Weiwu Wang1, Bo Fu2, Minwu Xu3, Yixiang Wang3, Guowei Li4, Tao Liu5, Rong Zhang5, Dingmiao Wang6, Meng Liyuan7, Wang Jieqiong7, Yang Hongfei7, Min Qing7*
1Hunan Ruitongtang Medical Co. Ltd., Changsha, Hunan, 437100.
2TCM department, Xianning Centreal Hospital, Xianning, Hubei, 437500.
3Tongshan County People's Hospital, Hubei, 437100.
4Chongyang County People's Hospital, Hubei, 437100.
5Xianning First People's Hospital, Hubei, 437100.
6Xianning Central Hospital, Hubei, 437100.
7School of Pharmacy, Hubei University of Science and Technology, Xianning, China.
*Corresponding Author: Min Qing, School of Pharmacy, Hubei University of Science and Technology, Xianning, China.
Abstract
Objective: This retrospective cohort study aimed to investigate the therapeutic potential of FeiDuQing (FDQ), a traditional Chinese herbal decoction, in the management of Novel Coronavirus Pneumonia (NCP), particularly its impact on disease progression, length of hospitalization, and overall survival rates in NCP patients.
Methods: A cohort of 355 NCP patients consecutively admitted between January 15 and February 18, 2020, in Xianning City, China, was meticulously analyzed. Of these, 213 patients received FDQ treatment, while 142 patients did not. Disease progression, length of hospitalization, and overall survival rates were compared between the two groups. A hazard Ratio analysis was performed to assess the impact of FDQ therapy.
Results: Patients who received FDQ treatment exhibited a notable absence of disease progression to severe conditions, even among elderly patients, contrasting with a progression rate of 8.45% in the non-FDQ group. FDQ-treated patients experienced a significantly reduced length of hospitalization, with a lower percentage presenting mild residual symptoms upon discharge (1.40% vs. 11.19%). The cumulative survival rate was substantially higher in the FDQ group (79.04% vs. 32.60%), with a Hazard Ratio of 0.210 (95% CI: 0.123–0.357) favoring FDQ therapy. FDQ demonstrated a favorable safety profile with no reports of severe adverse effects.
Conclusion: This study provides substantial evidence supporting the potential therapeutic benefits of FDQ in managing NCP. Its efficacy in reducing disease progression, alleviating symptoms, and improving survival rates suggests that FDQ warrants further investigation as a promising candidate in the battle against COVID-19.
1. Introduction
The global outbreak of coronavirus disease 2019 (COVID-19), caused by the SARS-CoV-2 virus, has emerged as an unprecedented global health crisis. By September 6, 2020, this relentless pandemic had afflicted 213 countries, claiming a staggering 885,932 lives [1]. Alarming as this is, the situation is compounded by the absence of a definitive therapeutic agent approved by the U.S. Food and Drug Administration (FDA) for treating COVID-19. Remdesivir, the only FDA-authorized treatment, has yielded mixed results in pharmacological, pre-clinical, and clinical studies, often accompanied by a spectrum of side effects [2,3].
In stark contrast, China has garnered considerable success in curtailing the pandemic's impact, owing to a combination of stringent governmental measures, robust public surveillance, and the judicious integration of both conventional Western medicine and traditional Chinese medicine (TCM) [4,5]. While the principles of TCM may appear intricate and esoteric to Western medical practitioners and researchers, they have historically played a pivotal role in epidemic control within China. TCM was notably instrumental in addressing previous outbreaks, including the severe acute respiratory syndrome (SARS) and the H1N1 influenza pandemic [4,5].
This study embarks on a retrospective exploration of FeiDuQing (FDQ), a traditional Chinese herbal decoction employed in treating hospitalized COVID-19 patients in Xianning City, Hubei Province, China. Situated as the southern gateway to Wuhan, Xianning City is just 30 miles from the origin of the COVID-19 infections. The city, home to three million permanent residents, saw an influx of 200 thousand migrant workers returning from Wuhan just before the lockdown.
