Dhafer G. Alshehri1*, Amani Azizalrahman2, Ghada Al Qahtani3
1Adult Emergency Department, King Fahd Medical City, Riyadh, Saudi Arabia.
2Pediatric Emergency Department, King Fahd Medical City, Riyadh, Saudi Arabia.
3Pediatric Department, King Fahd Medical City, Riyadh, Saudi Arabia.
*Corresponding Author: Dhafer G. Alshehri, Adult Emergency Department, King Fahd Medical City, Riyadh, Saudi Arabia.
Abstract
The first presentation of congenital heart disease and managing critically ill neonates is sometimes challenging. One of the most common birth abnormalities is aortic coarctation (CoA), which is caused by narrowing the aortic isthmus. It is tough and complicated to make an accurate prenatal diagnosis of CoA. We continue to see a high number of false (+) and false (-) diagnoses. We report a five-day-old newborn boy who presented with coarctation of the aorta and atrial septal defect and underwent coarctectomy. He had decompensated shock and looked ill. Point- of-care ultrasound helps us to reach the diagnosis, which affects clinical decision-making, choosing an appropriate intervention, and early consultation with excellent service. In the instance presented, CoA was diagnosed postnatally. In the CoA diagnoses, incorrect ultrasonography dimensions of great vessels and PA/Ao ratio are critical. However, a broad differential diagnosis is necessary, including lung dilatation (due to pulmonary hypertension or fetal blood redistribution due to potential infection).
Keywords: Early Detection, Point of Care Ultrasound, Congenital Heart Disease, Critically ill Neonates, Case Report.
Introduction
Congenital heart diseases (CHD) are the most common congenital disability. It occurs approximately 8 in 1000 births and ranges from benign to life-threatening.[1] Coarctation of the aorta (CoA) is one of CHD and is defined as narrowing the aortic arch (AA).[2] The AA is typically located near the insertion of the ductus arteriosus just distal or proximal to the left subclavian artery, which can lead to obstruction of blood flow.[3] This obstructive lesion may limit blood flow in the fetal aortic arch, resulting in arch hypoplasia. However, this may not be clinically obvious until after delivery in certain circumstances.[4] CoA represents 4-6 % of CHD with a reported prevalence of approximately 4 per 10,000 live births [5] were more common in Caucasians than in the black or Hispanic population [6] and with male-to-female predominance (59 versus 41 %).[7]
At birth, Newborn children with coarctation of the aorta are usually asymptomatic.[8] The onset of symptoms is correlated to the closure of the ductus arteriosus within the first 7–10 days of life.[9] The symptom's severity and the age at the time of presentation rely on the site of the coarctation, the narrowing degree, and any other associated cardiac defects.[10] Newborn children with severe coarctation with ductal closure may initially present with a decrease in feeding and irritability; there are appearing pale or have differential cyanosis. Also, patients with severe coarctation present signs of shock and circulatory collapse with poor or no palpable pulse in the femoral area.[11] In the evaluation measuring blood pressure in the upper and lower extremities is necessary because low systolic blood pressure of the lower extremity to the upper should raise suspicion of Certification of the aorta.[12] Transthoracic echocardiography is initially the diagnostic tool for the coarctation of the aorta.[13] The suprasternal aortic arch view can reveal aortic coarctation, particularly when combined with color flow mapping.[14] The treatment of Certification of the aorta depends on the narrowing degree and severity of presentations. In the neonatal period, almost the definitive treatment is by surgery.[15]. Many pediatric POCUS guidelines do not cover the diagnosis of aortic coarctation. [16,17,18] Point-of-care ultrasonography (POCUS) is a focused ultrasonography accomplished and interpreted at the patient's bedside by a physician or health care provider in conjunction with clinical examination. In adult studies, POCUS can facilitate clinical decision-making, appropriate investigation and intervention, early consultation with the appropriate service, a short stay in the emergency department, and improved patient satisfaction. [19,20,21,22,23,24]
To encourage using POCUS as a diagnostic tool in the pediatric emergency department. This study presented a case of aortic coarctation detected in utero in which blood flow imaging gave critical information on the location and type of this aortic arch defect. [25] This case showed how important it is to use POCUS in managing critically ill neonates, which helped us in clinical decision-making, choosing an appropriate intervention, and early consultation with good service.
