Vandana Garg1*, Aida Gadzhieva-Moore2, An Yi Wong3, Joseph Lin4, Sheryl S. L. Tan5
1Haleon, GlaxoSmithKline Consumer Healthcare Pte Ltd, 23 Rochester Park, Singapore 139234. ORCID: https://orcid.org/0000-0001-8836-6928
2IQVIA Solutions Asia Pte Ltd, 79 Anson Road, Singapore 079906. ORCID: https://orcid.org/0009-0008-2757-6233
3Haleon, GlaxoSmithKline Consumer Healthcare Pte Ltd, 23 Rochester Park, Singapore 139234. ORCID: https://orcid.org/0009-0005-2011-9425 4Haleon UK Services Limited Taiwan Branch, 24F, No.66, Sec. 1 Zhongxiao W. Rd. Taipei 100507 Taiwan. ORCID: https://orcid.org/0009-0005- 5959-707X
5Haleon, GlaxoSmithKline Consumer Healthcare Pte Ltd, 23 Rochester Park, Singapore 139234. ORCID: https://orcid.org/0000-0002-7485-7037
*Corresponding Author: Vandana Garg, Haleon, GlaxoSmithKline Consumer Healthcare Pte Ltd, 23 Rochester Park, Singapore 139234. ORCID: https://orcid.org/0000-0001-8836-6928.
Abstract
Objectives:
This study aimed to examine the impact of joint symptoms on daily mobility and validate the perceived effectiveness of undenatured collagen type II's (Caltrate® UC-II) regular intake on improving joint mobility in healthy individuals experiencing joint symptoms.
Methods
A 2-phase retrospective cross-sectional study was conducted using self-administered online questionnaires. Phase 1 (n=111) included individuals aged 35 to 60 experiencing joint symptoms with no history of osteoarthritis or recent bone fracture to assess the impact of joint symptoms, specifically identifying areas which were most affected by joint conditions. Phase 2 (n=400) focused on regular users of Caltrate® UC-II's who consumed the product at least three times a week in the past three months prior to recruitment, while also meeting the same criteria as set in phase 1, to evaluate the perceived effectiveness of Caltrate UC-II on those specific areas identified in Phase 1. Participants who took part in phase 1 were not eligible to participate in phase 2. Continuous variables were summarized using mean and standard deviation (SD), while categorical variables were presented using frequency and proportion. Chi-square tests were used to compare categorical variables between groups, while Student's t-tests were applied for continuous variables. Statistical significance was determined at the 5% level (p < 0.05). All data analyses were conducted using IBM SPSS Statistics software, version 28.0.
Results:
Phase 1 has shown that joint symptoms significantly impact daily activities, with most respondents experiencing challenges with posture change (57.7%), climbing stairs (49.5%), squatting (40.5%), bending (30.6%), running (24.3%), and hiking (23.4%). Improvements in these activities was the most important benefit expected by respondents (62.2%) from supplements such as UC-II. In Phase 2, respondents reported a positive impact on daily activities after taking UC-II for 90 days, including more comfortable sitting up and down or changing posture (85.5%); climbing more stairs with less discomfort (84.5%), squatting with less discomfort (84.8%), more comfortable bending (87.8%); running longer distances with less discomfort (82.9%), improvement in the duration, discomfort, and speed while walking (84.5%). Overall, up to 90% of respondents experienced perceived improvements in all 6 daily mobility activities evaluated. Corresponding to these improvements, up to 86.3% reported positive emotional impact and improved quality of life (QoL).
Conclusion:
Our study findings complemented the existing science of UC-II and demonstrated how regular UC-II intake relieves joint symptoms and improves daily mobility.
Keywords: joint symptoms, quality of life, real-world evidence, supplements
Introduction
According to the Global Burden of Disease (GBD) 2019 analysis, around 1.71 billion individuals worldwide are affected by musculoskeletal conditions, including joint disorders (such as osteoarthritis and rheumatoid arthritis) and low back pain.[1] Consequently, musculoskeletal conditions are recognized as substantial contributors to global disability, accounting for approximately 149 million years lived with disability (YLDs), constituting 17% of all YLDs worldwide. This burden was expected to escalate due to the aging and expanding world population.[1] These conditions, impacting joints, bones, muscles, and supporting structures in limbs, neck, and back, often manifested as pain, accompanied by body aches, malaise, and stiffness in affected patients. [2,3]
Joint pain is considered one of the most common types of chronic pain,[4] and among the most affected joints are the knee joints. [5-7] It is also the primary cause of joint discomfort.[4,8] For instance, an 8-year longitudinal cohort study from the Osteoarthritis Initiative (OAI), consisting of 3,053 individuals, reported mild knee pain in at least 67 %.[9] A cross-sectional study conducted on 2,233 adults aged 40 years and older in Germany found that 75% of respondents had experienced joint pain in the current 1-month period, and around one- third reported knee pain.[10] In comparison, the prevalence of knee pain among the Asian population was in the range of 11% to 56% in China,[11,12] 21% to 33% in Malaysia, [7,13] 21% to 23% in Korea [14,15] in adults. Available prevalence data from worldwide studies therefore suggest the possibility of geographical locations in joint pain prevalence.
Several components of the musculoskeletal system play a crucial role in maintaining joint health. With age, bones and muscles gradually weaken over time due to a decline in both bone and muscle mass, as well as power.[16] Additionally, tendons and ligaments become less elastic over time, resulting in reduced flexibility and restricted joint movement.[17] These age-related changes in connective and muscle tissues are linked to a decreased maintenance of tissue homeostasis due to cell senescence and alterations in circulating factors among other cellular changes.[16] Additionally, the cumulative impact of repeated joint use over one's lifetime can gradually wear down the cushioning cartilage between joints, contributing to joint discomfort.[17] Several studies report that a noticeable decline in mobility becomes evident at 60 years of age, although signs of reduced mobility can be detected as early as 20 years of age when more challenging mobility tasks were performed.[18] Similarly, engaging in certain sports poses a risk factor for developing joint.[19] This irony arises as various guidelines advocate for exercise to manage age-related changes to joint health.[17,20] However, vigorous physical activity increases the likelihood of sustaining joint injuries. Sports such as wrestling and powerlifting entail excessive loading of joints, while activities like baseball and ice hockey involve high intensity joint impact, thereby elevating the risk of joint injury.[19] Common symptoms of joint discomfort include stiffness and a restricted range of movement around the affected joint.[19] These impede daily physical activities and jeopardize the individual's quality of life.
Considering knee pain or general joint discomfort as a perceived impediment to daily physical activities, a common strategy to improve mobility and flexibility involves addressing and alleviating discomfort associated with joint movement. Viable and readily accessible approaches for joint protection may include exercise and dietary interventions,[21,22] along with the use of nutraceutical supplements.[23,24] Undenatured collagen stands out as one such dietary supplement that can be utilized in individuals experiencing activity-related joint discomfort, potentially reducing the progression of issues like limited mobility.
