D. Nemsadze, MD, PhDs*, V. Tkeshelashvili, MD, JD, PhD, ScD, professor, G. Nemsadze, MD, PhD, ScD, professor. M. Mchedlishvili MD, PhD, Professor, A. Shemerovskiy MD, PhD
The University of Georgia, The Institute of Clinical Oncology, Tbilisi, Georgia
*Corresponding Author: D. Nemsadze, MD, PhDs, The University of Georgia, The Institute of Clinical Oncology, Tbilisi, Georgia.
Abstract
Phyllodes tumors of the breast are among sporadic tumors. Their frequency does not exceed 1%. There are benign, borderline, and malignant forms of tumors. The latter is the rarest form associated with a high recurrence rate. At the same time-frequency of this disease in children and adolescents is extremely rare. According to the information we obtained, malignant phyllodes tumor in children in Georgia was observed only in our clinic during the last 10 years.
We present a literature review and case report of a 13-year-old female adolescent diagnosed with a malignant phyllodes breast tumor.
Keywords: breast sarcoma, phyllodes tumors, rare oncologic disease, pediatric malignant tumor.
Introduction and literature review
Phyllodes tumors (PT) of the breast are rare, accounting for less than 0.5 % of all breast tumors. These tumors comprise stromal and epithelial elements, classified into benign, borderline, and malignant subtypes. The mild variant is the most common and is close to fibroadenoma but is usually larger and recurs more frequently [1]. PTs can mimic fibroadenoma in clinical presentations. Breast imaging is also like fibroadenoma. Cytological diagnosis of PTs by biopsy is usually unreliable. However, a core needle biopsy is superior to fine-needle aspiration [7].
Phyllode tumors occur in relatively young women compared with the classical adenocarcinoma of the breast [1]. Phyllodes tumors can be detected in all ages; however, the median age of presentation is 45 years [7].
They tend to reach large sizes without nodal metastasis [4]. The mainstay of treatment of non-metastatic phyllodes tumors of the breast is complete surgical resection with wide resection margins.
The surgical management of phyllodes tumors is still controversial. But there are lots of studies that suggest surgical margins ≥ 1 cm. Negative margins could be appropriate regardless of their width. Thus, free surgical margins are necessary to treat PT, and at least 1 mm margins might be sufficient to prevent recurrence [6,9].
Lumpectomy or partial mastectomy is the preferred surgical therapy. The local failure rate may be high, and 22 % of malignant tumors may give rise to hematogenous metastases. The most frequent site of distant metastases is the lungs [1]. Rarer are liver, adrenal, brain, and bones cases by hematogenous route [3]. Several predictive factors of recurrence and metastases, such as positive surgical margins, increased stromal cellularity, stromal overgrowth, stromal atypia, and increased mitotic activity, have been described in the literature [1].
The use of adjuvant chemotherapy and radiation therapy for malignant PTs is controversial [7]. Radiation therapy (RT) and chemotherapy (CT) did not significantly improve the long-term survival of PT patients [8].
Without solid evidence of survival benefit, adjuvant radiation therapy has revealed a more favorable local control rate than the observation group. Malignant and borderline phyllodes tumors with positive margins after surgery have high local recurrence rates. After treatment by margin-negative breast conservation treatment alone, patients with significant (≥ 5 cm) malignant phyllodes tumors also have a higher risk of local recurrence. Thus, such patients should be considered for additional local therapy [2]. The trend of survival benefit from adjuvant RT existed in patients with > 50 mm tumor and receiving breast-conserving surgery [8].
For patients with borderline/malignant phyllodes tumors, adjuvant radiation therapy significantly improved local recurrence-free survival after margin-negative wide local excision; however, patients treated with mastectomy did not attain the same benefit from adjuvant irradiation. But adjuvant radiation therapy did not improve overall survival [8].
To sum up, today, there is a lot of controversial data and limited guidelines, given the rare occurrence of this pathology [10]. But it can be said unequivocally that despite the patients' higher aggressiveness and younger breast cancer age, old ideas that "nothing but extensive surgery will help" have lost their relevance. Compliance with general oncological principles such as "clean margins," adjuvant chemotherapy, and radiotherapy are also significant in the case of phyllodes tumors.
Case Description
A 13-year-old female patient presented to the clinic in August 2021 complaining of a rapidly growing tumor in the right breast. Examination of the breast revealed a large, moving, painless lump, which, according to the patient and her relatives, had grown within about 1 month.