FDQ, the focal point of our investigation, comprises a composite of 12 herbal components, encompassing Divaricate saposhniovia root, Stir-fried atractylodes macrocephaly, Houttuynia cordata thumb, Radix paeonies rubra, Chinese thorax root, Platycodon grandiflorum, Fritillaria acuminate, Winter mulberry leaf, Ramulus Cinnamomi, Stir-fried white paeony root, Radix Isatidis, and Glycyrrhiza uralensis. Its inception occurred in the nascent stages of the COVID- 19 outbreak on January 20th. Following the successful treatment of a 70-year-old woman with a lengthy history of diabetes and the resolution of a family cluster infection involving five individuals, FDQ was officially incorporated into the COVID-19 treatment guidelines across various counties within Xianning City [6].
Remarkably, Xianning City achieved a milestone on March 17, 2020, becoming the first prefecture-level city in Hubei Province to discharge all its COVID-19 patients, boasting an impressive cure rate of 98.21% [6]. This feat was accomplished across seven tertiary hospitals within the city, with most patients benefiting from FDQ treatment. The core objective of this cohort study is to elucidate the pivotal role of FDQ in treating COVID-19, thereby advancing a promising therapeutic strategy for combating this formidable disease.
2. Methods
2.1 Study Design and Patient Selection
In this retrospective cohort study, we meticulously reviewed the medical records of all patients diagnosed with Novel Coronavirus Pneumonia (NCP) who were admitted to seven tertiary hospitals in Xianning City, Hubei Province, China, during the period between January 15 and February 18, 2020. The study timeline was defined by the interval from the first admission of NCP patients to our hospitals until the commencement of FeiDuQing (FDQ) treatment for all NCP patients in Xianning City.
Our inclusion criteria encompassed patients aged between 10 and 90 years who received a diagnosis of COVID-19 by the interim guidelines established by the World Health Organization. These patients were required to exhibit at least one relevant NCP symptom. Additionally, patients included in the FDQ treatment group were subjected to continuous FDQ treatment in the hospital for a minimum duration of 5 days. Exclusion criteria comprised asymptomatic infected individuals, pregnant women, patients treated with FDQ for less than 5 days, and those in critical condition with organ dysfunction stemming from other underlying medical conditions.
Consequently, our cohort comprised 355 NCP patients, with 213 in the FDQ treatment group and 142 in the non-FDQ treatment group. All patients provided written informed consent for their data to be collected and analyzed.
2.2 Treatment Procedures
Upon hospital admission, all patients, regardless of their group assignment, received the standard treatments recommended by the Chinese National Health Commission's Diagnosis and Treatment Protocol for COVID-19 (4th to 7th Edition). Additionally, patients in the FDQ treatment group received FeiDuQing (FDQ), an herbal decoction composed of 12 specific herbs, which was orally administered twice daily at a dose of 150 mL per administration. The FDQ was prepared by the Department of Medication Preparation at each hospital using a Decoctable Packaging Machine (Samyam, LCK2000).
Upon discharge, patients were required to meet specific criteria, including being afebrile for at least 3 consecutive days, significant improvement in respiratory symptoms, resolution of radiological abnormalities on chest radiographs or CT scans, and two successive negative COVID-19 RT-PCR tests conducted more than 24 hours apart.
3. Study Variables and Assessments
Our data collection encompassed various parameters, including patient demographics, presenting symptoms, length of hospital stay, laboratory results, and therapeutic interventions, all obtained from the patient's medical records. For symptoms and laboratory examinations, we considered values at the time of admission and at the time of discharge for assessment.
This study examined several vital features, including age, gender, duration of hospitalization, the proportion of patients progressing to a severe condition during their treatment course, as well as symptoms and laboratory values upon admission and discharge.
4. Statistical Analysis
Continuous variables were expressed as means with standard deviations (SD) and were compared using t-tests when they adhered to a normal distribution. For variables that did not exhibit normal distribution, medians along with interquartile ranges (IQRs) were presented, and comparisons were made using the Mann–Whitney U test. Categorical variables were reported as numbers and percentages and were subjected to chi-square tests for comparison.