Undenatured collagen type II (UC-II) is a natural ingredient derived from chicken sternum, which contains a glycosylated, undenatured type II collagen.[25] It is believed that UC-II exerted its effect via a mechanism in the gastrointestinal tract known as oral tolerance, which is the immune system's ability to become tolerant or less responsive to specific antigens when they are introduced through the oral route.[26,27] UC-II appears to simulate the regulatory T cells (immune cells) in secreting anti-inflammatory cytokines and inducing articular cartilage repair.[28] Compared to other types of collagen, UC-II contains active epitopes—smaller antigenic segments with the potential to elicit an immune response.[25] In vivo studies demonstrated that UC-II enhanced the mechanical function of a compromised joint, prevented undue degradation of articular cartilage, and reduced circulating levels of inflammatory cytokines.[27, 29, 30] Randomized controlled trials (RCTs) demonstrated that daily small doses (10mg to 40mg) of UC-II ameliorated joint symptoms in the population suffering from osteoarthritis (OA).[25,31,32] In healthy individuals experiencing joint discomfort or pain, intake of UC-II enhanced joint symptoms.[26,33-35] Daily intake of 40mg of UC-II for 90 days resulted in improved knee range of motion and reduced pain following a step mill performance test.26 Similarly, healthy individuals with activity-related joint discomfort exhibited significant enhancements in joint mobility (daily step count) and reduced discomfort (subjective discomfort level after repetitive stepping up and down from a platform) after 24 weeks of daily UC-II consumption compared to a placebo group.[33]
Given the significant impact of joint discomfort on everyday mobility activities, as well as the strong show of evidence supporting the benefits of UC-II intake, our cross-sectional study seeks to further validate the real-world effectiveness of regular UC-II intake on mobility health amongst existing product users (Caltrate® UC-II) in Taiwan.
Material and methods
Study design
This was a two-phase cross-sectional study conducted in Taiwan using self-administered, structured online questionnaires. Phase 1 involved respondents who had experienced joint symptoms, focusing on the activities impacted by their joint symptoms and the benefits sought when choosing joint health supplements. Phase 2 involved respondents who regularly used Caltrate® UC-II (daily dose of one tablet contains UC-II 40 mg; Vitamin C 45 mg; Magnesium 52.5 mg; Zinc 7.5 mg; Copper 0.5 mg; Manganese 1.8 mg) to assess their perceived improvement in joint symptoms. There was no overlap in respondents in both phases.
Participants
In the Phase 1 study, eligible respondents were residents of Taiwan aged between 35 and 60. They were required to be self-declared individuals who had experienced joint discomfort, pain, or stiffness in the past 3 months and were open to health supplements or over-the- counter medication for joint relief. Exclusion criteria encompassed having been diagnosed with chronic illnesses such as osteoarthritis, fibromyalgia, gout, or lupus, as well as experiencing bone fractures or broken bones within the preceding 6 months. In Phase 2 of the study, respondents were recruited under the same eligibility criteria as in Phase 1, with the additional criteria that they had been using Caltrate® UC-II for joint relief at least three times a week in the past three months. Sample sizes were calculated using Cochran's (1977) formula. For Phase 1, a minimum required sample of 100 respondents was determined with a 10% margin of error, a standard deviation (SD) of 0.5, and a 95% confidence interval (CI); the final sample for analysis in Phase 1 included data collected from N=111 respondents. For Phase 2, a sample of 400 respondents was determined with a 5% margin of error, SD of 0.5, and a 95% CI; the final sample for analysis in Phase 2 included data collected from N=400 respondents.
Data collection and procedure
Respondents were recruited via an online consumer panel representative of the general population in Taiwan using an online link. The recruitment process also employed quota sampling, with adaptable quotas for age and gender closely monitored throughout. In both phases of the study, interested respondents who accessed the online link were first presented with an informed consent explaining the use of their data and a pre-screening questionnaire. Only those who met the inclusion criteria in the pre-screening questionnaire and aligned with age and gender quotas were invited to complete the remainder of the questionnaire.
Respondents in the Phase 1 study were asked to detail the daily activities impacted by their joint symptoms, their activity metrics performance (e.g., in daily steps, squatting, running performance and daily stairs count), and the key benefits they sought from joint health supplements. In Phase 2, respondents were asked about their perceived effectiveness and satisfaction with the use of Caltrate® UC- II in alleviating joint discomfort, pain, or stiffness and improvement in joint mobility and physical endurance. Respondents were also asked to provide the perceived quantitative changes in daily metric performance encompassing metrics such as daily steps count in walking, running frequency, distance, and duration per session, and squat counts, both before and after incorporating Caltrate® UC-II into their routine. For instance, the perceived quantitative changes in daily steps count for walking were captured by the question: "Thinking about the time before you started taking Caltrate® UC-II, how many steps did you use to walk on an average day? and after taking it for 90 days?" The questionnaire was constructed based on the analysis of a social media listening study involving approximately 6,000 spontaneous online conversations about joint health and mobility, sourced from various blogs, forums, and social networks.
Data analysis
The study endpoints for both phases remain unchanged during the study. In both study phases, only completed questionnaires were analyzed. Respondent characteristics and demographics were summarized using descriptive statistics. Continuous variables were summarized using mean and standard deviation (SD), while categorical variables were presented using frequency and proportion. When comparing between groups, the chi-square test was used for categorical variables. For continuous variables, a Student's t-test was used. The significance of differences between groups was assessed at a 5% significance level (p < 0.05). All data analysis was performed using IBM SPSS Statistics software, version 28.0.
Results
Demographic and joint treatment usage profile
The respondents' characteristics and health supplement usage in both phases were displayed in Table 1. In Phase 1 of the study, a total of 111 respondents completed the questionnaire. The respondents recruited were fairly representative in sex distribution with 48.6% being male and 51.4% female. The majority were between the ages of 40 to 49 years (49%). Approximately two-thirds (64.9%) of the respondents reported experiencing joint bruising, and slightly over one-third (37.8%) had joint sprains in the past 6 months. Additionally, approximately two-thirds reported experiencing headaches in the past three months. Respondents had also reported treatment to alleviate their joint symptoms in the past three months, with the majority utilizing topical medications and health supplements (66.7% and 55.9%, respectively). Amongst those who had consumed health supplements to relieve their joint symptoms, the most commonly reported options were Glucosamine (65.2%) and type 2 collagen (UC-II) (46.4%). The distribution of type of glucosamine and UC-II consumed by participants are presented in Table 2.
