Anurag Srivastava1, Anmol Randhawa1*, Tshering Sherpa1, Jitendra Verma2, Shiteez Agrawal2, B S Sharma3
1MS, Senior resident, Department of Neurosurgery, Mahatma Gandhi Hospital, Jaipur, Rajasthan, India.
2MCh, Associate Professor, Department of Neurosurgery, Mahatma Gandhi Hospital, Jaipur, Rajasthan, India.
3MCh, Emiretus professor, Department of Neurosurgery, Mahatma Gandhi Hospital, Jaipur, Rajasthan, India.
*Corresponding Author: Anmol Randhawa, MS, Senior resident, Department of Neurosurgery, Mahatma Gandhi Hospital, Jaipur, Rajasthan, India.
Keywords: Intramedullary Spinal Cord Tumors (IMSCTs), Gross Total Resection (GTR), Subtotal Resection (STR), Ependymoma, Astrocytoma.
Introduction
Intramedullary Spinal Cord Tumors (IMSCTs) are a rare variety of tumors having an incidence of around 1.1 cases per 100 000 people and comprise only 2% to 4% of the overall neoplastic tumours in the central nervous system [1]. The commonly diagnosed tumors are astrocytomas, ependymomas, and other miscellaneous tumors like melanocytomas, epidermoid cysts, and hemangioblastomas [2]. Previous studies have depicted that intraoperative tumor resection should be based on the identification of a plane of dissection, which is most often dependent on the histology of the tumor [2-5]. Typically, ependymomas demonstrate a clear interface between the spinal cord and the tumour, in contrast to astrocytomas which exhibit an extensive infiltrative nature [4,5]. If there is the absence of a clearcut plane of dissection (POD), it leads to subtotal resections (STR) of the astrocytoma [6]. The surgical skill required to have a gross total resection (GTR) has been illustrated by recent reports and has questioned the definitive role of histology in the extent of tumour resection [7]. Hence, a retrospective analysis was performed to determine the impact of 3 parameters- the preoperative neurological condition of the patients, histological differentiation of the IMSCTs, and the extent of surgical resection in a series of operated cases of IMSCTs. According to our knowledge, this is the first of its kind study conducted in Western India with such a large series of patients.
Methods
Data collection
Between January 2017 through December 2022 (6 years), 68 patients were treated for spinal intramedullary tumors at the Department of Neurosurgery at Mahatma Gandhi University of Medical Sciences and Technology, a tertiary care centre in Western India. The data was collected retrospectively from the hospital records and further analysis was done on the basis of clinical features, patient demographics, radiological findings, extent of surgical resection, tumour histopathology as well as the functional outcome postoperatively was assessed in the follow-up visits. The extent of tumour resection was reported as gross total resection (GTR i.e. complete tumor removal) and subtotal resection (STR) or partial decompression (decompression only due to inoperable nature of the tumor) written informed consent was obtained from all the patients and this study has been approved by the Institutional Ethics Commitee.
Assessment of the pre and postoperative functional status was done according to the McCormick grade[8]:
Grade I – Neurologically intact, ambulates normally, may have minimal dysesthesia.
Grade II – Mild motor or sensory deficit; patient can maintain functional independence.
Grade III – Moderate deficit, limitation of function, independent without external aid
Grade IV – Severe motor or sensory deficit, limited function, dependent patient
Grade V – Paraplegia or quadriplegia, even if there is a flicker of movement
Study Design - Retrospective Observational Study
Surgical Procedure
All the patients were operated using the standard microsurgical techniques. Access to the spinal canal was made through a laminotomy or laminectomy. Microscopically opening the duramater is crucial, keeping the arachnoid mater as intact as possible. Then, the arachnoid layer is opened by cutting with micro scissors and freed from the lateral or posterior spinal cord gently, as it is necessary to keep it intact for replacement at the end of the operation. A subpial discoloration due to the tumor might be seen under magnification.
Ependymomas seen were mostly located centrally and were accessed through the posterior midline, where a careful inspection of the dorsal nerve root entry zone revealed a central spinal fissure separating the two posterior columns. Even though astrocytomas were seen to be eccentric in location, in most of the patients the approach was the same as the one utilised for ependymomas, especially due to the reason that the type of the tumor is not known before biopsy. The extent of resection was identified on the basis of the dissection plane between the normal spinal architecture and the tumor. An attempt to perform a GTR was advocated in cases where such a plane was identified. In the infiltrative tumors, an attempt was made for the maximum possible resection, at the surgeon’s discretion, by paying attention to the changes in motor and sensory evoked potentials. Resection using the microsurgical method along with electrophysiological monitoring (motor and sensory evoked potentials) was performed in all the patients.