Survival analysis was conducted using the Kaplan-Meier method and Cox proportional-hazard model (CPHM) to investigate the impact of each variable on the outcome (i.e., discharge status by February 26). Hazard ratios (HR) and their corresponding 95% confidence intervals (CI) were reported. The distinction in outcomes between the two groups was assessed using the Cox regression survival curve and hazard ratio.
All statistical tests were two-tailed, and statistical significance was determined at a P-value ≤0.05. Data analysis was carried out using SPSS version 24.0 (IBM Corp, Armonk, NY), and graphical representations were generated using Prism version 8.0 (GraphPad Software Inc, La Jolla, CA).
3. Results
3.1 Demographic Characteristics
This study encompassed a total of 355 patients diagnosed with COVID-19 Novel Coronavirus Pneumonia (NCP). Among them, 142 patients exclusively received the standard treatment protocol stipulated by the Diagnosis and Treatment Protocol for COVID-19 by the Chinese National Health Commission, while 213 patients were subjected to FDQ treatment in conjunction with the standard protocol. Table 1 provides a concise summary of the demographic characteristics of both groups.
The demographic profile between the FDQ and non-FDQ groups exhibited remarkable similarity. In the FDQ group, the average age was 45.65 years, with a standard deviation of 14.22 years, spanning an age range from 13 to 82 years. Notably, 60.1% of the FDQ group were male. Comparatively, the non-FDQ group had an average age of 47.74 years, with a standard deviation of 13.44 years and an age range from 15 to 79 years. In this group, 60.6% of the patients were male (Table 1).
To further refine our analysis, we stratified patients into three distinct age subgroups: those aged ≤ 30 years, those aged 31 to 60 years, and those aged ≥ 61 years. It is noteworthy that there were no statistically significant differences in terms of age or gender distribution among these age-defined subgroups.
Table 1: Basic information of all patients
|
characteristics |
FDQ (n=213) |
non- FDQ (n=142) |
P value |
|
Age (years), mean±SD |
45.65±14.22 |
47.74±13.44 |
0.166 |
|
≤30 years |
25.59±4.68 |
26.05±3.98 |
0.710 |
|
31-60 years |
45.44±8.414 |
47.47±8.02 |
0.067 |
|
≥61 years |
67.06±4.87 |
65.56±5.22 |
0.245 |
|
Sex (male), n(%) |
128(60.1%) |
86(60.6%) |
1.000 |
|
≤30 years |
25(67.6%) |
13(61.9%) |
0.776 |
|
31-60 years |
83(59.3%) |
57(60.6%) |
0.892 |
|
≥61 years |
20(55.6%) |
16(59.3%) |
0.802 |
3.2 Clinical Presentations
Upon admission, the most prevalent symptoms among COVID-19 patients included fever, cough, fatigue, and poor appetite. Notably, the prevalence rates of these common symptoms were comparable between the FDQ and non-FDQ treatment groups, as indicated in Table 2. Less common symptoms reported included chest pain or pressure, bodily soreness, chills, headache, diarrhea, nasal congestion or a runny nose, throat discomfort, dizziness, and nausea or vomiting. Meanwhile, other symptoms, such as abdominal pain and feeling flustered, were exceedingly rare, occurring in less than 1% of patients. Upon discharge, a noticeable discrepancy in symptom improvement was observed between the FDQ-treated group and the non-FDQ group. Specifically, only 3 out of the 213 patients (1.41%) who received FDQ treatment still exhibited symptoms. Conversely, among the non-FDQ group, a substantial proportion, consisting of 16 patients (11.19%), continued to experience various symptoms. These lingering symptoms in the non-FDQ group encompassed cough in 10 patients (7.0%), fatigue in 4 patients (2.8%), poor appetite in 1 patient (0.7%), shortness of breath in 2 patients (1.4%), nausea in 1 patient (0.7%), feeling flustered in 1 patient (0.7%), dyspnea in 1 patient (0.7%), and insomnia in 1 patient (0.7%) (Table 2).