According to performed ultrasound, a mass with a maximum diameter of 10 cm was noted in the lower lateral quadrant of the right breast. The preliminary clinical diagnosis was juvenile fibroadenoma of the right breast. The patient was soon subjected to surgical treatment, and wide excision of the tumor from the right breast was done.
Macroscopy: operative material was 14.0 X 11.0 11.0 cm well-circumscribed tumor nodule. Invasion of the tumor into the soft tissue was focally visualized. The cancer was white gray in color with necrosis and hemorrhages. Multiple tissue samples were embedded in paraffin blocks. The material's fixation duration does not exceed 24 hours (Figure. 1).

Figure 1. Macroscopy of tumor
Microscopically: a biphasic fibroepithelial tumor was found. The epithelial component was mainly observed at the periphery of the tumor. There was no atypia of epithelial cells. Attention was paid to the atypia of the mesenchymal part of the tumor: the cellular stroma was seen, and there was an overlap of cells, atypia. Cellular elements were mostly finger-shaped (Figure. 2,3,4).

Figure 2: Staining with hematoxylin and eosin. ok 10 X ob.10. Malignant phyllodes tumor; stromal component. A cellular stroma is noted; the superimposition of cells is visible. There is swelling focally.

Figure 3: Staining with hematoxylin and eosin. ok 10 X ob.20. Malignant phyllodes tumor; Necrobiosis, proliferation of finger-shaped tumor cells.
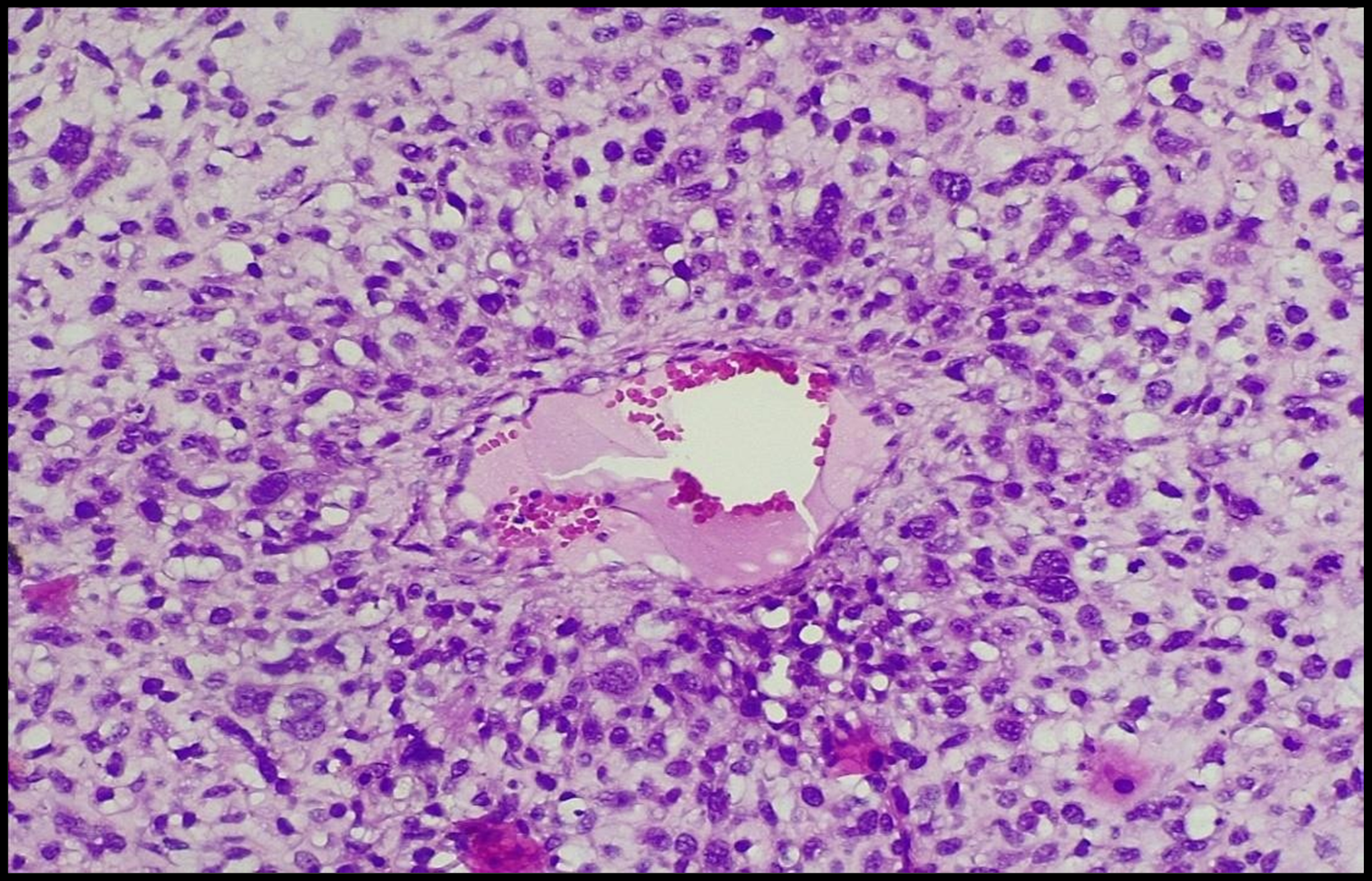
Figure 4: Staining with hematoxylin and eosin. ok10 X ob40. Malignant phyllodes tumor; stromal component. There is atypical cell proliferation around the blood vessel. Marked nuclear atypia is seen.