Table 1: Demographics and respondents’ usage profile in both study phases
|
Characteristics |
Phase 1 (N =111) |
|
Phase 2 (N=400) |
||
|
n |
% |
|
n |
% |
|
|
Gender |
|
|
|
|
|
|
Male |
54 |
48.6% |
|
250 |
62.5% |
|
Female |
57 |
51.4% |
|
150 |
37.5% |
|
Age |
|
|
|
|
|
|
Mean (SD) |
44.9 (6.6) |
|
46.6 (7.0) |
||
|
35-39 years old |
29 |
26.1% |
|
80 |
20.0% |
|
40-49 years old |
54 |
48.6% |
|
181 |
45.3% |
|
50-60 years old |
28 |
25.2% |
|
139 |
34.8% |
|
Monthly household income (NT$) |
|
|
|
|
|
|
Mean (SD) |
111,621.6 (29,062.3) |
|
133,300.0 (38,035.9) |
||
|
70,001 - 90,000 |
40 |
36.0% |
|
42 |
10.5% |
|
90,001 - 110,000 |
19 |
17.1% |
|
71 |
17.8% |
|
110,001 - 130,000 |
13 |
11.7% |
|
106 |
26.5% |
|
130,001 - 150,000 |
12 |
10.8% |
|
101 |
25.3% |
|
150,001 or above |
27 |
24.3% |
|
80 |
20.0% |
|
Condition Experienceda |
|
|
|
|
|
|
Joint stiffness/ pain/ discomfort |
111 |
100.0% |
|
400 |
100.0% |
|
Headache |
73 |
65.8% |
|
145 |
36.3% |
|
Sore throat |
56 |
50.5% |
|
146 |
36.5% |
|
Stomach-ache |
43 |
38.7% |
|
128 |
32.0% |
|
Atopic dermatitis/ eczema |
54 |
48.6% |
|
161 |
40.3% |
|
Injuries (P6M) |
|
|
|
|
|
|
Bruising |
72 |
64.9% |
|
90 |
22.5% |
|
Joint sprain |
42 |
37.8% |
|
97 |
24.3% |
|
Ligament trauma |
9 |
8.1% |
|
50 |
12.5% |
|
Others |
0 |
0.0% |
|
244 |
61.0% |
|
Joint treatment P3M |
|
|
|
|
|
|
Oral prescription medications |
24 |
21.6% |
|
152 |
38.0% |
|
Oral medications available without prescription/ over-the-counter |
19 |
17.1% |
|
237 |
59.3% |
|
Health supplements/ health food |
62 |
55.9% |
|
332 |
83.0% |
|
Topical medications – creams, gels, rubs etc. |
74 |
66.7% |
|
174 |
43.5% |
|
Natural remedies/ herbals/ TCM |
34 |
30.6% |
|
116 |
29.0% |
|
Physiotherapy/ massage |
55 |
49.5% |
|
166 |
41.5% |
|
TENS |
11 |
9.9% |
|
78 |
19.5% |
|
Others |
1 |
0.9% |
|
0 |
0.0% |
|
Nothing at all |
11 |
9.9% |
|
0 |
0.0% |
|
Supplement used P3Mb |
|
|
|
|
|
|
Undenatured Type II Collagen (UC-II) |
32 |
46.4% |
|
400 |
100.0% |
|
Glucosamine |
45 |
65.2% |
|
177 |
44.3% |
|
Caltrate® UC-II usage duration (months)*** |
|
|
|
|
|
|
Mean (SD) |
- |
|
6.1 (1.7) |
||
|
3 months |
- |
- |
|
44 |
11.0% |
|
4 months |
- |
- |
|
41 |
10.3% |
|
5 months |
- |
- |
|
87 |
21.8% |
|
> 6 months |
- |
- |
|
228 |
57.0% |
|
Caltrate® UC-II usage frequency (weekly)b |
|
|
|
|
|
|
Mean (SD) |
- |
|
5.1 (1.4) |
||
|
3 times/week |
- |
- |
|
65 |
16.3% |
|
4-5 times/week |
- |
- |
|
207 |
51.8% |
|
Daily |
- |
- |
|
128 |
32.0% |
Abbreviations: -, Non-applicable; P6M, past 6 months; P3M, past 3 months; TENS, Transcutaneous Electrical Nerve Stimulation; TCM,
Traditional Chinese Medicine
aCondition experienced was assessed for the past 3 months at Phase 1 and for the past 12 months at Phase 2
bThe number of respondents assessed for supplement brand in Phase 1 is 69
cCaltrate® UC-II usage profile was assessed in Phase 2 study only.
Table 2: Type of joint treatment supplements taken by participants in phase 1
|
Type of joint treatment supplements |
Phase 1 (n=69*) |
|
|
n |
% |
|
|
Caltrate UCII |
18 |
26.1% |
|
Move Free UCII |
13 |
18.8% |
|
Viatril-S Glucosamine |
27 |
39.1% |
|
Suntory Glucosamine |
24 |
34.8% |
|
Kirkland Glucosamine |
10 |
14.5% |
|
BRANDS UCII |
16 |
23.2% |
|
Others |
1 |
1.4% |
|
None of the above |
13 |
18.8% |
*A subset of participants in phase 1 who indicated that they consumed ‘Oral medications available without prescription/ over-the-counter’ or ‘Health supplements/ health food’
Respondents in Phase 2 comprised 400 UC-II users, within the age of 40 to 49 years (45.3%). More than one-third of participants reported occurrences of atopic dermatitis (40.3%), sore throat (36.5%), and headaches (36.3%) over the past 12 months. In addition, a major proportion of respondents (61.0%) indicated experiencing nonspecific joint-related injuries, followed by joint sprains (24.3%) and bruises (22.5%) within the previous six months. Four in five respondents (83.0%) reported consuming joint health supplements as part of joint health treatment over the past three months, and 57% had been using UC-II for over 6 months, typically at a frequency of 5 times a week.