Table 2: Comparison of symptoms and signs at admission and at discharge
|
|
FDQ |
(n=213) at discharge |
non-FDQ |
(n=142) |
||
|
symptoms and signs, n(%) |
|
|
|
|||
|
|
|
|
||||
|
|
at admission |
at admission |
at discharge |
|||
|
Fever |
155 (72.8%) |
0 |
(0%) |
101 (71.1%) |
0 |
(0%) |
|
Cough |
148 (69.5%) |
1 |
(0.5%) |
102 (71.8%) |
10 |
(7.0%) |
|
Fatigue |
70 (32.9%) |
1 |
(0.5%) |
35 (24.6%) |
4 |
(2.8%) |
|
Poor appetite |
39 (18.3%) |
0 |
(0%) |
15 (10.6%) |
1 |
(0.7%) |
|
Pain or pressure in the chest |
34 (16.0%) |
0 |
(0%) |
11 (7.7%) |
0 |
(0%) |
|
Sore body |
23 (10.8%) |
0 |
(0%) |
11 (7.7%) |
0 |
(0%) |
|
Chilly |
24 (11.3%) |
0 |
(0%) |
23 (16.2%) |
0 |
(0%) |
|
Headache |
17 (8.0%) |
0 |
(0%) |
5 (3.5%) |
0 |
(0%) |
|
Shortness of breath |
10 (4.7%) |
0 |
(0%) |
7 (4.9%) |
2 |
(1.4%) |
|
Congestion or runny nose |
10 (4.7%) |
1 |
(0.5%) |
5(3.5%) |
0 |
(0%) |
|
Diarrhea |
8 (3.8%) |
0 |
(0%) |
11 (7.7%) |
0 |
(0%) |
|
Throat discomfort |
6 (2.8%) |
0 |
(0%) |
10 (7.0%) |
0 |
(0%) |
|
Dizziness |
7 (3.3%) |
0 |
(0%) |
6 (4.2%) |
0 |
(0%) |
|
Nausea or vomiting |
5 (2.3%) |
0 |
(0%) |
10 (7.0%) |
1 |
(0.7%) |
|
Abdominal pain |
2 (0.9%) |
0 |
(0%) |
2 (1.4%) |
0 |
(0%) |
|
Dyspnea |
2 (0.9%) |
0 |
(0%) |
6 (4.2%) |
1 |
(0.7%) |
|
Flustered |
1 (0.5%) |
0 |
(0%) |
1 (0.7%) |
1 |
(0.7%) |
|
Excessive sweating |
1 (0.5%) |
0 |
(0%) |
0 (0%) |
0 |
(0%) |
|
Insomnia |
0 (0%) |
0 |
(0%) |
2 (1.4%) |
1 |
(0.7%) |
Furthermore, it is noteworthy that during the course of treatment, a concerning development occurred among the group of 142 patients who did not receive FDQ treatment. Specifically, 12 of these patients, constituting 8.45% of the non-FDQ treatment group, progressed to a severe condition, and unfortunately, one death was recorded.
Strikingly, in contrast to this, no patients who received FDQ treatment experienced progression to a painful condition. Remarkably, the clinical manifestations observed upon admission were comparable between the patients who received FDQ treatment and those who did not, as indicated in Table 3.
Table 3: Adverse events during treatment course
|
Adverse Event During Treatment Course, n(%) |
FDQ (n=213) |
non-FDQ (n=142) |
|
Developed to severe condition |
0 (0%) |
12 (8.45%) |
|
Death |
0 (0%) |
1 (0.70%) |
Laboratory tests were conducted both at the time of admission and upon discharge. White blood cell (WBC) count, lymphocyte count, and neutrophil count generally fell within the normal range for most patients in both groups. However, it's noteworthy that C-reactive protein (CRP) and high-sensitivity C-reactive protein (hs-CRP) levels exhibited considerable variation among individuals, primarily upon admission, as illustrated in Table 4.