The immunohistochemistry test examined the tumor. We used "Novocastra" antibodies. The following markers were used: AE1/AE3, ER, PR, p53, Vimentin, SMA, DESMIN, S100, CD34, CD68, CK5 and ki67. The epithelial component was intenselyactivated by AE1/AE3 (Figure. 5). The most prominent was DESMIN expression. The marker was expressed over 45% of the tissue, marking atypical finger-shaped cells (Figure. 6).

Figure 5: Malignant phyllodes tumor; Involvement of Ae1/Ae3 in the epithelial component. ok 10 X ob20.

Figure 6: Malignant phyllodes tumor; Intense involvement of the mesenchymal component DESMIN in myxoid areas. ok10 X ob10.
Pathology: phyllodes tumor of the breast, with areas of differentiation of rhabdoid sarcoma of the mesenchymal component (WHO-O code:9020/3).
The patient was referred to a pediatric oncologist for follow-up, where the observation mode was selected only. 8 months after the operation, the patient again visited the clinic complaining of a painless tumor in the same breast, this time on the border of the upper quadrants. A 5 cm tumor in the right breast was confirmed by ultrasound. A CT scan of the chest and abdomen was performed. No signs of disease spread, or progression were found. Surgical treatment was planned again. This time mastectomy was offered, which the patient and her relatives refused. We performed wide excision of the mass from the right breast. Recurrence of the malignant phyllodes tumor was diagnosed histomorphologically (WHO-O code: 9020/3).
The patient was advised to undergo adjuvant radiation therapy. 2 weeks after the operation, a CT scan of the chest was performed during the planning of the radiation therapy and showed a 1.7/1.5 cm mass revealed ventrally in the sternum on the right, which was not noted on the previous CT scan. Therefore, despite the atypical CT semiotics, this formation was considered a secondary lesion, and it was decided to irradiate this area along with the breast.
Adjuvant radiotherapy with IMRT technology was performed. The whole breast and suspicious bone lesion in the sternum with 2 grays- 30 fractions were treated. The total dose was 60gy.
The patient underwent radiation therapy and moved to another country with her family. According to the last telephone contact, the situation is still stable. There are no signs of disease progression.
Discussion
Breast tumors in children and adolescents are rare. Moreover, malignant diseases almost do not occur at this age. Phyllodes tumors are generally classified as rare breast diseases, mainly in older women. Moreover, malignant phyllodes tumors developed in children are presented only in cases. The case we describe will add to those and help with aspects of rare disease management.
Phyllodes tumor is morphologically a biphasic fibro-epithelial tumor, in which there is proliferation of the mesenchymal component and coexistence of the epithelial component. It should be noted that the epithelial part in a phyllodes tumor is benign, and according to the degree of maturity of the stromal component, phyllodes tumors are divided into three groups: benign phyllodes tumor, phyllodes tumor of borderline malignancy and malignant phyllodes tumor.
Assessment of the degree of differentiation of a phyllodes tumor is based on the morphological evaluation of the stromal component. These are stromal cellularity, stromal cell atypia, stromal component, overgrowth, and mitotic activity. Great importance is attached to the tumor margins.
Benign growths are usually well circumscribed, while tumors of borderline with malignant potential have a "pulling growth pattern." It should be noted that such types of tumors, including benign forms, can recur. In phyllodes tumors of borderline malignancy, local recurrence may occur in 46% of cases [11].
The risk of local recurrence in malignant phyllodes tumors is significantly higher. Local recurrence is possible in 23-30 %, and metastases in visceral organs are observed in 9 % [12].