Effect of joint symptoms on daily activities and desired benefits from joint supplements
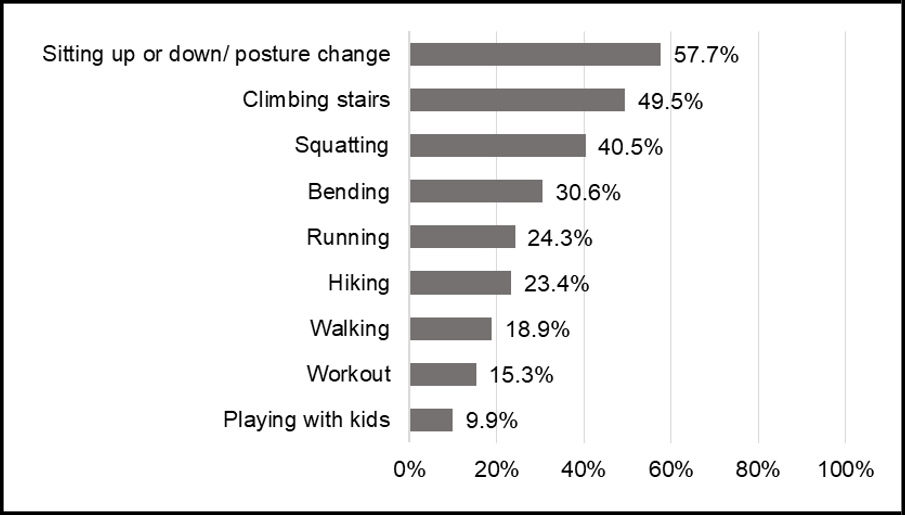
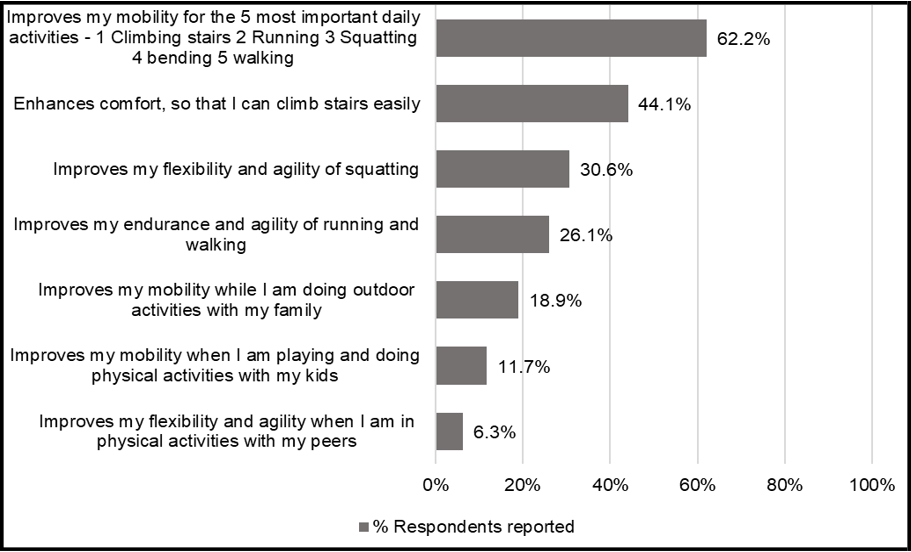
Respondents encountered difficulties in daily activities due to joint symptoms, with the most affected mobility aspects being posture change and climbing stairs (57.7% and 49.5%, respectively), followed by squatting (40.5%), bending (30.6%), running (24.3%), and hiking (23.4%) (Figure 1a). Consequently, the most important benefits that respondents sought in a joint health supplement included the improvement in the five daily mobility areas (Figure 1b). 62.2% of the respondents have rated "improves my mobility for the 5 most important daily activities -1 Climbing stairs 2 Running 3 Squatting 4 bending 5 walking" as their first or second priority when selecting a joint health supplement.
Figure 1a: Daily activities impacted by joint symptoms.
Figure 1b: Desired benefits from joint health supplements
Figure 1a&b. Daily activities impacted by joint symptoms and desired benefits from joint health supplements. (a) The aggregated percentages of daily activities most affected by joint symptoms reported by the respondents. (b)The aggregated percentages of the most important benefits that respondents sought for in a joint supplement. Data based on Phase 1 study (n = 111).
Perceived benefits in joint symptoms, mobility, and endurance following 90 days of UC-II consumption
In general, nine in ten respondents reported improvements in across all joint symptoms following their use of UC-II over the past 3 months, encompassing reduced joint discomfort (90.8%), decreased joint and knee joint stiffness (88.0% and 89.5%, respectively) as well as improvement in joint flexibility (90.3%) and range of motion (87.5%).
Additionally, 90% of UC-II users reported perceived improvement in the 6 most important daily mobility areas, including climbing stairs, walking, squatting, bending, running, and posture change. Specifically, over 90% of users observed a significant doubling in their daily walking steps to an average of 4,087 steps after UC-II intake (p < 0.001) (Table 3). Additionally, more than 80% of users reported enhancements in discomfort, duration, and walking speed (Table 3). Half of the respondents in Phase 1 reported significant limitations in climbing stairs due to joint symptoms, resulting in fewer than 5 flights of stairs being climbed daily. However, over 80% of UC-II users reported improved endurance and comfort when climbing stairs (Table 4). Regular UC-II users also noticed improvements in the duration, distance, and speed of running, with less discomfort (Table 4). Quantitatively, nine in ten users reported a significant increase in their running frequency to approximately five times per week, along with a two-fold increase in their average running distance and running time (p < 0.001) (Table 3). Moreover, users experienced reduced discomfort during squatting, with a significant two-fold increase in squatting frequency on average following UC-II usage (p< 0.001) (Table 3 and Table 4). Respondents also reported benefits in bending, providing increased comfort during daily activities and physical exercises such as yoga and stretching exercises (Table 4).
Table 3. Perceived quantitative changes in mobility benefits after taking Caltrate® UC-II
|
Mobility areas |
Before taking Caltrate® UC-II; Mean (SD) |
After taking Caltrate® UC-II; Mean (SD) |
Mean Difference; Mean (SD) |
p-value |
|
Walking |
|
|
|
|
|
Daily steps count |
3,367.5 (2,320.1) |
7,454.4 (2,594.1) |
4,086.9 (2,472.7) |
<0.001* |
|
Running |
|
|
|
|
|
Frequency/week |
2.3 (1.8) |
5.3 (3.4) |
2.9 (2.2) |
<0.001* |
|
Distance (km) / session |
1.8 (1.1) |
4.0 (1.5) |
2.3 (1.3) |
<0.001* |
|
Duration (min) / session |
16.4 (8.4) |
36.3 (12.1) |
20.0 (12.6) |
<0.001* |
|
Squatting activities |
|
|
|
|
|
Squat counts/ 30 seconds |
9.3 (7.4) |
18.1 (11.6) |
9.3 (7.6) |
<0.001* |
Abbreviations: SD, Standard deviation; km, kilometers; min, minutes
*Significant results
Table 4: Perceived benefits in 5 mobility areas (i.e., walking, climbing stairs, running, squatting, and bending) after 90 days of Caltrate® UC-II intake (N = 400)
|
Mobility Areas Attribute Statements |
Percentage reported (%) |
|||
|
Agree |
Neutral |
Disagree |
||
|
Walking |
|
|
|
|
|
1 |
Since I started taking Caltrate® UC-II, my mobility can last longer |
79.3% |
19.3% |
1.5% |
|
2 |
I can walk 30 min or more without discomfort, after taking Caltrate® UC-II for 90 days |
80.0% |
18.8% |
1.3% |
|
3 |
Since I started taking Caltrate® UC-II, I enjoy walking more |
80.0% |
17.5% |
2.5% |
|
4 |
After taking UC-II for 90 days, I can walk 7,000 steps a day or more without discomfort |
80.3% |
17.5% |
2.3% |
