Yibing Zhao1,2#, Yuanyuan Sun1,2#, Chuanqing Wang3, Yan Fu2, Shan Long4, Yingshu Cui5, Yuanyuan Xu6*, Xiaosong Li7*
1Graduate School, Jinzhou Medical University, Jinzhou.
2Department of Oncology, the Fourth Medical Center, Chinese PLA General Hospital; Beijing.
3Emergency Center, The Affiliated Qingdao Central Hospital of Qingdao University, Shandong.
4Graduate School of Nankai University, Tianjin.
5Graduate School of PLA Medical College, Beijing.
6Department of Laser, The First Medical Center of the PLA General Hospital; Beijing.
7Department of Oncology, the Seventh Medical Center, Chinese PLA General Hospital; Beijing.
*Corresponding Authors: Yuanyuan Xu, Department of Laser, The First Medical Center of the PLA General Hospital; Beijing. Xiaosong Li, Department of Oncology, the Seventh Medical Center, Chinese PLA General Hospital; Beijing.
#Co-first authors: Yibing Zhao and Yuanyuan Sun contributed equally to this article.
Abstract
As an anti-tumor drug, cinobufotalin has the effect of inducing apoptosis, inhibiting cell proliferation in malignant tumor cells, and regulating immune function. Few studies have addressed whether the effects of cinobufotalin can reduce cancer markers. Our purpose is to explore the effects of cinobufotalin on cancer patients with abnormal levels of CEA. Five cancer patients had the same characteristics after surgery and chemotherapy according to the National Comprehensive Cancer Network guide with tumor-free lesions and abnormal levels of carcinoembryonic antigen. They were monitored for carcinoembryonic antigen levels. Ten patients with similar conditions compared the role function, emotional function, cognitive function, and insomnia between the two groups. Three patients had carcinoembryonic antigen levels within normal limits. The EORTC QLQ-C30 Chinese version showed that role function, emotional function and cognitive function were statistically significant in 5 cancer patients (P=0.034, P=0.003, P=0.005, respectively). Emotional function and cognitive function were statistically significant between the two groups (p=0.000 and p=0.028, respectively). These preliminary study results support the hypothetical role of cinobufotalin in inhibiting the increasing level of carcinoembryonic antigen, to explore the feasibility of interventions in cancer patients, when abnormal tumor markers appear after the patients receive standard treatment.
Keywords: Cinobufotalin; CEA; carcinoembryonic antigen; tumor; cancer.
1. Abbreviations
AJCC, American Joint Committee on Cancer; AP, Appetite loss; CA, carbohydrate antigen; CEA, carcinoembryonic antigen; CF, Cognitive Function; CO, Constipation; DI, Diarrhea; DY, Dyspnea; EF, Emotional Function; EORTC QLQ-C30, the European organization for the research and treatment of cancer Quality of life questionnaire-C30; FA, Fatigue; FDA, Food and Drug Administration; FI, financial difficulty; NCCN, National Comprehensive Cancer Network; NV, Nausea vomiting; OS, overall survival; PA, Pain; PF, Physical Function; PFS, progression-free survival; QL, Global quality of life; RF, Role Function; SF, Social Function; SL, sleep.
2. Introduction and hypothesis
The aging population, environmental change, and unhealthy lifestyle have led to the rapid growth of the global cancer burden. Potential years of life lost and disability-adjusted life years have also shown an increasing trend and rank first in various diseases. [1,2]Therefore, cancer has become a major killer that seriously endangers people's health and affects the productivity of social labor. The warning roles of tumor markers in diagnosis, relapse, and progression of cancer are also becoming areas of increasing interest. Lois A. Daamen et al showed that tumor markers could detect the recurrence of postoperative pancreatic cancer, especially carbohydrate antigen (CA19-9). [3] By monitoring the postoperative tumor markers carcinoembryonic antigen (CEA) and carbohydrate antigen (CA19-9) in 30 patients with stage II or III colon cancer, Claudia Burz et al showed the importance of predicting the recurrence of colorectal cancer. Produced in colorectal cancer tissue, one antigen, called a carcinoembryonic antigen, could elicit an immune response in patients and could be widely found in digestive system cancers of endoderm origin as well as normal human serum. [4] CEA could reflect the existence of a variety of tumors and, thus, is regarded as a broad-spectrum tumor marker. It is an effective tumor marker for judgments concerning the curative effect and disease progression, and it can be used to monitor tumor growth and reflect the prognosis of colorectal cancer, breast cancer, and lung cancer. [5,6] CEA is an important marker for all kinds of tumors, especially for patients who have undergone surgery. However, it must be considered that the specificity and sensitivity of this marker are not high, and early diagnosis of a tumor is not obvious.
