy is also recommended, which allows distinguishing between intramural or extramural injury, visualizing the depth and local involvement of the injury, and allowing taking a directed biopsy. [1,12,13] Ultrasound was useful as an initial non-invasive diagnostic tool in this patient. UDE and endoscopic ultrasound were performed to assess the characteristics of the lesion and guide the collection of a fine needle biopsy sample, as there was no thick needle. Subsequently, an abdominal tomography was indicated to look for lesions in other organs of the abdominal cavity.
UDE is the preferred method of taking a core needle biopsy or forceps biopsy with or without ultrasound guidance, due to concerns about the spread of tumors through other routes, such as percutaneous image-guided biopsy or surgical biopsy. Tumor seeding is highly associated with recurrence in pediatric GIST and should be avoided at all costs. If anatomy allows and an endoscopic biopsy approach is not possible, it is prudent to perform a primary resection as a means of tissue diagnosis. [1,6]
Histologically, GIST can present a variable architectural pattern, but in pediatrics patients, it has no prognostic relevance. [2] Diagnosis of these lesions is based on a microscopic study with immunohistochemical techniques.[7] GIST present three different morphologies: spindle cells, epithelioid cells, and mixed cell types. Spindle cell morphology is highly associated with classic adult GIST, whereas mixed epithelioid cell morphologies are more highly associated with pediatric / WT GIST tumors. Immunohistochemical CD117 (KIT) and anoctamin (ANO1) markers are the most sensitive and specific markers for the analysis of GIST lesions. (1) The case of pediatric GIST previously reported in Cuba [7] and the one in question, have presented characteristics histological and immunohistochemical different from that described in the current bibliography for pediatric patients, [1-4] presenting as in the adult form.
Initial diagnosis for pediatric patients should be coordinated with radiology, pathology, gastroenterology, and surgery teams to ensure prompt diagnosis and adherence to the most current clinical guidelines. [1]
It is difficult to make estimates of disease-free survival. The risk of relapse is 25 %, however, mortality from disease progression ranges from 10-14 %. Various studies show a better prognosis in pediatric patients compared to adults. [2] Scores have been developed to determine the risk of aggressive behavior, incorporating metastatic disease, tumor size, and mitotic count. Tumors are stratified into very low, low, intermediate, high risk, and metastatic disease categories. Others include the site of the primary tumor (gastric versus small intestine). [1] In the presented case, the tumor size and mitotic index are taken into account, an intermediate risk was documented.
Cytotoxic chemotherapy is ineffective. Before the introduction of tyrosine kinase inhibitors (TKIs) as adjuvant therapy, the prognosis for adult patients with metastatic disease was poor, with a median survival of fewer than 2 years. The prognosis improved dramatically after the introduction of specific treatments such as Imatinib. Most pediatric GIST / WT GIST do not express mutations in KIT and PDGFRA, and therefore have been shown to be less sensitive to traditional ITK treatment. However, the heterogeneous nature of these tumors means that these findings must be considered in light of the tumor biology on a case-by-case basis, since the sensitivities of the many different mutations found in these tumors are not well studied, in some mutations reports sensitivity to imatinib. Several studies have also shown that second-generation ITKs like sunitinib show better activity against pediatric GIST. There are currently multiple therapies in various stages of clinical trials that may benefit pediatric patients. [2,13,14] In the case of the presented patient, she should benefit from the use of imatinib if its use is necessary since it presents immunohistochemical characteristics similar to the adult type of GIST.
Surgery plays a fundamental role in patients with pediatric GIST, since it is the only curative treatment. The risk of relapse is 25 %, with mortality of 10-14 %. [2,9,15,16]
As in the treatment of GIST in adults, if the primary tumor is completely resected at the time of initial surgery, without tumor effusion, no adjuvant treatment is warranted.
Due to the complexity of the mutations involved in pediatric GIST and its consequences in therapeutic management, the importance of referral to specialized centers should be emphasized when determining adjuvant therapies for the pediatric patient presenting with GIST. [15]
Taking into account all of the above, we can conclude that pediatric GISTs are extremely rare, therefore, diagnoses are usually made with delays. GISTs should be considered as a differential diagnosis in patients with anemia and gastrointestinal bleeding. The diagnostic approach for this type of tumor is multidisciplinary and should be referred to specialized centers to determine the therapy to be followed.