|
5 |
I can walk longer distance after taking Caltrate® UC-II for 90 days |
80.8% |
16.5% |
2.8% |
|
6 |
I can walk longer distances without discomfort after taking Caltrate® UC-II for 90 days |
80.8% |
16.3% |
3.0% |
|
7 |
I can walk for longer time after taking Caltrate® UC-II for 90 days |
82.0% |
16.5% |
1.5% |
|
8 |
I enjoy walking more often after taking Caltrate® UC-II for 90 days |
83.8% |
14.5% |
1.8% |
|
9 |
I have noticed improvement in the duration, discomfort, and speed while walking since I started taking Caltrate® UC-II |
84.5% |
14.8% |
0.8% |
|
Climbing stairs |
|
|
|
|
|
1 |
I can climb 10 flights of stairs with no discomfort after taking Caltrate® UC-II for 90 days |
82.0% |
10.8% |
3.0% |
|
2 |
I can climb 5 flights of stairs with no discomfort after taking Caltrate® UC- II for 90 days |
82.5% |
11.0% |
1.8% |
|
3 |
After taking Caltrate® UC-II for 90 days, I now choose to take the stairs more often instead of elevator |
83.8% |
10.5% |
2.5% |
|
4 |
After taking Caltrate® UC-II for 90 days, I can comfortably climb stairs |
84.0% |
11.3% |
2.3% |
|
5 |
After taking Caltrate® UC-II for 90 days, I can climb more floors, with less discomfort |
84.5% |
10.3% |
1.8% |
|
6 |
I can climb stairs with less discomfort after taking Caltrate® UC-II for 90 days |
86.0% |
8.3% |
1.8% |
|
Running |
|
|
|
|
|
1 |
I can run longer distance after taking Caltrate® UC-II |
78.1% |
19.4% |
2.6% |
|
2 |
I can run comfortably after taking Caltrate® UC-II for 90 days |
82.7% |
15.6% |
1.8% |
|
3 |
Since I started taking Caltrate® UC-II, I enjoy running more |
83.2% |
14.5% |
2.3% |
|
4 |
I have noticed an improvement in the duration, discomfort, and speed while running since I started taking Caltrate® UC-II |
79.9% |
18.1% |
2.0% |
|
5 |
I can run longer distances with less discomfort after taking Caltrate® UC-II for 90 days |
82.9% |
15.6% |
1.5% |
|
6 |
I can run with my friends and family and don’t have the fear of getting left behind after taking Caltrate® UC-II |
79.1% |
19.6% |
1.3% |
|
7 |
After taking Caltrate® UC-II for 90 days, I can run 2km or more a day with less discomfort |
79.9% |
18.6% |
1.5% |
|
8 |
After taking Caltrate® UC-II for 90 days, I can run for a longer time with less discomfort |
78.8% |
19.6% |
1.5% |
|
Squatting |
|
|
|
|
|
1 |
I can squat longer after taking Caltrate® UC-II for 90 days |
83.0% |
13.3% |
0.8% |
|
2 |
I can do a greater number of squats with less discomfort, after taking Caltrate® UC-II for 90 days |
84.8% |
11.5% |
1.5% |
|
3 |
I can squat comfortably after taking Caltrate® UC-II for 90 days |
87.3% |
9.8% |
0.8% |
|
4 |
After taking Caltrate® UC-II for 90 days, I can comfortably use / get on and off the toilet |
86.5% |
10.8% |
1.0% |
|
5 |
After taking Caltrate® UC-II for 90 days, I can comfortably squat and lift things off the floor |
86.5% |
10.3% |
1.0% |
|
6 |
After taking Caltrate® UC-II for 90 days, I can squat without discomfort and get things from bottom shelves in supermarkets/ from refrigerators |
88.3% |
8.3% |
0.5% |
|
7 |
After taking Caltrate® UC-II for 90 days, I can squat without discomfort to clean/ mop the floor |
85.8% |
11.3% |
1.0% |
|
8 |
After taking Caltrate® UC-II for 90 days, I can comfortably squat and feed the pets |
83.5% |
11.5% |
1.5% |
|
9 |
After taking Caltrate® UC-II for 90 days, I can comfortably squat and tie my shoelaces |
86.8% |
9.5% |
1.0% |
|
10 |
Standing up from a crouching/ squatting position is easier after taking Caltrate® UC-II for 90 days |
85.3% |
11.3% |
1.3% |
| Bending | ||||
|
1 |
After taking Caltrate® UC-II for 90 days, my bending movements are smoother and more comfortable |
87.8% |
9.5% |
0.5% |
|
2 |
After taking Caltrate® UC-II for 90 days, I can bend comfortably to put on/ take off socks or shoes |
86.8% |
11.5% |
0.8% |
|
3 |
After taking Caltrate® UC-II for 90 days, I can bend comfortably and pick up objects from the floor |
85.8% |
12.3% |
0.5% |
|
4 |
After taking Caltrate® UC-II for 90 days, I can bend comfortably and do stretching exercise |
86.3% |
10.5% |
0.8% |
|
5 |
After taking Caltrate® UC-II for 90 days, I can bend comfortably and sit in the car |
88.5% |
8.0% |
0.5% |
Perceptions and Satisfaction of Caltrate® UC-II
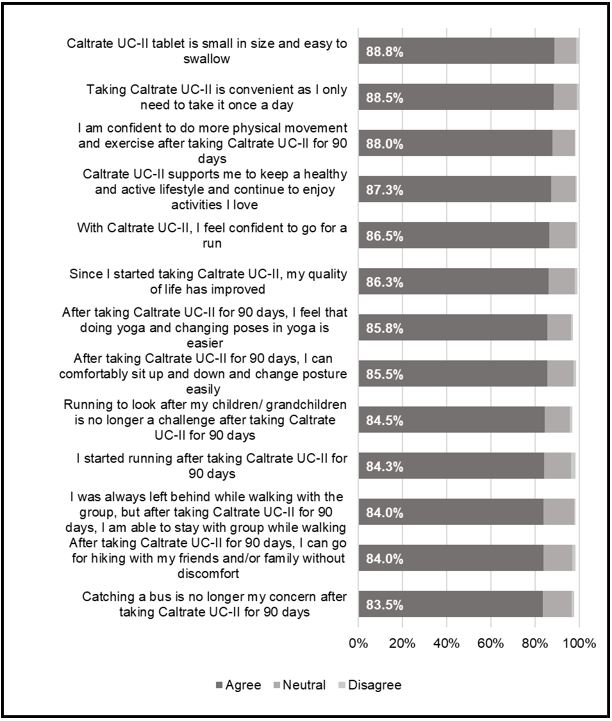
Approximately 90% of Caltrate® UC-II users appreciated the smaller tablet size and once-a-day intake regimen for its convenience (Figure 2). Corresponding to the improvements in joint symptoms with regular Caltrate® UC-II intake, users also reported enhanced QoL and lifestyle improvements. Over 80% of users reported a substantial positive emotional impact and improved QoL, as they are able to engage in increased physical activities and exercise, contributing to a healthier and more active lifestyle (Figure 2).