Early detection, early diagnosis, and early treatment are crucial for cancer patients, while early surgery is considered a standard for a possible cure. Postoperative patients are reviewed regularly, including routine blood tests, image examination, and tumor marker determination. Approximately one decade prior, people’s understanding of tumor markers was more superficial. After surgery and chemotherapy according to the National Comprehensive Cancer Network (NCCN) guide, cancer patients are usually monitored based on normal images with abnormal tumor markers during the long-term follow-up. Some studies have shown that tumor markers played a predictive role and may occur a few months earlier than imaging in some postoperative cancer patients. [7-10] The tumor markers are then found to be abnormal and the imaging normal when the patient completes the chemotherapy cycle according to the NCCN guide, or when the body is unable to tolerate chemotherapy. Thus, the question of how the doctor can choose a better treatment has been raised.
The cinobufotalin capsule is an anti-tumor agent that is used in traditional Chinese medicine. It has low toxicity and broad-spectrum anti-cancer characteristics, demonstrating significant effects for the treatment of advanced gastric cancer, rectal cancer, and lung cancer.[11] The main components of cinobufotalin capsule are extracted from Chinese toad husk, including quintamine, scorpion toxin, an indole alkaloid, and other substances. It has the functions of detoxification and eliminating heat, swelling, and diuresis. [12,13] Xiaochi Ma et al have shown that cinobufotalin is cytotoxic to hepatoma cells. [14] Some studies have revealed that cinobufotalin capsule combined with chemotherapy can reduce the level of CEA; however, little research has explored the use of cinobufotalin capsule alone for reducing the level of CEA and lowering the risk of recurrence. This article proposes the construction of an intervention to reduce CEA levels and improve the quality of life of cancer patients with abnormal CEA levels and normal imaging after routine surgery and chemotherapy, among whom the patients and their families have abnormal anxiety levels and require active treatment.
3. Cases and methods
Five cancer patients who underwent surgery and chemotherapy according to the NCCN guidelines were enrolled in our study. They were from the Oncology Department of the General Hospital of the Chinese People's Liberation Army from 2017 to 2018, with ages were between 45 and 65 years old. They were all treated with surgery and chemotherapy and then monitored regularly. Subsequently, the levels of the tumor marker CEA were found to be abnormal but the imaging was normal. The anxiety of the patients and their families, however, was apparent, and they did not participate in chemotherapy treatment due to poor physical health or a desire to wait to observe. Based on the literature and clinical experience, we recommended that the patients be treated with cinobufotalin and review their progress in two months. Of course, the patients and their families agreed on this intervention. All patients provided written informed consent prior to enrollment in the study. No ethical approval was obtained because this study did not involve a prospective evaluation, did not involve laboratory animals, and is noninvasive.
4. Dosage and Safety
The patients consumed 3 capsules twice daily, according to the instructions. The safety of cinobufotalin was assessed according to the response of each individual, using National Cancer Institute Common Toxicity Criteria, version 3.0. [15]
5. Assessment of efficacy
5.1 The level of the tumor marker CEA
In our study, the results were evaluated mainly by the level of the tumor marker CEA, for which the patients were assessed twice per month. In addition, routine imaging examination of patients was also considered essential. Normal imaging was a prerequisite, and the decline in the level of the tumor marker CEA was the standard for the assessment.
5.2 EORTC QLQ-C30 Chinese version
Another assessment standard was the quality of life of cancer patients according to the Chinese version of the EORTC QLQ-C30. Research on the quality of life of cancer patients has become mainstream in the medical field, and the FDA clearly stipulates that evaluations of the quality of life of cancer patients are one of the most important components of anticancer drugs. [16,17] EORTC QLQ-C30 is a core scale in the cancer patient quality of life measurement scale system that was developed by the European Cancer Research and Treatment Organization for the measurement of the quality of life of all cancer patients. Currently, QLQ-C30 has been translated into more than 50 languages and has been accepted by the medical community. [18-20] The EORTC QLQ-C30 Chinese version also better reflects the quality of life of cancer patients. [21-22]
6. Results
6.1 Patients characteristic
Five cancer patients, each with gastric cancer, cervical cancer, colorectal cancer, and throat cancer, were enrolled postoperation and post-chemotherapy. The detailed patient characteristics are shown in Table 1. Almost all the patients were followed-up according to the schedule, with only one patient interruption after two months of medication and a follow-up. Among the cancer patients, the median age was 58 years, and they were diagnosed with stage Ⅰ or stage II cancer(refer to the AJCC Cancer Staging Manual).