Conflict of interests: The authors declare that they have no conflict of interest in carrying out the study.
Source(s) of Financial Support: The authors did not receive any type of funding for the article.
Authors' Contributions
Dra. Sarah Esther Díaz Oliva: Drafting of the work draft and its final report.
Dra. Idalmis Aguilera Matos: she carried out the search, contribution in the writing of the draft and revision of the consulted bibliography. Review of the report.
Dra. Ángela Elvirez Gutierrez: she selected the images presented and wrote their captions. Review of the report.
Dr. Nélsido Luis Sánchez García: Critical review of the final version and approval of the one to be published
References
- Quiroz HJ, Willobee BA, Sussman MS, Fox BR, Thorson ChM, et al. (2018) Pediatric gastrointestinal stromal tumors—a review of diagnostic modalities. Transl Gastroenterol Hepatol. 3: 54.
- Morales PA, Millán VLO, Covarrubias EG, Galván RVG, Ríos GCG, et al. (2017) Tumor de GIST pediátrico. Presentación de dos casos y revisión de la literatura. Bol Clin Hosp Infant Edo Son. 34(2).
- Soreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, et al. (2016) Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 40: 39-46.
- Benesch M, Leuschner I, Wardelmann E, Thielen M, Schmid I, et al. (2011) Gastrointestinal stromal tumours in children and young adults: A clinicopathologic series with long-term follow-up from the database of the Cooperative Weichteilsarkom Studiengruppe (CWS). Europ Jour Can. 47(11): 1692-1698.
- Boikos SA, Pappo AS, Killian JK, LaQuaglia MP, Weldon CB, et al. (2016) Molecular Subtypes of KIT/PDGFRA Wild-Type Gastrointestinal Stromal Tumors: A Report From the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol. 2(7): 922-8.
- Mullassery D, Weldon CB (2016) Pediatric/"Wildtype" gastrointestinal stromal tumors. Semin Pediatr Surg. 25(5): 305-310.
- Verdecia Cañizares C, Villamil Martínez R, Montero Reyes I, Pineda Fernández D (2017) Tumor estromal gastrointestinal. Revista Cubana de Pediatría. 89(1).
- Prasad AA, Alex V. M, Anthony P, Madhavan V. P (2016) Gastrointestinal Stromal Tumors: A Review. American Journal of Therapeutics. 23(2): e550–e557.
- Raitio A, Salim A, Mullassery D, Losty PD (2021) Current treatment and outcomes of pediatric gastrointestinal stromal tumors (GIST): a systematic review of published studies. Pediatric Surgery International.
- Jakhetiya A, Garg P K, Prakash G, Sharma J, Pandey R, et al. (2016) Targeted therapy of gastrointestinal stromal tumours. World J Gastrointest Surg 2016 May 27; 8(5): 345-352.
- Vega J, Navarro Subiabre J, Lovera Riquelme C, Opazo H, Santamarina M (2017) Tríada de Carney. Una rara asociación de tumores infrecuentes. Caso clínico. Rev Med chile. 145(4): 533-537.
- Nishida T, Blay JY, Hirota S, Kitagawa Y, Kang Y-K (2016) The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 19(1): 3-14.
- Mullassery D, Weldon CB (2016) Pediatric/ “Wildtype” gastrointestinal stromal tumors. Sem Pedia Surg. 25(5): 305-310.
- Jakhetiya A, Kumar Garg P, Prakash G, Sharma J, Pandey R, et al. (2016) Targeted therapy of gastrointestinal stromal tumours. World J Gastrointest Surg. 8(5): 345-352.
- Willobee BA, Quiroz HJ, Sussman MS, Thorson CM, Sola JE, et al. (2018) Current treatment strategies in pediatric gastrointestinal stromal cell tumor. Transl Gastroenterol Hepatol. 3: 53.
- Benesch M, Wardelmann E, Ferrari A, Brennan B, Verschuur A (2009) Gastrointestinal stromal tumors (GIST) in children and adolescents: A comprehensive review of the current literature. Pediatr Blood Can. 2009; 53 (7): 1171–1179.



