Catherine Norise, MD1, Roberto Bomprezzi, MD, PhD2*, Kara M. Smith, MD, MSCI2, Khurshid A Khurshid, MD3
1University of Pennsylvania, Dept. of Neurology, Philadelphia, PA
2University of Massachusetts Chan Medical School, Dept. of Neurology, Worcester, MA
3University of Massachusetts Chan Medical School, Dept. of Psychiatry, Worcester, MA
*Corresponding Author: Roberto Bomprezzi, MD, PhD, Department of Neurology, University of Massachusetts Chan Medical School, Worcester, MA 01655.
Abstract
Background: Multiple sclerosis (MS) can have a widely variable clinical presentation and when cerebellar pathways are affected, severe functional impairment may result from limb and truncal ataxia, as well as rubral tremor. Therapeutic options for cerebellar tremor are limited as medications are often ineffective. Therefore, non-invasive brain stimulation treatments like Transcranial Magnetic Stimulation (TMS) may be explored as an option.
TMS is FDA approved for treatment of depression and obsessive-compulsive disorder, and it has been studied in various neurological conditions, such as stroke rehabilitation, movement disorders and multiple sclerosis. In this case report, we describe tolerability and tremor response to TMS in a patient with refractory rubral tremor secondary to MS.
Methods: In this case study, conducted at the University of Massachusetts Chan Medical School, TMS treatment was completed on a 45-year- old female with relapsing remitting MS. The patient had severe, refractory rubral tremor impacting truncal and appendicular movements. The TMS protocol involved daily sessions of sequential bilateral low frequency cerebellar TMSs for five days.
Clinical rating scales including Essential Tremor Rating Assessment Scale (TETRAS) and the Scale for the Assessment and Rating of Ataxia (SARA) were completed at baseline and on Day 5.
Results: The patient demonstrated improved scores on both rating scales. There were no side effects noted.
Conclusions: TMS may be a feasible therapeutic option for patients with refractory rubral tremor secondary to MS and should be explored in controlled trials with the goal of optimizing the stimulation protocol and further assessing safety and tolerability.
Keywords: cerebellar tremor; limb ataxia; truncal ataxia, multiple sclerosis, transcortical magnetic stimulation
Introduction
Multiple Sclerosis (MS) is an inflammatory-degenerative disease of the central nervous system with a known variable disease course and heterogeneous clinical manifestations [1-4]. The demyelinating process slows and impairs neuronal signaling affecting motor, sensory and cerebellar pathways. Cerebellar ataxia often occurs concurrently with rubral tremor, and patients with this clinical presentation may experience severe, disabling tremor of the limbs, head and trunk [5-6]. Rubral tremor and ataxia are particularly refractory to pharmacological treatments. Available therapeutics such as tremor medications and botulinum toxin injections are often ineffective or limited by side effects. Hence, there is a desperate need for treatment options to specifically address this disabling complication of MS.
TMS (transcranial magnetic stimulation) and transcranial direct current stimulation (tDCS) are non-invasive, office-based procedures that do not involve any anaesthesia or sedation. TMS is an FDA approved treatment for major depressive disorder, and it has been shown to provide benefits for other neurological and psychiatric conditions [7]. It involves delivering a magnetic stimulation to specific areas of the brain by placing a coil on the head, and when an electric current is passed through the wire of the coil, a powerful magnetic field is generated. The magnetic pulse is transmitted to the brain region under the coil and the intensity of the stimulus to the tissue can be modulated by the intensity of the electric current [8].
Case report
Patient was a 45-year-old female who was diagnosed with relapsing remitting MS when she was 28-years-old and her course of illness was characterized by several clinical relapses that were not controlled by various immune therapies. She failed to respond to interferon followed by glatiramer acetate, and after she showed disease activity while receiving natalizumab, the patient was treated with pulse doses of intravenous cyclophosphamide for about two years. Despite treatments, the patient developed cognitive complaints, right hemiparesis, bladder dysfunction, leg weakness and impaired balance. Moreover, starting approximately eight years after the diagnosis, she manifested a wide base ambulation due to gait ataxia, a mild amplitude kinetic tremor of both hands and an intermittent head tremor brought up by attention-requiring tasks. Within a year from the onset, the head tremor, the limb, truncal and gait ataxia progressed to a severely disabling, coarse postural and kinetic tremor that did not respond to levetiracetam, topiramate, L- dopa nor primidone, and all these medications were discontinued.
Under the immunology point of view, the patient’s disease achieved full stability with the treatment with ocrelizumab and there has been no evidence of neither clinical nor radiological progression while she has remained on B-cell depleting therapy.