Table 1. The characteristics of cancer patients.
|
NO. |
Age |
Cancer types |
Metastasis |
Staging |
Previous treatments |
|
1 |
58 |
Gastric Cancer |
none |
ⅡA |
Surgery + Chemotherapy |
|
2 |
63 |
Gastric Cancer |
none |
ⅡB |
Surgery + Chemotherapy |
|
3 |
48 |
Cervical Cancer |
none |
ⅡA |
Surgery + Chemotherapy |
|
4 |
52 |
Colorectal Cancer |
none |
ⅡA |
Surgery + Chemotherapy |
|
5 |
67 |
Throat Cancer |
none |
Ⅰ |
Surgery + Chemotherapy |
6.2 Assessment of Safety
If discomfort developed, the treatment was reduced or stopped. Fortunately, no serious adverse reactions were observed, although one cancer patient experienced a nauseated feeling. In the patient description, this event occurred only a few times and was gradually tolerated. Therefore, the drugs were found to be relatively safe in these five people.
6.3 Assessment of effectiveness
We determined the CEA levels in the patients after nearly 10 months of observation. The detailed information is shown in table 2. Surprisingly, 3 patients had CEA levels that were within normal limits. Of course, these findings were not absolute, with small fluctuations across the study. In 1 patient who had been interrupted at two months, the CEA level increased slightly, but the patient then continued to take the drug. The effect was satisfactory after two months of medication. As shown in Figure 1, the downward trend of the CEA level was quite obvious, thus providing significantly encouraging results.
Table 2. The level of CEA.a
|
|
First time |
Second time |
Third time |
Fourth time |
Fifth time |
|
Gastric Cancer 1 |
11.75 |
8.56 |
5.72 |
5.93 |
4.25 |
|
Gastric Cancer 2 |
6.73 |
3.52 |
3.34 |
|
|
|
Cervical Cancer |
5.57 |
3.35 |
3.37 |
3.25 |
|
|
Colorectal Cancer |
4.23 |
4.47 |
3.86 |
2.87 |
|
|
Throat Cancer |
3.75 |
3.38 |
3.82 |
3.35 |
|
aGenerally, CEA levels were tested every two months. The blue grid indicates a small elevation of the CEA level; the yellow grid indicates the CEA level after half a year; the green grid indicates the CEA level after interruption of cinobufotalin for two months.
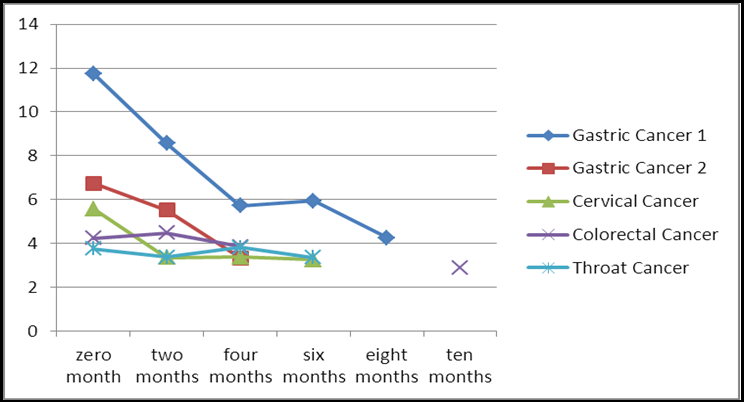
Figure 1: The trend of the tested CEA levels (the purple line was interrupted for half a year).
The EORTC QLQ-C30 Chinese version has been widely used to investigate the quality of life of cancer patients. Thirty items are listed on this scale, including the functional dimension, symptom dimension, and overall quality of life. It consists of two main evaluation results: a raw score (RS) and a standard score (SS). We could score points in the field by summing up the entries and dividing the number of entries included. The detailed information is summarized in Figure 2. As shown in Table 3, we performed the statistical analysis and found that the role function, emotional function, and cognitive function were statistically significant.