Using the international 10-20 electrode placement system, cork screws were inserted at C3 and C4 position. For stimulation, we used a six consecutive pulses of 0.5 ms duration with constant current of 70-200 mA with inter-stimulus time interval of 2-5 msec was applied. These parameters of stimulus used to obtain the baseline were kept the same throughout. For upper limb recording, the bipolar needle electrodes were inserted at the bilateral abductor pollicis brevis muscle, which receives innervation by median nerve C8, T1, whereas for the lower limbs, abductor hallucis muscle, which is innervated by medial plantar nerve L4 and L5. The distance between the two needle electrodes were kept at 2 cm. Transcranial Motor Evoked Potentials (TcMEP) were recorded at baseline in supine position (Ts), after prone positioning (Tp), before making any manipulation (Tm), subsequently on demand of the surgeon (Tm1, Tm2) and finally at the end of surgery (Te).
Results
Patient Demographics
Sixty-eight patients suffering from IMSCTs (intramedullary spinal cord tumors) were operated at our centre in the 6-yr study period out of which 41 (60%) were males and 27 (40%) were females. An average (mean) age of 36 years (range: 12–68 years) was observed, among which 7 (10.2%) were less than 18 years old.
Clinical Features
Most patients (77.9%) presented with pain, followed by paresthesia (75%), and weakness was found to be the most common motor symptom (55.8%). Urinary retention was the most frequent autonomic symptom (35%) (Table 1). There was no significant difference in the symptoms amongst patients with the different tumor variants.
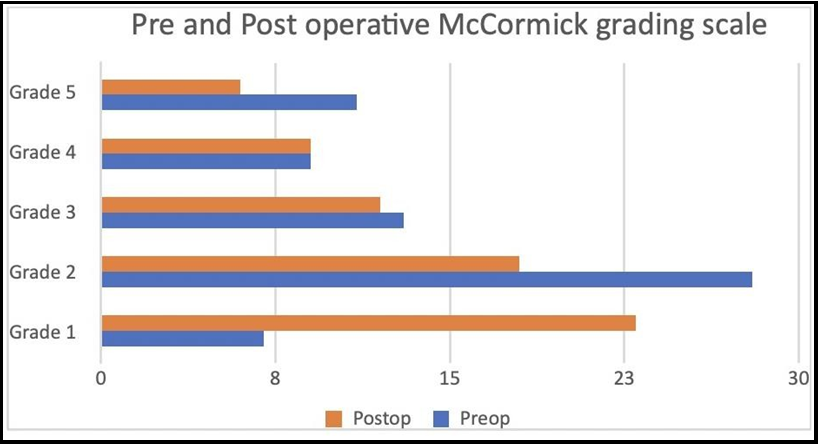
Assessment of the pre and postoperative state was done using McCormick grading scale, and most of the patients had grade 2 (41.2%) or grade 3 (19.1%) deficits (Figure 1). It was observed that that in patients with poorer preoprative neurological status, the postoperative result were also not favourable. Maximum improvement was seen in Grade 1 (33.8%) preop status, followed by Grade 2 (26.5%) patients. Beyond this, a declining trend was found in the higher McCormick grades (Table 2).
Table 1: Preoperative clinical features.