The residual disability conferred by the rubral tremor motivated the attempt to pursue possible benefits from TMS targeting the motor cerebellar pathways.
Methods
Tremor and Quality of Life assessments
Clinical assessments included the Essential Tremor Rating Assessment Scale (TETRAS) and the Scale for the Assessment and Rating of Ataxia (SARA). The TETRAS is a scale developed by the Tremor Research Group and it is composed of 10 areas of assessment, including head, face, tongue, voice, upper limb, lower limb, spirals, handwriting, dot approximation and standing. These areas are scored 0-4 based on the severity of the tremor, wherein a grade 4 tremor is defined as >20 cm for the upper limbs and > 5 cm for head and lower extremity tremor. The higher the score, the worse the subject’s performance, with a maximum possible score of 68.
The SARA consists of eight items assessing gait, stance, sitting, speech disturbance, finger chase (right and left), nose-finger test (right and left), fast alternating hand movements (right and left), and heel-shin slide (right and left). As with TETRAS, the higher the score, the worse is the subject’s performance with a maximum possible score of 56.
Quality of life was assessed using the Quality of Life in Essential Tremor Questionnaire (QUEST). The QUEST is a 30 item questionnaire, which allows subjects to self-report the degree to which their tremor affects their activities of daily living. All of these assessments were conducted prior to stimulation (baseline assessment) and after the final stimulation (Day 5).
TMS Stimulation
Informed consent for the treatment was obtained in writing from the patient and prior to the initiation of stimulation, the motor threshold was measured to determine the minimum magnetic stimulation intensity needed to activate the left motor cortex, specifically right thumb area. This is the same method used to determine motor threshold in patients undergoing TMS treatment for depression [9]. After motor threshold determination, the coil was placed on each cerebellar hemisphere by anatomic localization of 1cm inferior and 3 cm lateral to inion. The sites were marked on a cap that was worn by the subject during the procedure. Each cerebellar hemisphere was stimulated at 90% of the motor threshold. The subject received daily treatments for 5 days. Each session consisted of a total of 1200 pulses, 600 on each side. 1Hz frequency for 30 seconds with an interval of 10 seconds between stimulations (rest period) was utilized. The total duration of TMS treatment lasted about 30 minutes.
Results
TMS and Tremor Metrics
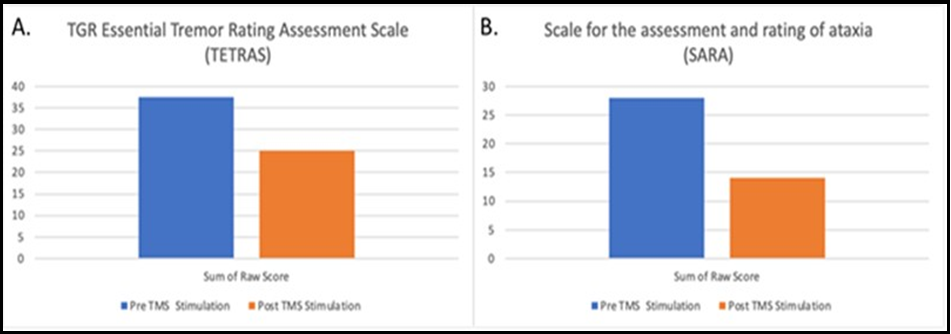
The participant’s performance on tremor metric (SARA and TETRAS) before and after TMS are presented in Table 1. In these different tremor assessment scales, improvement is demonstrated in each region of assessment. Table 1 presents 20 locations showing improvement, 6 locations showing no change, and 2 locations showing worsening following stimulation.