Table 3. Comparison of the standard score before and after medication.a
|
Before medicine After medicine Domain Mean SD Mean SD t p |
||||||
|
Physical Function* |
80.00 |
18.26 |
88.00 |
9.89 |
-2.058 |
0.109 |
|
Role Function* |
46.67 |
21.73 |
63.33 |
18.26 |
-3.162 |
0.034 |
|
Emotional Function* |
56.67 |
10.87 |
88.33 |
7.46 |
-6.516 |
0.003 |
|
Cognitive Function* |
70.00 |
13.94 |
93.33 |
9.13 |
-5.716 |
0.005 |
|
Social Function* |
86.67 |
13.94 |
90.00 |
14.91 |
-0.535 |
0.621 |
|
Global quality of life* |
58.33 |
18.63 |
68.33 |
12.36 |
-2.449 |
0.070 |
|
Fatigue * |
24.46 |
21.43 |
15.53 |
16.86 |
2.138 |
0.099 |
|
Nausea vomiting # |
6.67 |
9.13 |
3.33 |
7.46 |
|
0.317 |
|
Pain # |
3.33 |
7.46 |
0.00 |
0.00 |
|
0.317 |
|
Dyspnea # |
20.00 |
18.26 |
13.33 |
18.26 |
|
0.317 |
|
Insomnia # |
46.67 |
38.00 |
20.00 |
29.82 |
|
0.102 |
|
Appetite loss # |
13.33 |
18.26 |
20.00 |
29.82 |
|
0.317 |
|
Constipation # |
13.33 |
17.21 |
13.33 |
23.31 |
|
0.317 |
|
Diarrhea # |
6.67 |
14.91 |
0.00 |
0.00 |
|
0.317 |
|
financial difficulty # |
0.00 |
0.00 |
13.33 |
18.26 |
|
0.157 |
a*The data conform to the normal distribution and were calculated using the paired T-test. # The data do not conform to the normal distribution and were calculated by the U test.
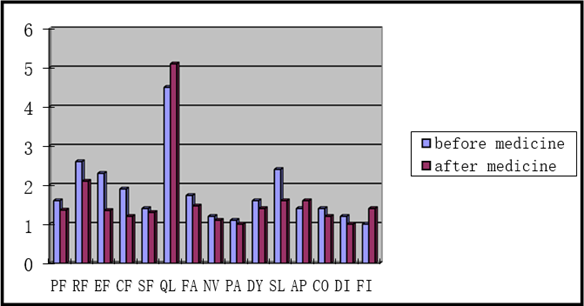
Figure 2: The mean raw score before medication and after medication.
Abbreviations: AP, Appetite loss; CF, Cognitive Function; CO, Constipation; DI, Diarrhea; DY, Dyspnea; EF, Emotional Function; FA, Fatigue; FI, financial difficulty; NV, Nausea vomiting; PA, Pain; PF, Physical Function; QL, Global quality of life; RF, Role Function; SF, Social Function; SL, sleep.
6.4 Comparison between patients with and without taking medicine
The significant differences prompted our further study. We followed up 10 similar patients who had surgery and chemotherapy according to the NCCN guidelines, with elevated CEA levels and normal imaging. However, they followed the routine without cinobufotalin, and the doctors and family members informed the patients regarding the relief of pressure. We asked them to fill out the EORTC QLQ- C30 Chinese version according to their memories. The three items with significant differences were compared between patients with and without taking medicine: role function, emotional function, and cognitive function. Although there was no significant difference in the insomnia field between patients with and without medicine, a clear improvement was observed in their oral description. Thus, we compared insomnia in the two groups. The specific results are shown in the table. Unfortunately, due to the lack of partial data, no statistical comparison of the CEA changes between those taking medicine and those not taking medicine was possible.
Table 4. Comparison of the standard score between people taking and not taking medicine.a
|
Taking medicine Not taking medicine Domain Mean SD Mean SD t p |
||||||
|
Role Function* |
16.67 |
11.79 |
1.67 |
11.57 |
1.794 |
0.096 |
|
Emotional Function* |
31.66 |
10.87 |
2.50 |
9.67 |
5.299 |
0.000 |
|
Cognitive Function # |
23.33 |
9.13 |
5.00 |
15.81 |
|
0.028 |
|
Insomnia # |
-26.67 |
27.89 |
-10.00 |
22.50 |
|
0.513 |
a*The data conform to the normal distribution and were calculated using the paired T-test. # The data do not conform to the normal distribution and were calculated by the U test.