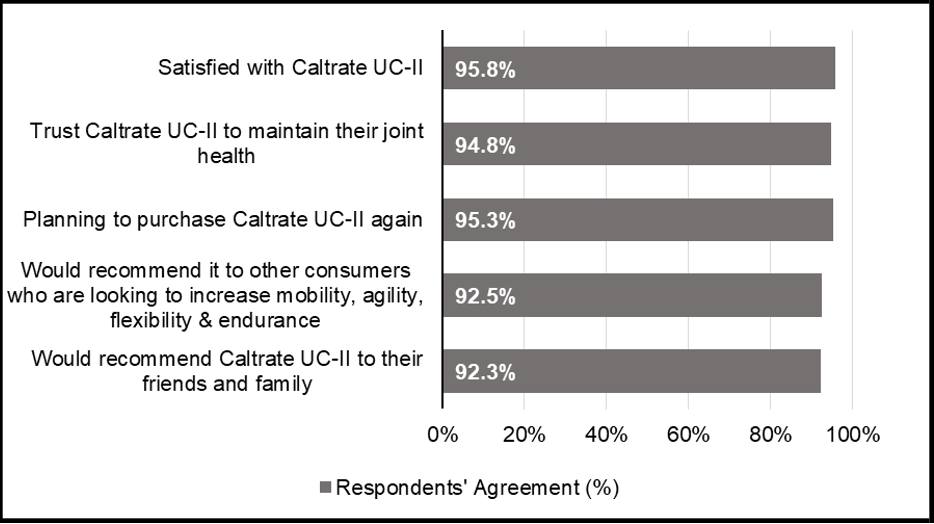
Overall, 95.8% of regular users were satisfied with Caltrate® UC-II to maintain their joint health, with over 90% of them trusting, planning to continue purchasing it, and willing to recommend it to others (Figure 3).
Figure 2: Lifestyle and quality of life (QoL) related perception and satisfaction among Caltrate® UC-II user. Data based on 400 respondents’ agreement (Phase 2) to the listed lifestyle and QoL statements. The aggregated score of respondents who selected ‘Somewhat Agree’ and ‘Completely Agree’ in a 5-point rating scale is presented.
Figure 3: Overall satisfaction with Caltrate® UC-II. Data based on 111 respondents’ agreement (Phase 1). The aggregated percentages of respondents’ satisfaction with Caltrate® UC-II are presented.
Discussion
This cross-sectional study conducted in Taiwan explored the impact of joint symptoms in daily mobility areas, the desired health benefits consumers are looking for in joint supplements and the potential benefits consumers experienced after consumption of a joint health ingredient – Type II undenatured collagen (UC-II). The study demonstrated that joint symptoms greatly affected different mobility areas, with posture changes such as standing up and sitting down as the most impacted area, followed by walking, climbing stairs, running, squatting, and bending. Respondents also valued joint supplements that could help improve in these six mobility areas. Regular users of UC-II (consumption of Caltrate® UC-II for more than 90 days) perceived positive changes in overall joint symptoms, mobility, as well as lifestyle and QoL-related benefits. This is corroborated by the findings of Lugo et al. [26] indicating that supplementation with 40mg of UC-II over a 90-day period enhanced joint mobility. In general, users reported high rates of satisfaction with Caltrate® UC-II, and thus, this confirmed the consumer acceptance of consuming Caltrate® UC-II to relieve their joint discomfort.
The results of the present study indicated that a substantial proportion, varying from two to five in every ten individuals within the general population in Taiwan with joint symptoms, encountered limitations in the five key mobility aspects: walking, climbing stairs, running, squatting, and bending. This finding is in line with existing literature where mobility limitations in daily activities are prominently associated with joint symptoms, encompassing pain, stiffness, and discomfort. [36,37] More importantly, these mobility limitations may eventually contribute to worsened mobility conditions and reduced quality of life. A cross-sectional study showed that decreased of physical activities with Rheumatoid Arthritis (RA) induced knee pain potentially speed age-related muscle that could eventually lead to loss of independence.[38]
Posture changes such as standing up and sitting down was also identified as the daily acitvitiy that was most impacted by joint symptoms, with over half of the respondents reporting challenges in standing up and/or sitting down due to joint symptoms.
The results of the perceived benefits of 90 days of regular consumption of UC-II on joint flexibility and mobility, reported by nearly 90% of the respondents in the present study, complement previous findings from RCTs conducted on healthy adults with no history of joint diseases. For instance, four to six months of UC-II supplementation improved knee flexibility on the range of motion extension and flexion in adults with activity-related joint discomfort aged < 65, compared to placebo control.[26,35] Schön, Knaub, Alt, Durkee, Saiyed and Juturu [35] further observed this improvement as early as 8 weeks (or 56 days). Another RCT that used daily step count to assess joint mobility, via a step counter, found that participants in the UC-II group took significantly more steps (increased by 217 steps) compared to the placebo group (decreased by 530 steps.[33] These findings could be attributed to the reduced joint discomfort rendering into increased mobility, where the same studies also found an increased time or more repetition before the onset of joint pain when subjected to intervention-controlled mobility tests (i.e., step mill test).[26,35] Therefore, the results of our study, where approximately 90% of respondents reported experiencing alleviation in joint discomfort and stiffness after 90 days regular use of UC-II, support existing published clinical findings. It further provided a real-world perspective to the use of UC-II on healthy adult populations.
Over 95% of consistent users of Caltrate® UC-II expressed satisfaction with their UC-II consumption. This satisfaction corresponded to the perceived positive impact on lifestyle changes and QoL following UC-II intake. Users reported increased confidence and comfort in daily activities, as well as a greater willingness to engage in an active lifestyle, including activities like yoga, running, and hiking. Previous studies have also shown that UC-II effectively enhanced joint comfort, subsequently improving the health-related QoL in OA patients.[31,39] Studies involving healthy adults with joint discomfort have also suggested a reduction in pain duration and recovery time within the UC-II group compared to the placebo group, potentially suggesting confidence of engaging in more physical movements and exercise in these populations.[26,33]
The primary strength of the current study was the investigation of real-world evidence, allowing insight into the perceived benefits of a product or intervention that has undergone thorough evaluation for safety and efficacy, emphasizing the relevance of this methodology. However, this approach resulted in a non-causal interpretation of the finding, and the study's outcomes necessitate cautious interpretation due to a few limitations. The study population predominantly comprised younger adults with an average age of 46, limiting generalizability to an older population, as observed in previous studies on healthy populations with joint symptoms.[26,33,35] Older adults often report a higher incidence of knee pain,[40] and a study suggested that the benefits of UC-II might extend more effectively to this demographic (> 50 years).[34] Furthermore, the Phase 2 study exclusively recruited respondents who were regular users of Caltrate® UC-II (> 90 days) in Taiwan, limiting the generalizability of the results to non-frequent users and individuals outside Taiwan. The recruitment method through an online survey panel, with the requirement of digital device access, may have excluded individuals with limited technology access. Additionally, findings related to Caltrate® UC-II consumption and perceived benefits on joint symptoms and daily mobility areas could be subject to self-reported bias, including recall bias and social desirability.[41] Lastly, there is an absence of a control group in this study, which could provide a more robust comparison to evaluate the effectiveness of Caltrate® UC-II. We encourage future studies to incorporate a control group to further validate and enhance the findings of this research.