Table 1: Pre and post stimulation scores on SARA and TETRAS tremor assessments
|
Region |
Location |
Scale |
Pre TMS |
Post TMS |
Status |
|
Head and Speech |
Head |
TETRAS |
4 |
2 |
Improvement |
|
Face |
TETRAS |
0 |
0 |
No change |
|
|
Voice |
TETRAS |
3 |
1 |
Improvement |
|
|
Speech |
SARA |
2 |
0 |
Improvement |
|
|
Upper Extermity |
Upper Limb Postural (Right) |
TETRAS |
3.5 |
2.5 |
Improvement |
|
Upper Limb Lateral (Right) |
TETRAS |
2 |
1.5 |
Improvement |
|
|
Upper Limb Kinetic (Right) |
TETRAS |
4 |
4 |
No change |
|
|
Upper Limb Postural (Left) |
TETRAS |
1 |
0 |
Improvement |
|
|
Upper Limb Lateral (Left) |
TETRAS |
1 |
0 |
Improvement |
|
|
Upper Limb Kinetic (Left) |
TETRAS |
1 |
1.5 |
Worsening |
|
|
Trunk |
Lower Limb (Right) |
TETRAS |
2 |
1 |
Improvement |
|
Lower Limb (Left) |
TETRAS |
2 |
1 |
Improvement |
|
|
Standing |
TETRAS |
2 |
0 |
Improvement |
|
|
Gait |
SARA |
3 |
0 |
Improvement |
|
|
Stance |
SARA |
3 |
1 |
Improvement |
|
|
Sitting |
SARA |
0 |
0 |
No change |
|
|
Coordiantion |
Finger Chase (Right) |
SARA |
3 |
3 |
No change |
|
Finger Chase (Left) |
SARA |
2 |
1 |
Improvement |
|
|
Nose Finger Test (Right) |
SARA |
3 |
3 |
No change |
|
|
Nose Finger Test (Left) |
SARA |
1 |
1 |
No change |
|
|
Fast Alternating Hand Movements (Right) |
SARA |
4 |
2 |
Improvement |
|
|
Fast Alternating Hand Movements (Left) |
SARA |
3 |
1 |
Improvement |
|
|
Heel Shin Slide (Right) |
SARA |
2 |
1 |
Improvement |
|
|
Heel Shin Slide (Left) |
SARA |
2 |
1 |
Improvement |
|
|
Writing |
Spirals (Right) |
TETRAS |
4 |
3 |
Improvement |
|
Spirals (Left) |
TETRAS |
3 |
2 |
Improvement |
|
|
Dot Approximation (Right) |
TETRAS |
2.5 |
4 |
Worsening |
|
|
Dot Approximation (Left) |
TETRAS |
2.5 |
1.5 |
Improvement |
Figure 1: (A) Pre and Post TMS stimulation scores on the TETRAS scale; high score indicates worse performance. (B) Pre and Post TMS stimulation scores on the SARA scale; high score indicates worse performance.
TMS and Quality of Life Metrics
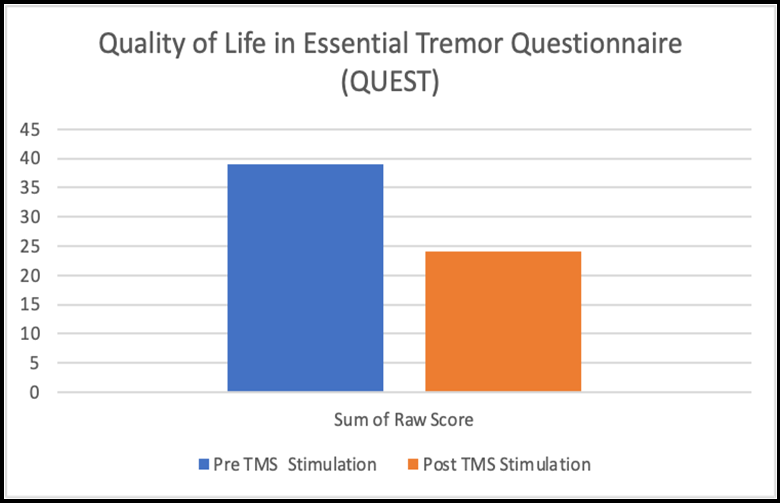
We found no significant change in self-report quality of life assessment (QUEST) in post stimulation compared to pre stimulation when using a Wilcoxon ranked sign test to assess the subjective change in performance. However, the subject’s responses demonstrate a trend toward improvement in the quality-of-life assessment.
Figure 2: Self-report quality of life assessment (QUEST) in pre stimulation compared to post stimulation.
Discussion
The development of limb ataxia and, in particular, rubral tremor in patients with MS represents a major treatment challenge as pharmacological options are too often ineffective. Arresting the advancement of the disease by implementing a disease modifying therapy proportional to the disease activity can be viewed as key to prevent the accumulation of fixed neurological deficits [10], but as illustrated by the case reported here, disability may not always be barred.
In the last decade, the applications of the TMS have expanded and specifically for movement disorders, the technique has provided a notable contribution to the understanding of the pathophysiology of tremor [11]. The cerebello-thalamo-cortical network has emerged as critical for tremor syndromes regardless of the underlying pathophysiology, (e.g. vascular, inflammatory).
Supported by growing experience with the application of TMS in neurological diseases [12], the disabling severity of the rubral tremor experienced by our patient motivated the attempt to treatment with the TMS. Targeting the cerebellum with the repetitive stimulation delivered for five consecutive days has the rationale of attempting to change the firing patterns of neurons involved in the circuitry and re-establish their physiologic oscillations.