Recent studies have established a connection between elevated CRP levels and an increased risk of composite poor outcomes (Reference). Surprisingly, our study did not identify a correlation between high CRP levels and a heightened risk of developing severe disease. This observation could be attributed to the limitations of our sample size or, intriguingly, the potential beneficial effects of FDQ treatment. Specifically, upon admission, 29 patients (13.55%) in the FDQ- treated group and 23 patients (16.20%) in the non-FDQ group exhibited CRP levels exceeding 50 mg/L. However, notably, none of the patients who received FDQ treatment progressed to a severe condition, whereas 12 (8.45%) of the patients treated solely with standard protocols developed extreme conditions during treatment. Encouragingly, upon discharge, both CRP and hs-CRP levels showed a significant decrease in most patients, signifying their recovery from NCP.
Table 4: Laboratory exam values at admission and at discharge
|
|
Normal Range |
FDQ At Admission |
(n=213) At Discharge |
non-FDQ At Admission |
(n=142) At Discharge |
|
White blood cell count, ˣ109/L |
3.5-9.5 |
5.01(3.74-6.08) |
5.41(4.40-6.63) |
4.13(3.40-5.51) |
5.29(4.21-6.66) |
|
Lymphocyte count, ˣ109/L |
1.1-3.2 |
1.16(0.96-1.60) |
1.58(1.28-1.83) |
1.08(0.77-1.42) |
1.44(1.20-1.73) |
|
Neutrophil count, ˣ109/L |
1.8-6.3 |
3.31(2.17-4.35) |
3.38(2.60-4.49) |
2.62(2.11-3.54) |
3.20(2.40-4.32) |
|
CRP, mg/L |
0-5 |
24.00(11.70-44.25) |
3.40(2.60-7.35) |
22.45(10.95-41.10) |
6.40(2.93-7.75) |
|
hs-CRP, mg/L |
<4.0 |
4.70(2.08-9.26) |
1.43(0.86-2.49) |
2.50(1.30-4.20) |
2.20(1.60-3.30) |
|
Data are median(IQR) |
|||||
3.3 The length of hospitalization
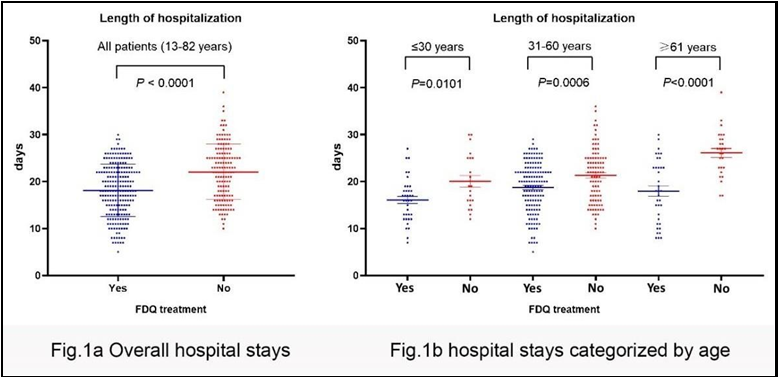
When comparing patients who received FDQ treatment to those without FDQ treatment, a significant difference in the length of hospital stay was evident, with the former group having a notably shorter stay (18.2 days vs. 22.1 days) (Figure 1a, Table 5). To discern whether age played a role in the duration of hospitalization, we stratified patients into three age categories: ≤30 years, 31-60 years, and ≥61 years. Within each age group, patients who received FDQ treatment exhibited significantly shorter hospital stays: 16.1 days for patients aged ≤30 years, 18.7 days for those aged 31-60 years, and 18.0 days for those aged ≥61 years (Figure 1b, Table 5).
Across all age groups, individuals aged ≤30 years had the briefest hospital stays, implying that younger patients generally experienced a more rapid recovery from COVID-19, irrespective of whether they received FDQ treatment. Intriguingly, in patients without FDQ treatment, there was a noticeable positive correlation between age and the length of hospital stay: 20.1 days for patients aged ≤30 years, 21.3 days for those aged 31-60 years, and an extended 26.1 days for those aged ≥61 years. This positive association, however, was not observed in FDQ-treated patients aged ≥30 years. Remarkably, even among FDQ-treated patients aged ≥61 years, the average hospital stay was merely 18.0 days (Table 5).