7. Discussion
Accumulating results suggest that cinobufacini plays a critical role in reducing the level of CEA. According to our study, there was a downward trend in CEA levels in patients. Among 5 patients using cinobufotalin, 3 patients’ CEA levels returned to normal, despite plateaus and small fluctuations. The postoperative review is a concern for each cancer patient and their family, as an even slightly abnormal condition makes cancer patients and their families extremely nervous. Therefore, an appropriate intervention is needed to improve this situation to reduce the psychological burden on cancer patients and help improve their quality of life. These findings are also the highlights of our study.
As an anti-tumor drug, cinobufotalin has the effect of inducing apoptosis, inhibiting cell proliferation in malignant tumor cells, and regulating immune function. In fact, we have already achieved good clinical efficacy through the use of cinobufotalin in recent years. Numerous studies have demonstrated that cinobufotalin can induce non-apoptotic death of lung cancer cells.[10] A study of 80 colorectal cancer patients post operation showed improved effects with the use of cinobufotalin combined with XELOX than XELOX alone, including therapeutic effects and life quality. Despite confirmation of the anti-tumor functions of cinobufotalin in many studies, few studies have addressed whether the effects of cinobufotalin can reduce cancer markers. In our recent study, we regularly observed tumor markers and imaging changes in cancer patients who had already periodically completed chemotherapy treatment after surgery. Our results showed that cinobufotalin could upregulate microRNA (miR)-494 expression in gastric cancer cells, whereas its potential mechanism remained unknown. We hypothesize that, on the one hand, cinobufotalin can promote the proliferation and activity of a variety of immune cells, including natural killer cells, T lymphocytes, and lymphokine- activated killer cells, and on the other hand, it can directly inhibit and kill tumor cells, which could induce tumor cell apoptosis and enhance phagocytic phagocytosis. More time and further experiments are needed to validate this hypothesis. In our study, we also found that gastric cancer, cervical cancer, colorectal cancer, and throat cancer were associated with increased levels of CEA, which would be effectively reduced by cinobufotalin. These results are inspiring.
In addition, we found that quality of life was improved in these cancer patients. Studies have shown that the EORTC QLQ-C30 Chinese version of the scale has good reliability, validity, and feasibility, as well as a certain degree of response, and it can be used as a tool for measurements of quality of life of patients with malignant tumors in China. [20,23] In our study, we found a statistically significant effect of role function, emotional function, and cognitive function on the quality of life of patients. Although insomnia was not statistically significant, patients reported improvements in insomnia. We also found a significant difference in emotional function and cognitive function between patients who did not take cinobufotalin and those who took cinobufotalin. There was no significant difference in role function and insomnia due to the small number of people. In fact, we determined that most cancer patients feel anxiety, especially when facing questions of suffering and relapsing, which is not conducive to treatment and improvement of the patient's quality of life. In contrast, it increases the burden on the patient's family and is a concern for clinicians as well. Of course, it is not the most important function of cinobufotalin, but it is strongly promoted to reduce the level of CEA, improve the quality of life of patients and reduce the burden on patients' families for postoperative patients with abnormal CEA.
In the past, people’s opinions have tended to be conservative until the disease progresses, and they believe that the intervention does not make much sense without the support of medical imaging. However, with the developments in medicine, the increasing favor among clinicians and clinicians for early intervention while tumor markers are abnormal has been a cause for alarm, especially when the level is higher than one. Robert M. Hoffman et al thought tumor markers were important for the diagnosis, prognosis, and targeting in tumor therapy.[24] For the early detection of metastatic breast cancer, Dorit Di Gioia et al found that CA15-3 and CEA were highly specific.[25] Wang Jingtao et al have shown that serum CEA, CA19-9, and CA242 levels play an important role in the surgery of treated colorectal cancer patients.[26] However, some people believe that tumor markers do not have as much of an effect as previously anticipated, and thus interventions should be treated with caution. Claudio Spinelli et al, in a 10-year study, demonstrated that approximately twenty percent of benign ovarian tumors also expressed high tumor marker levels. In that study, it was not a gold standard for radical surgical and conservative treatment. Regardless, the choice of these two treatments had a great impact on the quality of life of patients. [27] Therefore, how to intervene to maximize the patient's benefit remains a thought-provoking consideration. In our recent study, we not only considered the significance of tumor markers but also noted the quality of life of patients. Elevated tumor markers are not a beneficial sign for postoperative cancer patients. Additionally, the medical guide does not define how to treat this complex condition. Chemotherapy, observation, and medication are appropriate choices. Chemotherapy usually causes too much physical harm to cancer patients and results in some patients experiencing a poor physical condition or simply terminating the treatment. Observation seems to give cancer a chance to relapse. Therefore, it may be more appropriate to utilize anti-cancer drugs with definite therapeutic significance.