Conclusion
This 2-phase cross-sectional study demonstrated the substantial impact of joint symptoms on diverse mobility aspects as well as the perceived benefits and improvements in joint symptoms, mobility, and overall QoL following regular Caltrate® UC-II consumption. Our findings contribute to a better understanding of real-world benefits with regular intake of Caltrate® UC-II, amongst the healthy population in Taiwan experiencing joint symptoms. The high satisfaction rates among users confirmed the acceptance of consuming Caltrate® UC-II to optimize their joint and mobility health.
Acknowledgments
The authors would like to thank Shi Mun Yee for the support in data analysis and report writing; Christler John Real for the support in data processing and analysis; and Freda Jia Xin Jong for the support in report writing.
Authors’ contributions
All authors contributed to the conception of the work and interpreted the data for the work. They revised the work and approved the final version. They agreed to take responsibility for the accuracy or integrity of the work as a whole. They had complete access to the study data that supports the publication. All authors have read and agreed to the published version of the manuscript.
Disclosure Statement
IQVIA has received research funding from GlaxoSmithKline Consumer Healthcare Pte Ltd. The authors are employees of IQVIA and GlaxoSmithKline Consumer Healthcare Pte Ltd. The authors report no other potential conflicts of interest.
Funding
This research was funded by GlaxoSmithKline Consumer Healthcare Pte Ltd.
Ethics approval and informed consent
This was an observational study. No ethical approval and clinical trial registration was required due to the non-interventional nature of the study. This study was conducted according to the guidelines laid down in the Declaration of Helsinki. Informed consent was obtained from all individual respondents before their inclusion in the study. No personal identifiable information of the participants was collected in the course of this study.
About the authors
Vandana Garg is the Director, Medical & Scientific Affairs (Southeast Asia and Taiwan) at Haleon. She has worked in the healthcare industry for more than 15 years with a vast experience in medical affairs across multiple markets and therapeutic areas, in both pharmaceuticals & consumer healthcare. Vandana is passionate about bringing more science backed healthcare innovations to consumers and in her current role at Haleon, she provides medical leadership on innovation and evidence generation for brands such as Centrum and Caltrate.
Aida Gadzhieva-Moore is an Associate Principal at IQVIA Consumer Health in Singapore, where she leads real-world evidence and claim substantiation practice. She holds a BSc in Economics and Management from the London School of Economics and Political Science, UK. With over 15 years of experience in research and analytics across healthcare and other categories, her work focuses on establishing best practices for real-world data generation within Consumer Health industry.
An Yi Wong holds a diploma in Biomedical Science from Ngee Ann Polytechnic and is currently pursuing her Bachelor’s degree with Honours in Biomedical Science and Biobusiness from Nanyang Technological University. An Yi has experience conducting research at both academic and research institutions. An Yi is currently a Medical and Scientific Affairs Intern at Haleon.
Joseph Lin is the Medical and Scientific Manager in Haleon, with has medical and regulatory affairs experience in healthcare and pharmaceutical industries over 15 years, and currently support Southeast Asia and Taiwan business unit.
Sheryl S. L. Tan holds a Ph.D. from the Yong Loo Lin School of Medicine, National University of Singapore, and did her post- doctorate fellowship in Lung Diseases. She has 10 years of research experience in pre-clinical drug development, in the fields of immunology, cancer and respiratory. Sheryl is currently the Head of Medical & Scientific Affairs for Haleon Wider Asia, where she leads real world evidence generation and research partnerships.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author(s).
References
- Cieza A, Causey K, Kamenov K, Hanson SW, Chatterji S, et al. (2020) Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 396(10267): 2006-2017.
- El-Tallawy SN, Nalamasu R, Salem GI, LeQuang JAK, Pergolizzi JV, et al. (2021) Management of Musculoskeletal Pain: An Update with Emphasis on Chronic Musculoskeletal Pain. Pain Ther. 10(1): 181-209.
- World Health Organization. (2022) Musculoskeletal health.
- Woo T, Lau L, Cheng N, Chan P, Tan K, Gardner A (2017) Efficacy of oral collagen in joint pain-osteoarthritis and rheumatoid arthritis. J Arthritis. 6: 2.
- Dasgupta E, Yap JLL, Kunjunee KK, Choong XY, Soh WW, et al. (2021) Prevalence of musculoskeletal pain in two primary care clinics in a mid-sized town's urban population in Malaysia. Malays Fam Physician. 16(1): 93-102.
- Kopec JA, Heath AJ, Sayre EC, Cibere J, Li LC, et al. (2022) Prevalence of joint-specific osteoarthritis and joint pain in British Columbia, Canada. Rheumatology International. 42(9): 1623-1628.
- Mat S, Jaafar MH, Ng CT, Sockalingam S, Raja J, et al. (2019) Ethnic differences in the prevalence, socioeconomic and health related risk factors of knee pain and osteoarthritis symptoms in older Malaysians. PLOS ONE. 14(11): e0225075.
- Fransen M, Bridgett L, March L, Hoy D, Penserga E, et al. (2011) The epidemiology of osteoarthritis in Asia. International journal of rheumatic diseases. 14(2): 113-121.
- Törmälehto S, Aarnio E, Mononen ME, Arokoski JPA, Korhonen RK, et al. (2019) Eight-year trajectories of changes in health- related quality of life in knee osteoarthritis: Data from the Osteoarthritis Initiative (OAI). PLoS One. 14(7): e0219902.
- Thiem U, Lamsfuß R, Günther S, Schumacher J, Bäker C, et al. (2013) Prevalence of self-reported pain, joint complaints and knee or hip complaints in adults aged ≥ 40 years: a cross-sectional survey in Herne, Germany. PLoS One. 8(4): e60753.
- Kang X, Fransen M, Zhang Y, Li H, Ke Y, et al. (2009) The high prevalence of knee osteoarthritis in a rural Chinese population: the Wuchuan osteoarthritis study. Arthritis Rheum. 61(5): 641-7.
- Liu Q, Wang S, Lin J, Zhang Y (2018) The burden for knee osteoarthritis among Chinese elderly: estimates from a nationally representative study. Osteoarthritis Cartilage. 26(12): 1636-1642.
