Huber F. MD MSc1*, Gottsberger J. MD1, Schachner B. MD1, Grund M. MD2, Zierer A. MD Prof1
1Department of Cardiothoracic and Vascular Surgery, Johannes Kepler University, Linz, Austria
2Department of Cardiology, Johannes Kepler University, Linz, Austria
*Corresponding Author: Huber F. MD MSc, Department of Cardiothoracic and Vascular Surgery, Johannes Kepler University, Linz, Austria.
Abstract
Objective: Aortic valve disease is the most prevalent valvular abnormality in the developed world and carries a high risk of morbidity and mortality. Transcatheter aortic valve replacement (TAVR) is favored over open-heart surgery in high-risk patients, and its use is steadily expanded towards lower-risk groups. Redo-TAVR requires meticulous planning. The Acurate Neo is a nitinol, self-expanding, supra-annular transcatheter heart valve (THV) and is a relatively recent addition to the market. We report, to the best of our knowledge, the first case of redo transapical (TA) TAVR with a Sapien 3 Ultra in an early degenerated Acurate Neo.
Methods: An 83-year-old male patient was admitted with shortness of breath 4 years after TA-TAVR using an Acurate Neo, size L. Echocardiography revealed new severe valvular regurgitation. No paravalvular leakage was present. Cardiac catheterization confirmed the presence of coronary artery disease with a history of CABP and a patent LIMA graft. CT scan of the access route showed significant calcification and angulation of the iliac vessels, which made the TA approach necessary for initial TAVR. Trans-subclavian access was not an option because of the patent LIMA graft and a moderate kinking of the left subclavian artery. Therefore, we had to go for a true redo TA-TAVR.
Results: After careful preoperative screening and planning, an Edwards 26mm S3 Ultra balloon-expandible valve (Edwards Lifesciences, Irvine, CA, USA) was selected and positioned relatively high to pin the first THV in a fully open position. Post-deployment, no mean invasive gradient across the aortic valve was present. Echocardiogram showed neither transvalvular nor paravalvular regurgitation. This excellent result could also be demonstrated by contrast aortography. This straightforward procedure lasted 54 minutes. Postoperative recovery was uneventful, with extubation in the operating room and transferred to the regular ward on the first postoperative day. He was discharged home after another 8 days with excellent pre-discharge transthoracic echocardiographic findings.
Conclusion: This is the first reported case of a redo TA-TAVR procedure with an S3 Ultra balloon expandible valve due to a structurally destroyed Acurate Neo THV. Our successful case underlines the feasibility of redoing TA-TAVR even in an Accurate NEO despite its supra-annular profile.
Keywords: Case Report, Transcatheter aortic valve implantation, Structural valve deterioration, Redo TAVR, Accurate NEO, Sapien 3 Ultra
1. Introduction
Aortic valve disease is the most prevalent valvular disease and carries a high risk of morbidity and mortality. Transcatheter aortic valve replacement (TAVR) in 2002 revolutionized the management of aortic valve stenosis [1]. Since then, technology and devices have progressed at a tremendous speed. Although initially only utilized in “high-risk” patients who were not candidates for open heart surgery, the indications for TAVR are expanding worldwide, including populations with lower surgical risk [2,3].
Transcatheter heart valves are bioprosthetic valves and carry the risk of early structural valve deterioration (SVD) leading to valve dysfunction. The safety and efficacy of valve-in-valve procedures via a transcatheter approach has been shown [4,5]. This published data refers to valve-in-valve methods in degenerated surgical bioprosthesis. However, there needs to be more data in the literature about valve-in-valve TAVR for degenerated TAVI valves.
The Accurate Neo is a nitinol, self-expanding, supra-annular transcatheter heart valve (THV) and is a relatively recent addition to the market. As such, there have been very few reports of SVD complicating this valve and its management.
We report, to the best of our knowledge, the first case of a true redo transapical (TA) TAVR with a Sapien 3 Ultra in an early degenerated Acurate Neo.
2. Case Report
In January 2023, an 83-year-old man was admitted emergently with a short history of progressive exertional breathlessness and orthopnea. Clinical examination revealed evidence of severe heart failure with the need for drugs for circulatory support and the presence of acute kidney injury requiring hemofiltration. There was no clinical finding for infective endocarditis.
Four years ago, he underwent transapical (TA) TAVR with a 27 mm, size L Acurate Neo THV (Boston Scientific, Malborough, MA, USA) for severe symptomatic aortic stenosis. At the time, the patient was deemed to be at high risk for open heart surgery because of previous bypass surgery in 2000, with three patent grafts. Femoral access was not feasible because of severe calcified and angulated iliac vessels. Therefore, the Heart Team considered TA TAVR to be the optimal treatment. After TA TAVR in October 2019, transthoracic echocardiogram (TTE) showed normal aortic valve parameters with a mean gradient of 6 mm HG, no paravalvular leakage, and average left ventricular ejection fraction (LVEF 60%). The patient had been under regular follow-up. The latest TTE examination at our department in July 2021 showed a well-functioning THV prosthesis. His other relevant medical history included atrial fibrillation, ischemic stroke, and pacemaker implanted for symptomatic bradycardia.