|
Presentation |
Number |
Percentage (%) |
|
A) SENSORY |
|
|
|
Pain |
53 |
77.9 |
|
Paresthesia |
51 |
75 |
|
Numbness |
21 |
30.8 |
|
B) MOTOR |
|
|
|
Weakness |
39 |
55.8 |
|
Spasticity |
5 |
7.3 |
|
C) AUTONOMIC |
|
|
|
Bowel disturbance |
23 |
33.8 |
|
Urinary Symptoms |
38 |
45 |
|
1. Frequency |
3 |
4.4 |
|
2. Incontinence |
10 |
14.7 |
|
3. Hesitency |
1 |
1.4 |
|
4. Retention |
24 |
35.2 |
Figure 1: Preoperative and postoperative status using the McCormick grading scale
Table 2: Preoperative and postoperative status using the McCormick grading scale
|
McCormick Grade |
Preop |
Percentage (%) |
Postop |
Percentage (%) |
|
Grade 1 |
7 |
10.3 |
23 |
33.8 |
|
Grade 2 |
28 |
41.2 |
18 |
26.5 |
|
Grade 3 |
13 |
19.1 |
12 |
17.6 |
|
Grade 4 |
9 |
13.2 |
9 |
13.2 |
|
Grade 5 |
11 |
16.2 |
6 |
8.8 |
Tumour Radiological Findings and Histopathology
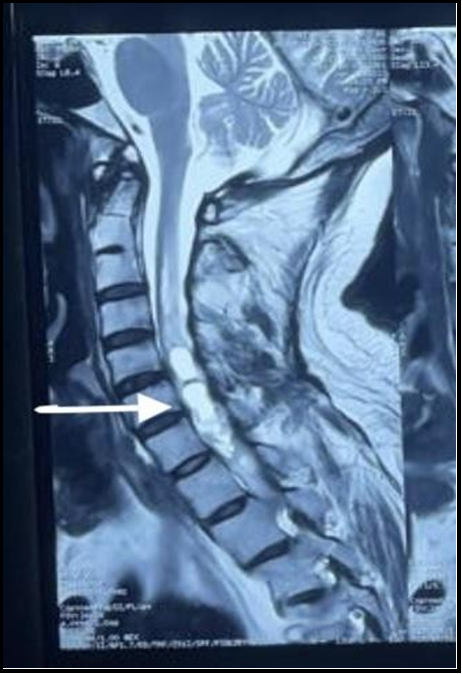
All patients were subjected to contrast-enhanced MRI. In our study, we observed that ependymomas were hypointense or isointense on T1-weighted MRI and hyperintense on T2-weighted imaging MRI with variable contrast enhancement (Figure 2).
Contrast enhancement was visualised in most. On the contrary, astrocytomas were mostly found to be hyperintense on T2-weighted MRI and hypointense on T1-weighted MRI. Syringohydromyelia is found to be the only characteristic which helps in distinguishing between ependymoma and astrocytoma[8].
Figure 2: Sagittal section of spinal cord showing T-2 weighted Hyperintensity (white arrow), suggestive of ependymoma.
Tumour Location and Histopathological Correlation
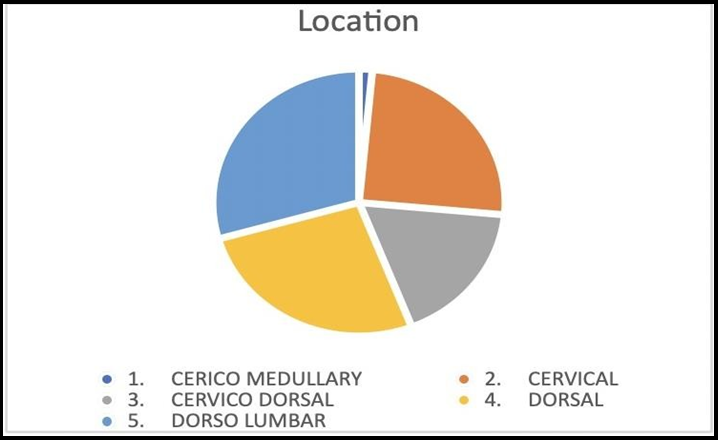
The tumor was located most commonly in the dorso-lumbar region (20 patients; 29.4%), followed by dorsal (18 patients; 26.4%), cervical (17 patients; 25%), cervico-thoracic (12 patients; 17.6%) and cervico-medullary region (1 patient; 1.4%) (Figure 3).
Overall, ependymomas (42.6%) constituted the most common tumor, followed by low-grade gliomas (19.1%). Dermoid cysts and glioblastomas were the most common type of intramedullary tumors in children (29%), however, in adults, ependymomas were observed to be more common (46%).
Gliomas were distributed evenly throughout the spinal cord, in contrast to ependymomas that were found to be located at the dorsal end of the spinal cord. Dermoid and epidermoid tumors were mostly located in the lower half of the spine, and hemangioblastomas were predominantly located in the dorso-lumbar region of the spinal cord (Table 3).
Figure 3: Location wise distribution of tumours.
Table 3: Histopathological distribution and correlation with age, extent of resection and location.