Table 5: Average length of hospitalization
|
Patients in age
≤30 years 31 - 60 years |
group
≥61 years |
All patient |
||
|
FDQ (n=213) (days) |
16.1±4.6 |
18.7±5.4 |
18.0±6.7 |
18.2±5.6 |
|
non- FDQ (n=142) (days) |
20.1±5.8 |
21.3±5.7 |
26.1±4.9 |
22.1±5.9 |
|
P value |
0.0101 |
0.0006 |
<0.0001 |
<0.0001 |
3.4 Cox-proportional hazard model analysis
To comprehensively evaluate the factors influencing patient outcomes, we conducted both univariate and multivariate Cox- proportional hazard model (CPHM) analyses. In this analysis, the event outcome was defined as "not discharged" from the hospital as of February 26. The results of these analyses consistently highlighted the absence of FDQ treatment as the sole risk factor associated with this outcome. In the univariate analysis, the hazard ratio (HR) was 0.20, with a 95% confidence interval (CI) ranging from 0.13 to 0.32. This relationship remained robust in the multivariate analysis, with an HR of 0.21 and a 95% CI spanning from 0.12 to 0.36 (Table 6).
Table 6: Hazard ratio (HR) of factors to the events
|
Factor |
Univariable HR(95% CI) |
analysis P value |
Multivariable |
analysis |
|
HR(95% CI) |
P value |
|||
|
Age |
1.02(1.00-1.03) |
0.052 |
1.02(1.00-1.03) |
0.053 |
|
Sex |
0.99(0.67-1.48) |
0.971 |
1.02(0.68-1.53) |
0.938 |
|
Treatment |
0.20(0.13-0.32) |
0.000 |
0.21(0.12-0.36) |
0.000 |
|
(FDQ vs Non-FDQ) |
||||
|
WBC |
1.05(1.02-1.09) |
0.078 |
1.04(1.00-1.09) |
0.074 |
|
LC |
1.03(0.78-1.36) |
0.839 |
1.09(0.83-1.42) |
0.547 |
|
NC |
1.13(1.01-1.25) |
0.289 |
1.06(0.94-1.19) |
0.364 |
|
CRP |
1.01(1.00-1.02) |
0.169 |
1.00(0.99-1.01) |
0.960 |
|
hsCRP |
1.00(0.95-1.06) |
0.998 |
1.00(0.95-1.06) |
0.915 |
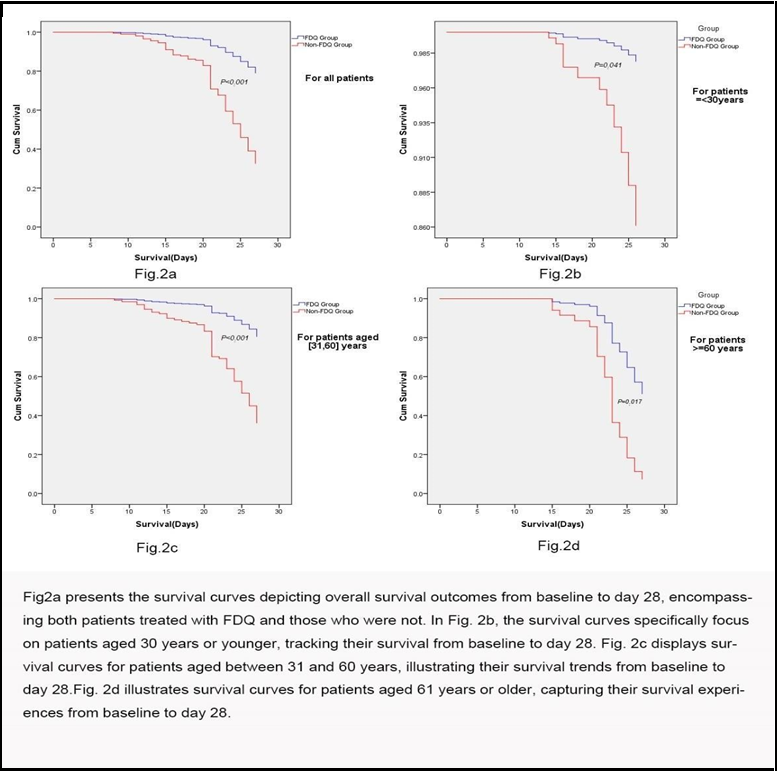
Additionally, we employed Cox regression survival curves to compare the treatment groups. These curves were generated for the entire patient cohort as well as for different age subgroups. The outcomes revealed a significantly higher discharge rate among patients receiving FDQ treatment compared to those without it (P<0.001) (Figure 2a). Cumulative probabilities were estimated, indicating that for all patients, the discharge rates were 79.04% for FDQ-treated patients and 32.60% for non-FDQ-treated patients, reinforcing the beneficial effects of FDQ treatment.