However, our study has some limitations. Because there were only five patients, we should expand the number of cases for improved statistical analyses. Moreover, the time of observation in this study was short, and we lacked a control group. The use of the cinobufotalin could certainly prolong PFS and OS in patients. In our study, it represented an effective treatment for postsurgical cancer patients with abnormal tumor markers and normal imaging. Thus far, few studies on anti-tumor drugs have been conducted in this respect, although apparently, it merits study. Moreover, the combination of multiple anti-tumor drugs and the relative specificity of each drug will become a worthy focus of concern. Some studies have also suggested that cinobufotalin could reduce the side effects of chemotherapy and improve the tolerability of cancer patients. [28,29] However, only a few studies have examined the combination of cinobufotalin and other drugs, thus it may present greater potential and deserves further exploration.
8. Reasoning and hypothesis
Cinobufotalin may reduce levels of CEA, relieve patient anxiety, and avoid the development of more severe adverse reactions. Cinobufotalin may be effective and safe for postoperative cancer patients with abnormal levels of CEA.
9. Funding
This article was supported by National Key Project (No:2018YFB0407200) Application demonstration of laser materials and devices in medical field.
The National Natural Science Foundation of China (No:61975239) The underlying mechanisms of immunogenicity induced by carbon nanotubes plus laser via dectin-1 receptor expressed on dendritic cells.
References
- Siegel RL, Miller KD, Jemal A(2017) Cancer Statistics, 2017. CA Cancer J Clin. 67(1): 7-30.
- Chandan KD, Mahitosh M, Donat K (2018) Pro-survival autophagy and cancer cell resistance to therapy. Cancer Metastasis Rev. 37(4): 749-766.
- Daamen LA, Groot VP, Heerkens HD, Intven MPW, van Santvoort HC, et al. (2018) Systematic review on the role of serum tumor markers in the detection of recurrent pancreatic cancer. HPB (Oxford). 20(4): 297-304.
- Kerr D, Laber D, Visweshwar N , Jaglal M (2018) Case Report: CEA Elevation Can Be a Marker of Increased Inflammation During Treatment with Oxaliplatin. Anticancer Res. 38(3): 1711- 1713.
- Bae U, Shim JY, Lee HR , Shin JY (2013) Serum carcinoembryonic antigen level is associated with arterial stiffness in healthy Korean adult. Clin Chim Acta. 415: 286-289.
- Bulut I, Arbak P, Coskun A, Balbay O, Annakkaya AN, et al. (2009) Comparison of serum CA 199, CA 125 and CEA levels with severity of chronic obstructive pulmonary disease. Med Princ Pract. 18(4): 289-293.
- Burz C, Aziz BY, Bălăcescu L, Leluţiu L, Buiga R, et al. (2016) Tumor markers used in monitoring the tumor recurrence in patients with colorectal cancer. Clujul Med. 89(3): 378-383.
- Zhi Q, Wang Y, Wang X, Yue D, Li K, et al. (2016) Predictive and prognostic value of preoperative serum tumor markers in resectable adenosqamous lung carcinoma. Oncotarget. 7(40): 64798-64809.
- Duffy MJ, Harbeck N, Nap M, Molina R, Nicolini A, et al. (2017) Clinical use of biomarkers in breast cancer: Updated guidelines from the European Group on Tumor Markers (EGTM). Eur J Cancer. 75: 284-298.
- Levy A, Popovici T, Bories PN (2017) Tumor markers in pancreatic cystic fluids for diagnosis of malignant cysts. Int J Biol Markers. 32(3): 291-296.
- Emam H, Zhao QL, Furusawa Y, Refaat A, Ahmed K, et al. (2012) Apoptotic cell death by the novel natural compound, cinobufotalin. Chem Biol Interact. 199(3): 154-160.