Given the nature of this study being a single subject case experiment, it is necessary to examine the patient’s responses on the TETRAS pre and post TMS stimulation in the context of prior research conducted by analyzing clinical significance pre and post treatment using the same scale. The focus is on the degree of improvement post-treatment, and in their pilot study for essential tremor by Handforth et al., the change in tremor performance, measured before and after perampanel administration in 11 patients, demonstrated a mean − 3.13 point score reduction, which appeared to be a significant change from baseline [13]. In our single subject study, the patient demonstrated a 12.5 point reduction in overall TETRAS score post TMS stimulation, which is suggestive of a clinically meaningful improvement in tremor reduction.
Similarly, with the SARA scale we evaluated our subject’s change in performance in the context of prior studies validating clinical outcomes of the SARA scale in persons with tremor due to spinocerebellar ataxia (SCA). Benussi et al. performed a sham controlled trial with 20 participants with degenerative ataxia, in which 12 subjects received intervention with anodal transcranial direct current stimulation and 8 received a placebo [14]. A clinically significant change from baseline was achieved with a 2.8-point change from baseline scores. In our experiment, the subject demonstrated a 14 point reduction in overall SARA score post TMS stimulation, which is suggestive of a clinically meaningful improvement. The possibility of an even more profound and perhaps durable effect could theoretically be achieved with administering the treatment in multiple sessions over a period of months [14]. Our case report supports the design of future clinical trials, including multiple and longer treatment periods as well as using placebo/sham-controlled study designs.
Conclusions
The management of tremor syndromes is expected to rely on the future of neuro-stimulation and TMS possesses a flexibility of treatment paradigms that offer opportunities for optimizations of stimulation parameters, and one could envision an expansion of its clinical utilizations. Based on our case report, we propose that TMS may be a safe and effective treatment for refractory head and limb tremor in MS patients.
Acknowledgements
The authors are thankful for the financial support of a private donation by eng. Marco Cherubini.
References
- Reich DS, Lucchinetti CF, Calabresi PA (2018) Multiple Sclerosis. NEJM. 378(2): 169-180.
- Dobson R, Giovannoni G (2019) Multiple Sclerosis- a review. Eur J Neurol. 26(1): 27-40.
- Kister I, Chamot E, Salter AR, Cutter GR, Bacon TE, et al. (2013) Disability in multiple sclerosis: a reference for patients and clinicians. Neurology. 80(11): 1018-1024.
- Bomprezzi R, Chen A, Hemond C (2022) Insights into the course of illness of MS: Clinical and radiological aspects. Clinic Neurol Neurosc. 6(2): 19-28.
- Alusi SH, Glickman S, Aziz TZ, Bain PG (1999) Tremor in multiple sclerosis. JNNP. 66(2): 131-134.
- Makhoul K, Ahdab R, Riachi N, Chalah MA, Ayache SS (2020) Tremor in Multiple Sclerosis-An Overview and Future Perspectives. Brain Sci. 10(10): 722.
- Ozturk H, Venugopal S (2022) Transcranial Magnetic Stimulation as a Therapeutic Option for Neurologic Diseases and Psychiatric Disorders: A Systematic Review. Cureus. 14(8): e28259.
- Valero-Cabré A, Amengual JL, Stengel C, Pascual Leone A, Coubard OA (2017) Transcranial magnetic stimulation in basic and clinical neuroscience: A comprehensive review of fundamental principles and novel insights. Neurosci Biobehav Rev. 83: 381-404.
- Johnson KA, Baig M, Ramsey D, Lisanby SH, Avery D, et al. (2013) Prefrontal rTMS for Treating Depression: Location and Intensity Results from the OPT-TMS Multi-Site Clinical Trial. Brain Stimul. 6(2): 108-117.
- Chansakul C, Moguel-Cobos G, Bomprezzi R (2011) Head tremor secondary to MS resolved with rituximab. Neurol Sci. 32(6): 1157-1160.
- Frey J, Hess CW, Kugler L, Wajid M, Wagle Shukla A (2021) Transcranial Magnetic Stimulation in Tremor Syndromes: Pathophysiologic Insights and Therapeutic Role. Front Neurol. 12: 700026.
- Somaa FA, de Graaf TA, Sack AT (2022) Transcranial Magnetic stimulation in the treatment of neurological diseases. Front Neurol. 13: 793253.
- Handforth A, Tse W, Rodger JE (2020) A Pilot Double‐Blind Randomized Trial of Perampanel for Essential Tremor. Mov Disord Clin Pract. 7(4): 399–404.
- Benussi A, Dell’Era V, Cotelli MS, Turla M, Casali C, et al. (2017) Long term clinical and neurophysiological effects of cerebellar transcranial direct current stimulation in patients with neurodegenerative ataxia. Brain Stimul. 10(2): 242-250.