This trend persisted across age subgroups as well: in patients aged ≤30 years, the discharge rates were 98.36% for FDQ-treated individuals and 86.11% for those without FDQ treatment (P=0.041) (Figure 2b); in patients aged 31-60 years, the rates were 80.56% for FDQ-treated patients and 36.17% for non-FDQ-treated patients (P<0.001) (Figure 2c); in patients aged ≥61 years, the rates were 51.19% for FDQ-treated individuals and 7.36% for those without FDQ treatment (P=0.017) (Figure 2d).
4. Discussion
This retrospective study evaluated the safety and effectiveness of FeiDuQing (FDQ), a traditional Chinese herbal decoction, in treating Novel Coronavirus Pneumonia (NCP) patients. The clinical data revealed a significant improvement in symptoms following FDQ treatment. Upon discharge, only 3 (1.41%) of the 213 patients treated with FDQ still exhibited mild symptoms, such as a light cough. In contrast, 16 (11.19%) out of 142 patients who did not receive FDQ continued to experience various symptoms, with some presenting more than one symptom. This favorable symptom improvement was also observed in a separate study involving a particular population, where common COVID-19-related symptoms, such as cough and fever, improved or disappeared within 48 hours after initiating FDQ treatment.
Furthermore, FDQ treatment exhibited excellent tolerability and was not associated with severe adverse events during the study period. Significantly, no patients in the FDQ group progressed to extreme conditions, while 12 (8.45%) patients who did not receive FDQ developed severe conditions, and one death occurred. These findings underscore the potential efficacy of FDQ as a valuable treatment for COVID-19.
Additionally, we observed a noteworthy reduction of approximately 17.6% in the total duration of hospital stay among FDQ-treated patients (18.2±5.6 days) compared to those in the non-FDQ treatment group (22.1±5.9 days). Consistent with other studies (reference: https://www.cdc.gov/mmwr/volumes/69/wr/mm6918e1.htm), we observed that hospitalization duration increased with age, particularly in patients without FDQ treatment. However, no such age-related trend was observed among FDQ-treated patients aged 31 years and older. Remarkably, the average hospital stay for patients aged 61 years and older in the FDQ treatment group was only 18.0 days, which was not significantly different from the 18.7 days observed in patients aged 31-60 years, highlighting the effectiveness of FDQ in treating NCP. It is important to note that the overall duration of hospital stays in this cohort study was longer than the reported median length of 10-13 days (https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical- guidance-management-patients.html) [1,6,8], possibly due to differences in thresholds for hospitalization.
Furthermore, our study employed Cox-proportional hazard model analysis to provide further evidence of the positive impact of FDQ treatment on patient outcomes. We defined the event outcome as "not discharged" from the hospital as of February 26. The results consistently identified the absence of FDQ treatment as the only significant risk factor for this event outcome in our study. These findings were reinforced by the Cox regression survival curve, which illustrated a substantially higher discharge rate in FDQ-treated patients compared to those without FDQ treatment. The estimated discharge rate at day 28 increased from 45.20% to 84.70% with FDQ treatment. Importantly, this beneficial effect was observed across all age subgroups and was particularly notable among elderly patients, which has significant implications for developing effective therapy plans for this demographic.