- Kai S, Lu JH, Hui PP, Zhao H (2014) Pre-clinical evaluation of cinobufotalin as a potential anti-lung cancer agent. Biochem Biophys Res Commun. 452(3): 768-774.
- Li JK, Ma XC, Li FY, et al. Rbufadienolides from Chinese traditional medicine of ChanSu using high-speed counter-current chromatography. J Sep Sci. 2010;33: 1325-1330.
- Ma XC, Xin XL, Liu KX, Han J, Guo D (2008) Microbial Transformation of Cinobufagin by Syncephalastrum racemosum.J Nat Prod. 71(7): 1268-1270.
- US Department of Health and Human Services, National Institutes of Health, National Cancer Institute, Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis: Common terminology criteria for adverse events v3.0 (CTCAE).
- Matsumoto T, Ohashi Y, Morita S, Kobayashi K, Shibuya M, et al. (2002) The quality of life questionnaire for cancer patients treated with anticancer drugs (QOL-ACD): Validity and reliability in Japanese patients with advanced non-small-cell lung cancer. Qual Life Res. 11(5): 483-493.
- Johnson JR, Temple R (1985) Food and drug administration requirements for approval of new anti-cancer drugs. Cancer Treat Rep. 69(10): 1155-1159.
- Fayers P, Bottomley A; EORTC Quality of Life Group, Quality of Life Unit. (2002) Quality of life research within the EORTC— the EORTC QLQ-C30. Eur J Cancer. 38(Suppl 4): S125-133.
- Fitzsimmons D, Kahl S, Butturini G, van Wyk M, Bornman P, et al. (2005) Symptoms and Quality of Life in Chronic Pancreatitis Assessed by Structured Interview and the EORTC QLQ-C30 and QLQ-PAN26. Am J Gastroenterol. 100(4): 918-926.
- Anota A, Mariet AS, Maingon P, Joly F, Bosset JF, et al. (2016) Cross-cultural adaptation and validation of the French version of the Expanded Prostate cancer Index Composite questionnaire for health-related quality of life in prostate cancer patients. Health Qual Life Outcomes. 14(1): 168.
- Zhao H, Kanda K (2000) Translation and validation of the Standard Chinese version of the EORTC QLQ-C30. Quality of Life Research. 9(2): 129-137.
- Wan C, Meng Q, Yang Z, Tu X, Feng C, et al. (2008) Validation of the simplified Chinese version of EORTC QLQ-C30 from the measurements of five types of inpatients with cancer. Ann Oncol. 19(12): 2053-2060.
- Magaji BA, Moy FM, Roslani AC, Law CW, Sagap I (2015) Psychometric Validation of the Malaysian Chinese Version of the EORTC QLQ-C30 in Colorectal Cancer Patients. Asian Pac J Cancer Prev. 16(18): 8107-8112.
- Hoffman RM, Guadagni F (2018) Expression and Targeting of Tumor Markers in Gelfoam® Histoculture: Potential Individualized Assays for Immuno-Oncology. Methods Mol Biol.1760: 29-37.
- Di Gioia D, Blankenburg I, Nagel D, Heinemann V, Stieber P (2016) Tumor markers in the early detection of tumor recurrence in breast cancer patients: CA 125, CYFRA 21-1, HER2 shed antigen, LDH and CRP in combination with CEA and CA 15-3. Clin Chim Acta. 461: 1-7.
- Wang J, Wang X, Yu F, Chen J, Zhao S, et al. (2015) Combined detection of preoperative serum CEA, CA19-9 and CA242 improve prognostic prediction of surgically treated colorectal cancer patients. Int J Clin Exp Pathol. 8(11): 14853-14863.
- Spinelli C, Pucci V, Buti I, Liserre J, Messineo A, et al. (2012) The Role of Tumor Markers in the Surgical Approach of Ovarian Masses in Pediatric Age: A 10-Year Study and a Literature. Ann Surg Oncol. 19(6): 1766–1773.
- Zheng Z, Cho WC, Xu L, Wang J, Man-Yuen Sze D (2013) Lessons learnt from evidence-based approach of using chinese herbal medicines in liver cancer. Evid Based Complement Alternat Med. 2013: 656351.
- Xie M, Chen X, Qin S, Bao Y, Bu K, et al. (2018) Clinical study on thalidomide combined with cinobufagin to treat lung cancer cachexia. J Cancer Res and Ther. 14(1): 226-232.