The patient´s current presentation was consistent with a diagnosis of heart failure. Urgent TTE showed severe aortic regurgitation. Transoesophageal echocardiogram (TEE) confirmed severe transvalvular aortic regurgitation due to prolapse of a THV cusp. No findings for paravalvular leakage or infective vegetation were given. LVEF was still expected (LVEF 60%). Cardiac catheterization revealed three patent bypass grafts. A diagnosis of rapid, early SVD causing severe aortic regurgitation was made.
The patient was discussed by the Heart Team. A true redo of TA TAVR was considered the optimal therapy. A transfemoral access route was still impossible for the same reasons as in 2019. A trans axillary course was ruled out because of the patent LIMA graft and a kinking of the left subclavian artery.
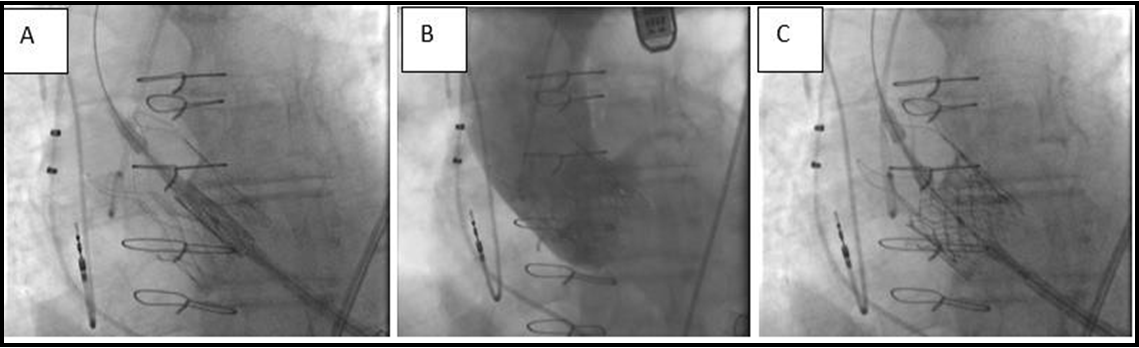
After evaluation, we performed a redo TA TAVR with a 26-mm Sapien S3 Ultra valve (Edwards Lifesciences, Irvine, CA, USA). The intention was to position the Sapien S3 Ultra high within the Acurate Neo to ensure complete coverage of the leaflets and eliminate the risk of residual degenerative leaflet mobility, which could impair the outflow and normal function of the new THV leaflets (Figure. 1). We did not take much care of coronary ostia obstruction because of the patent bypass grafts. A 26 mm Sapien S3 Ultra valve (nominal volume) was positioned to overlap the top of the Sapien 3 frame with the commissures of the Acurate Neo. Post-deployment, no mean invasive gradient across the aortic valve was present. Echocardiogram showed neither transvalvular nor paravalvular regurgitation. This excellent result could also be demonstrated by contrast aortography (Figure. 2). This straightforward procedure lasted 54 minutes. Postoperative recovery was uneventful, with extubation in the operating theatre and transfer to the regular ward on the first postoperative day. He was discharged home after another 8 days with excellent pre-discharge transthoracic echocardiographic findings.
Figure 1: Alignment of S3 transcather heart valve in relation to Acurate Neo THV [3].
Figure 2: (A) Fluoroscopy showing positioning of the Sapien 3 Ultra. (B) Following redo-transcatheter aortic valve replacement. (C) Final aortogram showing an excellent result
3. Discussion
With the aging population, aortic stenosis is gradually increasing. The treatment of choice has been surgical aortic valve replacement for most patients with low surgical risks. TAVR has been increasingly used in recent years, and it is seen that it will be used in low-risk patients [6-8]. This means that TAVR will be done on younger patients, and we will see degenerated TAVI valve cases in the coming years [9]. In cases of BPV deterioration, infective endocarditis, vegetation, thrombus, and dehiscence need to be excluded, as in our case. SVD can be present as stenosis or regurgitation.
Transcatheter aortic valve replacement in TAVR is attractive. The most extensive observational study of redo TAVR has demonstrated good safety [10]. In the 5-year follow-up of the randomized PARTNER 2 trial, in which TAVR was compared with open heart surgery in intermediate-risk patients, aortic valve reinterventions were more frequently observed in the TAVR group (3.2% vs. 0.8%). Of the 21 reinterventions after TAVR, 17 were treated with redo TAVR [11]. The low rate of redo TAVR reflects the excellent durability of THV. However, facing the continuous shift towards younger and low-risk patients, we will have to face the problem of degenerated THV shortly.