While this study has provided valuable insights, it is not without limitations inherent to its retrospective observational design. Firstly, it lacks the randomized comparison characteristic of controlled clinical trials, introducing the possibility of treatment assignment and patient-related biases. Secondly, the study's population is limited to patients within Xianning City, Hubei Province, China. Thirdly, certain cases were initially diagnosed in outpatient settings where medical information was briefly documented, and some laboratory testing was incomplete. Lastly, the study did not capture clinical features during the disease course, and data for important COVID-19 biomarkers, such as IL-6 and D-dimer, were unavailable due to a shortage of laboratory supplies and medical staff. Despite these limitations, the significant reduction in hospital stays and symptom improvement observed after FDQ treatment serve as promising surrogate indicators of the drug's effectiveness and safety in clinical practice.
To our knowledge, this is the first large-scale cohort study focusing on the specific use of Chinese herbal medicine in treating COVID-19, even though Traditional Chinese Medicine (TCM) has been widely applied in China and has shown promising results. In conclusion, this study demonstrates that FDQ is an effective and safe treatment option for NCP patients, including elderly individuals. Remarkably, due to its outstanding clinical efficacy, FDQ has been widely used by outpatients, including at home, in senior care houses, and mental health centers (as described in Dr. Wang's other manuscript). Xianning City achieved an impressive 98.21% cure rate in treating COVID-19 patients, making it the first prefecture-level city in Hubei Province to treat all COVID-19 patients [8, 9] successfully. Moreover, FDQ is a cost-effective treatment that can be easily administered by simply drinking it twice daily. The observed side effects of FDQ were minimal, with a few patients reporting mild diarrhea or abdominal pain, which typically resolved within 2-3 days. This observational study suggests that FDQ may offer significant clinical benefits. However, these findings should be validated through future randomized controlled trials.
5. Conclusion
FDQ emerges as a pivotal factor influencing the progression of COVID-19. Its utilization not only accelerates recovery but also serves as a safeguard against severe outcomes. Notably, the risk of hospitalization for COVID-19 patients is significantly reduced when FDQ is incorporated into their treatment regimen. The traditional Chinese medicine compound "Fei-Du-qing" demonstrates remarkable promise and warrants further in-depth investigation and widespread adoption in the fight against COVID-19.
Funding Sources
This research was financially supported by:
1. Key Projects in Xianning Science and Technology Project: "Research and Development of Traditional Chinese Medicine Modernization of Novel Coronavirus Pneumonia Prescription FDQ" (2020SFYF01).
2. Natural Science Foundation of Hubei Province: "Study on Pharmacological Action and Tablet Preparation Technology of FDQ" (2020CFB868).
Competing Interests: The authors affirm that there are no conflicts of interest to declare.
Ethical Statement
The authors bear full responsibility for all aspects of this study, ensuring that any concerns regarding the accuracy or integrity of the research are thoroughly examined and addressed.
All procedures involving human subjects have been granted approval by the Institutional Review Board at the Ethical Committee of Hubei College of Science and Technology.
References
- Countries where COVID-19 has spread.
- Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment.
- Singh AK, Singh A, Singh R, Misra A (2020) Remdesivir in COVID-19: A critical review of pharmacology, pre-clinical and clinical studies. Diabetes Metab Syndr. 14(4): 641-648.
- SARS: Clinical Trials on Treatment Using a Combination of Traditional Chinese Medicine and Western Medicine, World Health Organization, 2004.
- Wang C, Cao B, Liu QQ, Zou ZQ, Liang ZA, et al. (2011) Oseltamivir compared with the Chinese traditional therapy Maxingshigan-Yinqiaosan in the treatment of H1N1 influenza: a randomized trial. Ann. Intern. Med. 155(4): 217–225.
- http://www.xinhuanet.com/local/2020-03/04/c_1125659745.htm (in Chinese).