|
HISTOPATHOLOGY |
|
|
Low grade gliomas |
High grade glioma |
MISCELLANEOUS |
|||||
|
|
Ependymo ma |
Anaplastic ependymo ma Gr 3 |
Astrocyt oma |
Pilocytic astrocytoma Gr 1. |
Glioblasto ma Gr 4 |
Epiderm oid |
Dermoid Cyst |
Lipoma |
Hemangio blastoma |
Cavernous Hemangio ma |
|
TOTAL (Presentation) |
29 (42.6%) |
5 (7.3%) |
8(7.3%) |
5(11.7%) |
6 (8.8%) |
2 (2.9%) |
7 (10.2%) |
2 (2.9%) |
3 (4.4%) |
1 (1.4%) |
|
AGE |
|
|
|
|
|
|
|
|
|
|
|
1 |
0 |
1 |
0 |
2 |
0 |
2 |
1 |
0 |
0 |
|
28 |
5 |
7 |
5 |
4 |
2 |
5 |
1 |
3 |
1 |
|
|
|
|
|
|
|
|
|
|
|
|
16 |
1 |
2 |
0 |
0 |
1 |
6 |
0 |
3 |
1 |
|
13 |
4 |
6 |
5 |
6 |
11 |
1 |
2 |
0 |
0 |
|
|
|
|
|
|
|
|
|
|
|
|
- |
- |
|
|
1 |
|
|
|
|
|
|
3 |
3 |
5 |
3 |
2 |
|
|
|
1 |
|
|
3 |
1 |
2 |
|
3 |
|
2 |
|
|
1 |
|
14 |
- |
- |
2 |
|
|
|
2 |
|
|
|
9 |
1 |
1 |
|
|
2 |
5 |
|
2 |
|
Surgical Resection
GTR was achieved in 30 patients (44.1%) and sub-total excision in 38 (55.9%). Ependymomas were more amenable to gross total resection (GTR) (55.2%) in comparison to astrocytomas (25%). In cases of anaplastic ependymomas, STR (80%) was used as they are more aggressive tumours and complete surgical resection poses a higher risk of postoperative complications. GTR was achieved in 100% of patients with haemangioblastoma, but lipomas were not radically resected in any patient. GTR was achieved in all 3 patients with hemangioblastoma.
Clinical assessment of the operated patients was performed in the immediate postoperative period, at discharge and the 6 month follow- up visit. There were three postoperative deaths, two related to respiratory complication and one due to deep vein thrombosis.
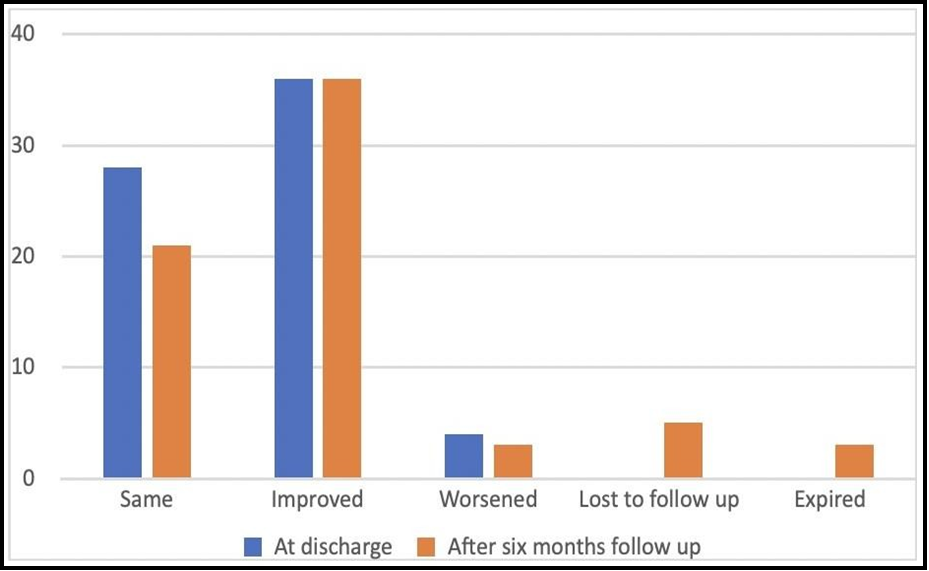
At discharge, 4 patients (6%) had deteriorated from their preoperative grades, whereas 36 patients (52.9%) showed improvement (Figure 4). The follow-up hospital records were available for 60 patients. The outcome of the patients’ at the final follow-up was correlated to the preoperative neurological status, histopathology of tumour as well as the extent of surgical resection. The complete functional outcome of a patient can be thoroughly assessed only by a long follow-up duration.
At 6 months, 55.17% of the patients having ependymomas showed a better functional outcome. This can be attributed to the fact that most of them had undergone Gross Total Resection (Table 4). Out of 5 patients of anaplastic ependymoma, 3 had undergone STR and had the same postop fucntional status as preop, the other 2 operated by GTR had a better functional outcome. 80% of the patients of pilocytic astrocytoma underwent GTR and their postop status was much better, owing to the fact that it is low grade tumour. In the other low grade astrocytoma, 57% patients showed an improvement even with a subtotal resection and 28% remained the same. 100% of cases of epidermoid cyst operated by subtotal resection showed better postoperative functional outcome.