The risk of metastasis increases 1 year after surgery. However, such patients require follow-up for 10 years [13].
In the case of malignant phyllodes tumor, morphologically, it is possible to encounter malignant heterogeneous elements such as liposarcoma, osteosarcoma, rhabdomyosarcoma, and chondrosarcoma [14]. The literature review shows only three cases of phyllodes malignant tumors with heterogeneous rhabdomyosarcoma [15].
As heterogeneous malignant elements are rare, information in the literature to evaluate the tumor behavior in the case of these elements is scarce. There is insufficient information on whether tumor aggressiveness increases when we detect heterogeneous elements in a malignant phyllodes tumor.
The issue of treatment in the presence of such elements is also relevant. Should we use chemotherapy at such times? Especially if the matter concerns children and adolescents.
Future studies are needed to examine issues such as disease incidence in childhood and its clinical management.
Conclusion
Rapidly growing breast tumors in childhood require caution and proper clinical management. Surgical treatment remains the primary method of treatment for phyllodes tumors. The issue of radiation therapy in treating malignant phyllodes tumors remains controversial.
The relevance of chemotherapy treatment in heterogeneous malignant phyllodes tumors is an issue for future studies. Management of recurrent disease is also critical.
References
- Khosravi-Shahi P (2011) Management of non-metastatic phyllodes tumors of the breast: review of the literature. Surg Oncol. 20(4): e143-8.
- Choi N, Kim K, Shin KH, Kim Y, Moon HG, et al. (2018) Malignant and borderline phyllodes tumors of the breast: a multicenter study of 362 patients (KROG 16-08). Breast Cancer Res Treat. 171(2): 335-344.
- Vempuluru VS, Mishra DK, Kaliki S (2021) Malignant phyllodes tumor of the breast with metastasis to the orbit: A Rare Case Report. Ophthalmic Plast Reconstr Surg. 37(1): e5-e7.
- Demian GA, Fayaz S, El-Sayed Eissa H, Nazmy N, Samir S, et al. (2016) Phyllodes tumors of the breast: Analysis of 35 cases from a single institution. J Egypt Natl Canc Inst. 28(4): 243-248.
- Toussaint A, Piaget-Rossel R, Stormacq C, Mathevet P, Lepigeon K, et al. (2021) Width of margins in phyllodes tumors of the breast: the controversy drags on? - a systematic review and meta- analysis. Breast Cancer Res Treat. 185(1): 21-37.
- Tremblay-LeMay R, Hogue JC, Provencher L, Poirier B, Poirier É, et al. (2017) How Wide Should Margins Be for Phyllodes Tumors of the Breast? Breast. 23(3): 315-322.
- Rayzah M (2020) Phyllodes Tumors of the Breast: A Literature Review. Cureus. 12(9): e10288.
- Zhang H, Tang S, Biskup E, Zhang Y, Yong L, et al. (2022) Long-term Survival After Diverse Therapeutic Modalities inMalignant Phyllodes Tumors of the Breast Technol Cancer Res Treat. 15330338221121086.
- Ogunbiyi S, Perry A, Jakate K, Simpson J, George R (2019) Phyllodes tumour of the breast and margins: How much is enough. Can J Surg. 62(1): E19-E21.
- Bogach J, Shakeel S, Wright FC, Hong NJL (2022) Phyllodes Tumors: A Scoping Review of the Literature. Ann Surg Oncol. 29(1): 446-459.
- Kim S, Kim JY, Kim DH, Jung WH, Koo JS (2013) Analysis of phyllodes tumor recurrence according to the histologic grade. Breast Cancer Res Treat. 141(3): 353-63.
- Tan BY, Acs G, Apple SK, Badve S, Bleiweiss IJ, et al. (2016) Phyllodes tumours of the breast: a consensus review. Histopathology. 68(1): 5-21.
- Grabowski J, Salzstein SL, Sadler GR, Blair SL (2007) Malignant phyllodes tumors: a review of 752 cases. Am Surg. 73(10): 967- 9.
- Koh VCY, Thike AA, Nasir NDM, Yip GWC, Bay BH, et al. (2018) Size and heterologous elements predict metastases in malignant phyllodes tumours of the breast. Virchows Arch. 472(4): 615-621.
- Barnes L, Pietruszka M (1978) Rhabdomyosarcoma arising within cystosarcoma, phyllodes; case report and literature review. Am J Surg Pathol. 2(4): 423-9.