Redo TAVR would have a safety profile like first-time TAVR. There were no mortalities in patients who underwent redo TAVR in the 5-year PARTNER 2 follow-up [11].
A recently recognized problem with redo TAVR is impaired access to the native coronary arteries due to displaced leaflets of the first THV. The risk is most significant in patients with supra-annular THVs. The displaced brochures of the first THV can form a physical barrier to the coronary ostia. This difficulty can be overcome by deeper placement of the initial THV into the left ventricular outflow tract. This might be associated with more pacemaker requirements. Furthermore, it is essential to consider, especially in younger patients, which type of THV prosthesis to choose for the first-time intervention (intra-annular vs. supra-annular profile). In the present case, we had patent coronary bypass grafts.
The distance between the valve and aorta (VTA) above the right and left main stem indicates the feasibility of future coronary access. The VTA is measured as the shortest distance between the THV frame at the commissures and the aortic wall at the sinotubular junction or above the LMCA. The risk is generally considered high when the VTA is <2mm [3].
Limited data exist for redo TA TAVR. According to Landes et al., who published a comprehensive evaluation of redo TAVR, only 6.9% had transapical access [10]. This case underlines the efficacy and safety of a transapical approach for redoing TAVR.
4. Conclusion
This is the first reported case of a redo TA-TAVR procedure with an S3 Ultra balloon expandible valve due to a structurally destroyed Acurate Neo THV. Our successful case underlines the feasibility of redoing TA-TAVR even in an Accurate NEO despite its supra-annular profile. With steadily rising numbers of implanted TAVRs worldwide and an expected continuous shift towards younger and lower-risk patients, the problem of how to treat degenerated THV needs to be addressed thoroughly. Against this background, the international multicentre „ReDo-TAVI“ registry has been initiated with the participation of our unit.
Authors Contributions
All authors have made substantial contributions to the conception and design, have been involved in drafting the manuscript and revising it critically for important intellectual content, and have given final approval of the version to be published.
Funding Sources: The authors have no funding sources to declare.
Disclosures
Prof. Zierer is a proctor for Edwards Lifesciences. Dr. Grund is a proctor for Abott and Medtronic. The other authors have no conflicts of interest to declare.
List of abbreviations
TAVR: Transcatheter aortic valve replacement
THV: Transcatheter heart valve
TA: transapical
CABP: coronary artery bypass grafting
LIMA: left internal mammary artery
SVD: structural valve deterioration
TTE: transthoracic echocardiogram
TEE: transoesophageal echocardiogram
References
- Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, et al. (2002) Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 106(24): 3006-8.
- Jawitz OK, Gulack BC, Grau-Sepulveda MV, Matsouaka RA, Mack MJ, et al. (2020) Reoperation After Transcatheter Aortic Valve Replacement: An Analysis of the Society of Thoracic Surgeons Database. JACC Cardiovasc Interv. 13(13): 1515-1525.
- Savvoulidis P, Nadir A, Ludman PF, Doshi SN (2022) Early Acurate Neo transcatheter heart valve degeneration in a haemodialysis patient successfully managed with Sapien 3 Ultra: a case report. Eur Heart J Case Rep. 6(7): ytac279.
- Dvir D, Webb JG, Bleiziffer S, Pasic M, Waksman R, et al. (2014) Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA. 312(2): 162-70.
- Tam DY, Vo TX, Wijeysundera HC, Dvir D, Friedrich JO, et al. (2018) Transcatheter valve-in-valve versus redo surgical aortic valve replacement for the treatment of degenerated bioprosthetic aortic valve: A systematic review and meta-analysis. Catheter Cardiovasc Interv. 92(7): 1404-1411.
- Thyregod HG, Steinbrüchel DA, Ihlemann N, Nissen H, Kjeldsen BJ, Petursson P, et al. (2015) Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Severe Aortic Valve Stenosis: 1-Year Results From the All-Comers NOTION Randomized Clinical Trial. J Am Coll Cardiol. 65(20): 2184-94.
- Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, et al. (2019) Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med. 380(18): 1706-1715.
- Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, et al. (2019) Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 380(18): 1695-1705.
- Duran Karaduman B, Ayhan H, Bulguroğlu S, Keleş T, Bozkurt E (2020) Transcatheter valve-in-valve implantation Edwards Sapien XT in a direct flow valve after early degeneration. J Card Surg. 35(12): 3592-3595.
- Landes U, Webb JG, De Backer O, Sondergaard L, Abdel-Wahab M, et al. (2020) Repeat Transcatheter Aortic Valve Replacement for Transcatheter Prosthesis Dysfunction. J Am Coll Cardiol. 75(16): 1882-1893.
- Makkar RR, Thourani VH, Mack MJ, Kodali SK, Kapadia S, et al. (2020) Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. N Engl J Med. 382(9): 799-809.