On the other hand, dermoid cyst had the same outcome in 71% of the patients and they all had undergone GTR. All the cases of hemangioblastoma showed a better outcome as they were operated using GTR. 75% of patients of glioblastoma were operated by STR and they had a better functional outcome, however the one case where extensive resection was done, suffered from a worse outcome postoperatively.
Overall, at the 6 month clinical examination, the McCormick grades of 36 patients (53%) were improved, in 21 patients (31%)- it had not changed, and in 3 patients (4.4%)- it had worsened. Tumor recurrence was detected in 7 cases (10.3%) at the last recorded follow-up.
In 11 patients (16.2%) of anaplastic ependymoma and high grade glioma, adjuvant radiotherapy was given. For patients with a low- grade glioma along with residual tumor, our protocol was to follow- up the patient clinically with serial MRI scanning or a second surgery.
Figure 4: Postoperative change in McCormick grading at regular follow-up.
Table 4: Functional outcome co-relation with various variables.
|
FUNCTIONAL OUTCOME (after 6 months) |
Better |
Same |
Worsened |
|
A. AGE |
|
|
|
|
< or = 18 |
2 |
4 |
0 |
|
>18 |
34 |
17 |
3 |
|
B. Extent of resection |
|
|
|
|
GTR |
15 |
8 |
2 |
|
STR |
21 |
13 |
1 |
|
C. Histopathology |
|
|
|
|
1. Ependymoma gr 2 |
16 |
9 |
1 |
|
2. Anaplastic ependymoma |
2 |
3 |
0 |
|
3. LGG |
|
|
|
|
1. Astrocytoma |
4 |
2 |
1 |
|
2. Pilocytic astrocytoma Gr 1. |
4 |
1 |
0 |
|
4. HGG |
|
|
|
|
3 |
0 |
1 |
|
5. Miscellaneous |
|
|
|
|
a. Epidermoid |
2 |
0 |
0 |
|
b. Dermoid cyst |
2 |
5 |
0 |
|
c. Lipoma |
1 |
1 |
0 |
|
d. Hemangioblastoma |
2 |
0 |
0 |
|
e. Cavernous hemangioma |
0 |
0 |
0 |
|
D. Location |
|
|
|
|
1 |
0 |
0 |
|
10 |
3 |
0 |
|
6 |
4 |
0 |
|
12 |
4 |
0 |
|
7 |
10 |
3 |
Discussion
Operative techniques for intramedullary spinal cord tumors have undergone many changes since the successful removal of an IMSCT by Cushing [9] (an ependymoma), advocating the use of operating microscopes and bipolar cautery. Various surgical approaches have been described for the management of these tumours in order to maintain a good quality of life postoperatively [10].
The male to female ratio in our series was 1.5 to 1, which is insignificant and found usually in primary intramedullary spinal cord tumors.
Intramedullary spinal cord tumors present with motor, sensory, or sphincter disturbances. In our study, pain was the predominant symptom present in 77% of the patients, followed by paraesthesia (75%), weakness (55.8%) and urinary symptoms (45%). This is concordance with a study conducted by Kane et al. [11], who noted sensory and motor disturbances on physical examination in 72% of patients, and incidence of sphincteric disturbances in their series was found to be 44%. Another study showing a high percentage of neurological deficits has been described by Hachicha et al. [12] in which motor weakness was seen in 77.5%, spinal pain in 29%, sensory loss in 29% and sphincter involvement in 46.7%.
Clinical grading in preoprative period of intramedullary tumours can be performed using various scoring systems like- (i) McCormick’s grading scale [1] (ii) Klekamp and Samii’s clinical scoring system[13]; and (iii) Cooper and Epstein’s scale [3,14]. Although every scale has its advantages and disadvantages, we have used the McCormick grade because of its simplicity, easy applicability, and its common use in literature. Most of our patients were found in McCormick grade 2 (41.2%) and McCormick grade 3 (19.1%) at presentation.
Brotchi et al. [15] found that 36 of 65 patients (55.3%) had tumors in the cervical region, as compared to Kane et al. [11] who observed that 33% of patients had tumors in the same region. In our study, tumors were mostly located in the thoraco-lumbar area, in 20 cases (29.4%), followed by 18 cases with tumors in the thoracic region (26.4%), 17 in cervical region (25%), 12 cases with a tumor in the cervico-dorsal region (17.6%) and lastly 1 patient with a tumour in the cervio- medullary region (1.4%).