- Chia YC, Beh HC, Ng CJ, Teng CL, Hanafi NS, et al. (2016) Ethnic differences in the prevalence of knee pain among adults of a community in a cross-sectional study. BMJ Open. 6(12): e011925.
- Jhun HJ, Sung NJ, Kim SY (2013) Knee pain and its severity in elderly Koreans: prevalence, risk factors and impact on quality of life. J Korean Med Sci. 28(12): 1807-13.
- Lee A, Lae KS (2015) Prevalence and Risk Factors of Knee Pain in Korean Adults: Results from Korea National Health and Nutrition Examination Survey, 2010-2012. Journal of health informatics. 40(3): 129-139.
- Freemont A, Hoyland J (2007) Morphology, mechanisms and pathology of musculoskeletal ageing. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland. 211(2): 252-259.
- American Academy of Orthopaedic Surgeons. Effects of Aging. 2009.
- Ferrucci L, Cooper R, Shardell M, Simonsick EM, Schrack JA, et al. (2016) Age-related change in mobility: perspectives from life course epidemiology and geroscience. Journals of gerontology series a: biomedical sciences and medical sciences. 71(9): 1184- 1194.
- Bestwick-Stevenson T, Ifesemen OS, Pearson RG, Edwards KL (2021) Association of sports participation with osteoarthritis: a systematic review and meta-analysis. Orthopaedic journal of sports medicine. 9(6): 23259671211004554.
- World Health Organization. Osteoarthritis.
- Chen T, Or CK, Chen J (2020) Effects of technology-supported exercise programs on the knee pain, physical function, and quality of life of individuals with knee osteoarthritis and/or chronic knee pain: A systematic review and meta-analysis of randomized controlled trials. Journal of the American Medical Informatics Association. 28(2): 414-423.
- Lima YL, Lee H, Klyne DM, Dobson FL, Hinman RS, et al. (2023) How Do Nonsurgical Interventions Improve Pain and Physical Function in People With Osteoarthritis? A Scoping Review of Mediation Analysis Studies. Arthritis Care & Research. 75(6): 467-481.
- Aghamohammadi D, Dolatkhah N, Bakhtiari F, Eslamian F, Hashemian M (2020) Nutraceutical supplements in management of pain and disability in osteoarthritis: a systematic review and meta-analysis of randomized clinical trials. Scientific Reports. 10(1): 20892.
- D’Adamo S, Cetrullo S, Panichi V, Mariani E, Flamigni F, et al. (2020) Nutraceutical Activity in Osteoarthritis Biology: A Focus on the Nutrigenomic Role. Cells. 9(5): 1232.
- Bagchi D, Misner B, Bagchi M, Kothari SC, Downs BW, et al. (2002) Effects of orally administered undenatured type II collagen against arthritic inflammatory diseases: a mechanistic exploration. Int J Clin Pharmacol Res. 22(3-4): 101-10.
- Lugo JP, Saiyed ZM, Lau FC, Molina JP, Pakdaman MN, et al. (2013) Undenatured type II collagen (UC-II®) for joint support: a randomized, double-blind, placebo-controlled study in healthy volunteers. J Int Soc Sports Nutr. 10(1): 48.
- Tong T, Zhao W, Wu YQ, Chang Y, Wang QT, et al. (2010) Chicken type II collagen induced immune balance of main subtype of helper T cells in mesenteric lymph node lymphocytes in rats with collagen-induced arthritis. Inflammation Research. 59(5): 369-377.
- Gencoglu H, Orhan C, Sahin E, Sahin K (2020) Undenatured type II collagen (UC-II) in joint health and disease: a review on the current knowledge of companion animals. Animals. 10(4): 697.
- Bagi CM, Berryman ER, Teo S, Lane NE (2017) Oral administration of undenatured native chicken type II collagen (UC-II) diminished deterioration of articular cartilage in a rat model of osteoarthritis (OA). Osteoarthritis and Cartilage. 25(12): 2080-2090.
- Orhan C, Juturu V, Sahin E, Tuzcu M, Ozercan IH, et al. (2021) Undenatured Type II Collagen Ameliorates Inflammatory Responses and Articular Cartilage Damage in the Rat Model of Osteoarthritis. Original Research. Frontiers in Veterinary Science. 8: 617789.
- Crowley DC, Lau FC, Sharma P, Evans M, Guthrie N, et al. (2009) Safety and efficacy of undenatured type II collagen in the treatment of osteoarthritis of the knee: a clinical trial. Int J Med Sci. 6(6): 312-21.
- Lugo JP, Saiyed ZM, Lane NE (2016) Efficacy and tolerability of an undenatured type II collagen supplement in modulating knee osteoarthritis symptoms: a multicenter randomized, double-blind, placebo-controlled study. Nutr J. 15: 14.
- Knaub K, Schön C, Alt W, Durkee S, Saiyed Z, et al. (2022) UC- II® Undenatured Type II Collagen Reduces Knee Joint Discomfort and Improves Mobility in Healthy Subjects: A Randomized, Double-Blind, Placebo-Controlled Clinical Study. J Clin Trials. 12(1): 1000492.
- Saiyed Z, Durkee S, Bowman J, Juturu V (2021) Efficacy of UC- II® undenatured type II collagen on knee joint function in healthy subjects: an exploratory post hoc analyses of a randomized double blind, placebo - controlled trial. The FASEB Journal. 35(S1).
- Schön C, Knaub K, Alt W, Durkee S, Saiyed Z, et al. (2022) UC- II Undenatured Type II Collagen for Knee Joint Flexibility: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Clinical Study. J Integr Complement Med. 28(6): 540-548.
- Clynes MA, Jameson KA, Edwards MH, Cooper C, Dennison EM (2019) Impact of osteoarthritis on activities of daily living: does joint site matter? Aging Clinical and Experimental Research. 31(8): 1049-1056.
- Yu H, Huang T, Lu WW, Tong L, Chen D (2022) Osteoarthritis Pain. International Journal of Molecular Sciences. 23(9): 4642.
- Torii M, Hashimoto M, Hanai A, Fujii T, Furu M, et al. (2019) Prevalence and factors associated with sarcopenia in patients with rheumatoid arthritis. Mod Rheumatol. 29(4): 589-595.
- Sadigursky D, Magnavita VFS, Sá CKC, Monteiro HS, Braghiroli OFM, et al. (2022) Undenatured Collagen Type Ii For The Treatment Of Osteoarthritis Of The Knee. Acta Ortop Bras. 30(2): e240572.
- McAlindon TE, Cooper C, Kirwan JR, Dieppe PA (1992) Knee pain and disability in the community. Br J Rheumatol. 31(3): 189- 92.
- Bauhoff S (2014) Self-Report Bias in Estimating Cross-Sectional and Treatment Effects. In: Michalos AC, ed. Encyclopedia of Quality of Life and Well-Being Research. Springer Netherlands. 2014: 5798-5800.