Francesca Ambrogio1*, Nicoletta Cassano2, Gino Antonio Vena2, Domenico Bonamonte1, Mauro Grandolfo1, Paolo Romita1, Anna Bosco2, Silvia Mazzotta1, Caterina Foti1
1Dermatological Clinic, Department of Biomedical Science and Human Oncology, University of Bari “Aldo Moro”, Bari, Italy.
2Dermatology and Venereology Private Practice, Bari, Italy.
*Corresponding Author: Francesca Ambrogio, Dermatological Clinic, Department of Biomedical Science and Human Oncology, University of Bari “Aldo Moro”, Bari, Italy.
Dear Editor,
Imiquimod is an imidazoquinoline derivative with a direct agonistic activity towards toll-like receptors (TLR)-7 and TLR-8, resulting in the production of proinflammatory cytokines and stimulation of innate and acquired Th1- and Th17-weighted immune responses. [1] Additional effects of imiquimod include changes in the epidermal barrier functions, induction of migration of epidermal Langerhans cells to regional lymph nodes, and stimulation of plasmacytoid dendritic cells. [2,3]
In clinical practice, local reactions are frequently seen at the site of application of topical imiquimod as an expected consequence of the drug's pro-inflammatory and immunostimulating mode of action that can also trigger immune-mediated cutaneous adverse events in a few subjects. For instance, exacerbation of psoriasis and de novo psoriasis have been reported in patients treated with topical imiquimod. [1,4] A minority of patients can experience adverse events at non- application sites, including systemic symptoms (e.g., headache, fever, fatigue, myalgias, or nausea). [4,5]. Widespread cutaneous reactions, such as erythema multiforme, seem to be rare, [6] as well as distant inflammatory mucosal reactions. [5]
Case Report
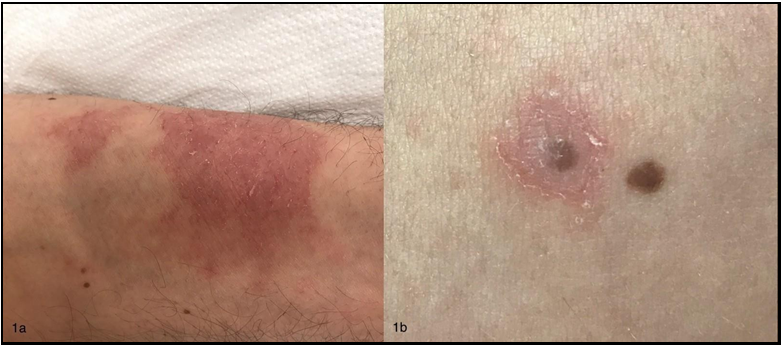
A 36-year-old non-atopic male presented with an itchy eczematous dermatitis involving initially the perianal region and then other areas. He was suffering from perianal warts that were treated with imiquimod 5 % cream. The patient had initially applied the cream three times a week for 15 days and then decided to discontinue treatment that was resumed after one month. Perianal dermatitis started after about 15 days from the beginning of the second treatment course. The patient was reported to be sensitized to nickel sulfate. He applied the cream only on perianal warts and denied any accidental transfer of the cream to the other affected sites (e.g., by scratching), as well as the use of compounds containing nickel in the last weeks. Physical examination revealed eczematous dermatitis on the left forearm (Figure 1A) and perianal region, and small patches with mild erythema and fine scaling around (Figure 1B) or near some melanocytic nevi of the trunk and upper limbs. Dermoscopy did not show atypical features of such nevi.
Figure 1: 1A) Eczematous dermatitis on the left forearm; 1B) Mild erythema and fine scaling around a melanocytic nevus of the trunk resembling Meyerson’s phenomenon
Therefore, our patient developed perianal dermatitis at the site of application of imiquimod and subsequent distant eczematous lesions with halo dermatitis around some melanocytic nevi, resembling Meyerson’s phenomenon (MP).
MP is an uncommon condition characterized by an eczematous halo surrounding a preexisting melanocytic nevus (Meyerson nevus) or numerous nonmelanocytic lesions, and even melanoma.[7,8] MP can involve multiple nevi, either separately over time or simultaneously, and generally resolves spontaneously or with the use of topical steroids or excision. Even though the pathogenesis is unknown, substantial evidence suggests that MP is immune-mediated, with the recruitment of a CD4+ T cell response. Interestingly, MP was observed in patients undergoing interferon-alpha-2b treatment. [7] There are limited data concerning imiquimod-related regional adverse effects with remote inflammatory skin reactions. Epicutaneous application of imiquimod has been used to create a murine model of psoriasis-like skin inflammation. [1] In human skin, imiquimod was shown to induce an inflammatory reaction similar to acute contact dermatitis rather than psoriasis whose peculiar hallmark is the primary involvement of plasmacytoid dendritic cells leading to a Th17-mediated response. [3]
The literature contains only anecdotal reports of eczema in patients treated with imiquimod. Taylor et al. described a severe recurring eczematous reaction at sites previously affected by eczema during imiquimod treatment, in the absence of any reaction at the site of application, suggesting a possible systemic absorption of imiquimod with enhanced T cell response and flare of the past eczematous dermatitis. [9]
It is conceivable that the Th1/Th17 microenvironment created by imiquimod might stimulate distant eczematous reactions in predisposed patients.
Keywords: imiquimod, eczema, Meyerson phenomenon
Financial Disclosure: None reported.
Conflict of Interest Disclosure: None Declared.
References
- van der Kolk T, Assil S, Rijneveld R, Klaassen ES, Feiss G, et al. (2018) Comprehensive, Multimodal Characterization of an Imiquimod-Induced Human Skin Inflammation Model for Drug Development. Clin Transl Sci. 11(6): 607-615.
- Schön MP, Schön M (2007) Imiquimod: mode of action. Br J Dermatol. 157(Suppl 2): 8-13.
- Garzorz-Stark N, Lauffer F, Krause L, Thomas J, Atenhan A, et al. (2018) Toll-like receptor 7/8 agonists stimulate plasmacytoid dendritic cells to initiate TH17-deviated acute contact dermatitis in human subjects. J Allergy Clin Immunol. 141(4): 1320- 1333.e11.
- Hanna E, Abadi R, Abbas O (2016) Imiquimod in dermatology: an overview. Int J Dermatol. 55(8): 831-844.
- Hammerl V, Parlar B, Navarini A, Gantenbein L, Väth H, et al. (2021) Mucosal side effects in patients treated with topical imiquimod-A scoping review of the literature. Dermatol Ther. 34(1): e14355.
- Chan MYL, Kennedy J, Oakley A (2017) Erythema multiforme triggered by imiquimod 5% cream. Australas J Dermatol. 58(4): e257-e258.
- Loh J, Kenny P (2010) Meyerson phenomenon. J Cutan Med Surg. 14(1): 30-2.
- Pižem J, Stojanovič L, Luzar B (2012) Melanocytic lesions with eczematous reaction (Meyerson's phenomenon) - a histopathologic analysis of 64 cases. J Cutan Pathol. 39(10): 901- 910.
- Taylor CL, Maslen M, Kapembwa M (2006) A case of severe eczema following use of imiquimod 5 % cream. Sex Transm Infect. 82(3): 227-8.