Although our aim was to perform a GTR, it was achievable in 30 patients (44%) only and STR was done in the rest 38 patients (56%). Most of the authors [16-20] have suggested radical resection as the ultimate surgical goal. Conservative surgical management is not usually recommended because a radical resection is not known to increase the morbidity, rather it may provide a cure. Recent advances in the surgical armamentarium have helped to achieve this goal. As early as the 1980s, Cooper et al. [3,15] had a 60% radical excision rate in their studies. Epstein’s group [4,15] has had a higher resection rate, and in their latest series, it was 73%.
Overall, ependymomas (42.6%) constituted the most common tumor, followed by astrocytomas (11.7%) in our study. In children, dermoid and glioblastoma Gr 4 were the mostly seen in our study (28.5%), whereas in adults, ependymomas were found to be more common (45.9 %). Rajkumar et al. [20] noted that astrocytomas and ependymomas contributed to 50% of intramedullary tumors and that the non-neoplastic malformations such as epidermoid and dermoid cyst had a higher incidence (23%) in the developing countries in comparison to the Western world, where all the congenital intramedullary tumors account for 5% to 8% only.
Our study showed that 55.17% of the patients having ependymomas showed a better functional outcome. This can be attributed to the fact that most of them had undergone Gross Total Resection, which is in accordance with Brotchi et al. (16), who noted an improvement in 53% cases, no change in 37% and a worsening of symptoms in 10% of patients. On the other hand, Constantini et al. [21] have reported an improvement in 16% of patients, no change in 60%, and a deterioration in 24% among the 164 subjects studied. Comparing the preoperative status and 6 months follow-up in a research performed by Cannizzaro et al. [22], the modified McCormick scale showed neurological stability in 30 patients (52.63%), worsening in 7 patients (12.28%) and an improvement in 20 patients (35.08%)
Altogether, in our series, at the final clinical examination, 53% of patients were improved, 31% of the patients remained at the same grade and 4.4% of patients had worsened, thus showing better results than most international series. Another Indian study reported that 79.27% of the patients having spinal tumours had improvement in their functional status, 15.31%, remained same, and 5.4% were worse at time of their last follow-up., which correlates with our study as well [23].
It was observed in our study that a good preoperative neurological status is extremely important for a favorable outcome. Along with that, we have to keep in mind the type of tumour histology while operating these cases. Complete surgical removal of the lesion is, of course, the primary goal. A low grade tumour with GTR will respond much better than a higher grade tumour operated by GTR. Similarly, if an STR is performed in a higher grade tumour, it will give a much more favourable outcome, rather than striving for extensive tumour clearance, which might lead to worse outcomes. For this, intraoperative frozen section sampling can be used, but the accuracy of these is not very well documented [24,25].
Sandalcioglu et al. [26] found that preoperative neurological condition of the patients was a crucial factor in affecting the long- term outcome. Drastic variations amongst patients who lived independently prior to surgery and the ones who required assistance were identified. Studies have shown that a more compromised spinal cord is extremely vulnerable to the additional trauma of the surgical procedure. Most of the authors [17-24] advocate early surgery for intramedullary tumors as soon as the diagnosis is made. Hence, it is not advisable to undertake a very extensive resection at the cost of a compromised spinal cord, which might lead to a poor quality of life in the post operative period.
Age of the patients did not have a significant effect on the outcome although the chance of improvement is more with astrocytomas in children [28]. There are variations in incidence of tumor type in adults as well as children, hence it is not exactly comparable, as astrocytomas are more common in the paediatric age group. However, if only the patients with astrocytas were analyzed, age is known to be a significant factor [27]. Poor preoperative clinical grading with any tumor histopathology translates into a poorer functional outcome. Furthermore, tumor histopathology is seen to be more relevant when we talk in in terms of surgical resectablity, as depicted in our study. Ependymomas are more suitable for complete resection as compared to astrocytomas. Tumors that are well-defined tumors like ependymomas and hemangioblastoma were observed to be more amenable to total resection than the infiltrative tumors like astrocytomas and lipomas in our study, and this has also been the experience of others [19-24,26]. Presently, maximum authors have suggested that a GTR or a radical excision must be the aim for all management of all intramedullary lesions, especially in the management of ependymomas [19-24,26]. Epstein’s group [14] consistently achieved a GTR in low grade astrocytomas. Garido and Stein [28] have achieved GTR in 3 of 5 low grade astrocytomas; in their other 2 patients, total removal was done. This series indicates that a radical surgery for a low grade astrocytoma is feasible without subjecting the patient to significant post surgical morbidity. An extended symptom-free survival is possible postoperatively, even when the resection done was subtotal. Long-term benefits can be manifested with Gross Total Resection of ependymomas.
Transcranial Motor Evoked Potentials (TcMEP) involves motor cortex stimulation through the cranium to elicit compound muscle action potentials (CMAP) generated from peripheral muscles so as to test whether the motor pyramidal pathway is intact or not. Various studies have shown that the use of TcMEP in spinal surgeries is of huge benefit to the patients being operated on for spinal cord tumors [29-30]. Hence, TcMEP is now considered as a gold standard with sensitivity of 91% and specificity of 96% [31-32]. Recent multi- modality monitoring mechanisms allow for better intra-operative assessment of functional integrity of ventral efferent motor tracts, dorsal afferent sensory tracts in spinal cord as well as the nerve roots [33].
Conclusion
It was observed in our study that a good preoperative neurological status is extremely important for favourable outcome. Along with that, we have to keep in mind the type of tumour histology while operating these cases. Complete surgical removal of the lesion is, of course, the primary goal. A low grade tumour with GTR will respond much better than a higher grade tumour operated by GTR. Similarly, if an STR is performed in a higher grade tumour, it will give a much more favourable outcome, rather than striving for extensive tumour clearance, which might lead to worse outcomes. But being a spinal tumour, the accuracy of intraoperative frozen sections is also not proven [25]. Hence the decision to perform a GTR or an STR lies with the operating surgeon and the on-table appearance of the tumour.
According to our knowledge, this retrospective study is the first of its kind conducted in Western India, which is comparing three parameters to assess the postoperative functional outcome in IMSCTs. From our experience in managing this huge series of patients with IMSCTs (intramedullary spinal cord tumors), we have concluded that a strong predictor of the postoperative functional outcome in these operated cases was the preoperative neurological status of the patient, along with the decision to undertake a GTR or an STR depending upon the aggressiveness of the tumour, which is ultimately the surgeon’s discretion. Along with that, it is extremely important to take into consideration the use of intra-operative TcMEP monitoring in order to prevent postoperative neurological defect. Recent multi-modality monitoring mechanisms allow for better intra- operative assessment of functional integrity of ventral efferent motor tracts, dorsal afferent sensory tracts in the spinal cord as well as the nerve roots.
Acknowledgement:
We would like to thank the Department of Neurosurgery for providing us with the necessary data for this study to be conducted and our Head of Department for guiding and motivating us to conduct this study.
Conflicts of Interest: The authors declare that there is no conflict of interest regarding the publication of this paper.
Funding Statement: No funding sources.
References
- McCormick P, Stein B (1990) Intramedullary Tumors in Adults. Neurosurgery Clinics of North America. 1(3): 609-630.
- Alvisi C, Cerisoli M, Giulioni M (1984) Intramedullary spinal gliomas: long-term results of surgical treatments. Acta Neurochir (Wien). 70(3-4): 169-79.
- Cooper PR (1989) Outcome after operative treatment of intramedullary spinal cord tumors in adults: intermediate and long-term results in 51 patients. Neurosurgery. 25(6): 855-9.
- Cooper PR, Epstein F (1985) Radical resection of intramedullary spinal cord tumors in adults. Recent experience in 29 patients. J Neurosurg. 63(4): 492-9.
- Fischer G, Mansuy L (1980) Total removal of intramedullary ependymomas: follow-up study of 16 cases. Surg Neurol. 14(4): 243-9.
- Bansal S, Ailawadhi P, Suri A, Kale SS, Sarat Chandra P, et al. (2013) Ten years' experience in the management of spinal intramedullary tumors in a single institution. J Clin Neurosci. 20(2): 292-8.
- Xu QW, Bao WM, Mao RL, Yang GY (1996) Aggressive surgery for intramedullary tumor of cervical spinal cord. Surg Neurol. 46(4): 322-8.
- Kim DH, Kim JH, Choi SH, Sohn CH, Yun TJ, et al. (2014) Differentiation between intramedullary spinal ependymoma and astrocytoma: comparative MRI analysis. Clin Radiol. 69(1): 29- 35.
- Dasenbrock HH, Pendleton C, Cohen-Gadol AA, Wolinsky JP, Gokaslan ZL, et al. (2011) "No performance in surgery more interesting and satisfactory": Harvey Cushing and his experience with spinal cord tumors at the Johns Hopkins Hospital. J Neurosurg Spine. 14(3): 412-20.
- Takami T, Naito K, Yamagata T, Ohata K (2015) Surgical management of spinal intramedullary tumors: radical and safe strategy for benign tumors. Neurol Med Chir (Tokyo). 55(4): 317- 327.
- Kane PJ, el-Mahdy W, Singh A, Powell MP, Crockard HA (1999) Spinal intradural tumours: Part II - Intramedullary. Br J Neurosurg. 13(6): 558–63.
- Hachicha A, Belhaj A, Karmeni N, Slimane A, Bouali S, et al. (2021) Intramedullary spinal cord tumors: A retrospective multicentric study. J Craniovertebr Junction Spine. 12(3): 269- 278.
- Klekamp J, Samii M (1993) Introduction of a score system for the clinical evaluation of patients with spinal processes. Acta Neurochir (Wien). 123(3-4): 221-3.
- Epstein FJ, Farmer J-P, Freed D (1993) Adult intramedullary spinal cord ependymomas: The result of surgery in 38 patients. J Neurosurg. 79: 204–209.
- Brotchi J, Noterman J, Balériaux D (1992) Surgery of intramedullary spinal cord tumors. Acta Neurochir (Wien). 116(2-4): 176–178.
- Alkhani A, Blooshi M, Hassounah M (2008) Outcome of surgery for intramedullary spinal ependymoma. Ann Saudi Med. 28(2): 109-13.
- M Das J, Hoang S, Mesfin FB (2023) Intramedullary Spinal Cord Tumors. 2023 Apr 24. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
- Mechtler LL, Nandigam K (2013) Spinal cord tumors: new views and future directions. Neurol Clin. 31(1): 241-68.
- Endo T, Inoue T, Mizuno M, Kurokawa R, Ito K, et al. (2022) Current Trends in the Surgical Management of Intramedullary Tumors: A Multicenter Study of 1,033 Patients by the Neurospinal Society of Japan. Neurospine. 19(2): 441-452.
- Kumar R, Banerjee S (2014) Management and functional outcome of intramedullary spinal cord tumors: A prospective clinical study. Asian J Neurosurg. 9(4): 177-81.
- Constantini S, Miller DC, Allen JC, Rorke LB, Freed D, et al. (2000) Radical excision of intramedullary spinal cord tumors: Surgical morbidity and long-term follow-up evaluation in 164 children and young adults. J Neurosurg. 93(2 Suppl): 183–93.
- Cannizzaro D, Mancarella C, Nasi D, Tropeano MP, Anania CD, et al. (2022) Intramedullary spinal cord tumors: the value of intraoperative neurophysiological monitoring in a series of 57 cases from two Italian centers. J Neurosurg Sci. 66(5): 447-455.
- Arora RK, Kumar R (2015) Spinal tumors: Trends from Northern India. Asian J Neurosurg. 10(4): 291-7.
- Hongo H, Takai K, Komori T, Taniguchi M (2018) Intramedullary spinal cord ependymoma and astrocytoma: intraoperative frozen-section diagnosis, extent of resection, and outcomes. J Neurosurg Spine. 30(1): 133-139.
- Kobayashi K, Ando K, Ito K, Tsushima M, Morozumi M, et al. (2018) Accuracy of intraoperative pathological diagnosis using frozen sections of spinal cord lesions. Clin Neurol Neurosurg. 167: 117-121.
- Sandalcioglu IE, Gasser T, Asgari S, Lazorisak A, Engelhorn T, et al. (2005) Functional outcome after surgical treatment of intramedullary spinal cord tumors: experience with 78 patients. Spinal Cord. 43(1): 34–41.
- Gerges N, Fontebasso AM, Albrecht S, Faury D, Jabado N (2013) Pediatric high-grade astrocytomas: a distinct neuro-oncological paradigm. Genome Med. 5(7): 66.
- Garrido E, Stein BM (1977) Microsurgical removal of intramedullary spinal cord tumors. Surg Neurol. 7(4): 215-9.
- Ohashi M, Watanabe K, Furutani K, Hirano T, Katsumi K, et al. (2017) False-negative transcranial motor evoked potentials (TcMEPs) during surgery for congenital lumbar kyphoscoliosis: a case report. Spinal Cord Ser Cases. 3: 17053.
- Lall RR, Lall RR, Hauptman JS, Munoz C, Cybulski GR, et al. (2012) Intraoperative neurophysiological monitoring in spine surgery: indications, efficacy, and role of the preoperative checklist. Neurosurg Focus. 33(5): E10.
- Bose B, Sestokas AK, Schwartz DM (2007) Neurophysiological detection of iatrogenic C-5 nerve deficit during anterior cervical spinal surgery. J Neurosurg Spine. 6(5): 381–385.
- Lieberman JA, Lyon R, Feiner J, Hu SS, Berven SH (2008) The efficacy of motor evoked potentials in fixed sagittal imbalance deformity correction surgery. Spine. 33(13): E414–E424.