Case Description
This is a 5-day-old full-term baby boy, 3.5kg, with the outcome of expected vaginal delivery with regular antenatal follow-up and uneventful prenatal and immediate postnatal history. The baby was discharged home with the mother on the second day of life in stable condition. On day five, the baby presented to our pediatric emergency department in King Fahad Medical City in Riyadh with complaints of fever, poor feeding, irritability, and shortness of breath for 1 day. No risk factor for CHD in the history. Those symptoms occur only on the day of the presentation, the parents are not related, and the newborn metabolic screening program was not remarkable.
On arrival at our Department, he was found to be febrile (38.6°C.), tachypneic (respiratory rate 60 breaths per minute) with severe work of breathing, oxygen saturation of 99 % on room air, tachycardiac (heart rate of 158 beats per minutes), cooled extremities with poor perfusion, Initial Systolic blood pressure on all upper and lower extremities was 50 mmHg and bilateral both femoral pulses were decreased. He was conscious, although she was looking pale and ill- appearing.
Physical examination revealed non-dysmorphic features with a weight of 3.5 kg. He was found to have chest retraction with good air entry and no added sounds, and the apex beat was not displaced with normal heart sounds. Abdomen examination revealed no hepatosplenomegaly, no sign of abuse or surgical abdomen, and the upper and lower extremities exam was unremarkable.
The initial impression was to roll out sepsis as the patient was febrile and ill, and a decrease in femoral pulse could be possible due to hypotension. Resuscitation started, and the broad-spectrum intravenous antibiotic was given after sending the initial lab and culture profile.
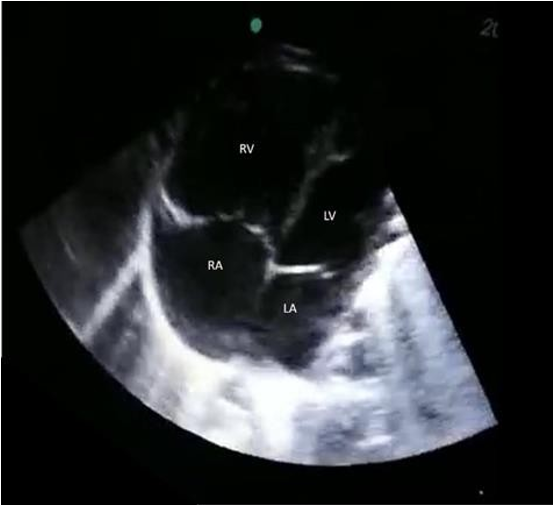
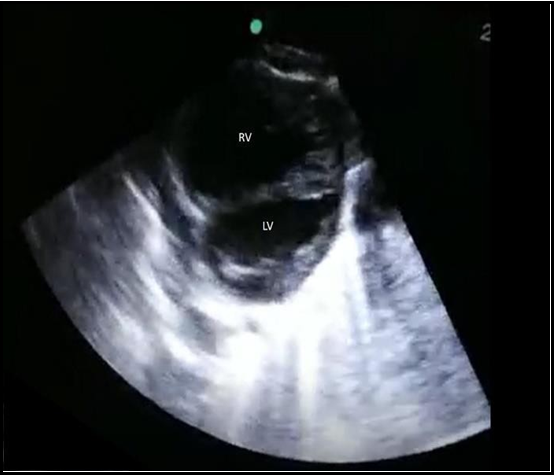
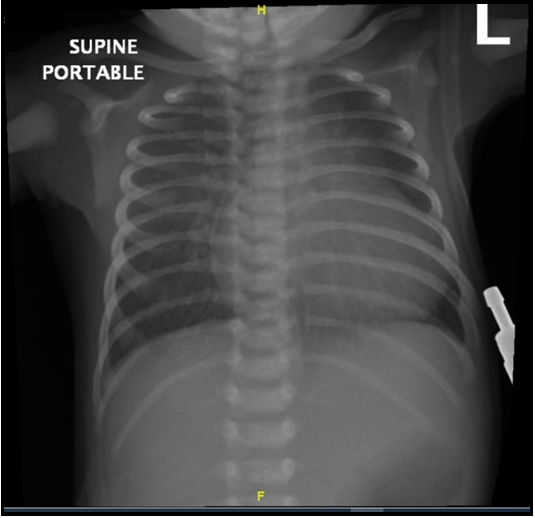
Bedside glucose level was low. The initial Blood Gas showed severe metabolic acidosis, pH 6.9 with Severe hyperlactatemia (Lactate as high as 21), and Bicarbonate as low as 5. child's condition did not change, and bedside POCUS was done after a while of assessments which revealed an abnormally dilated right ventricle (Figure 1a) with septum pushed to the left and a small left ventricle with poor function (Figure 1b), which increased the suspicion of cardiac defect leading to shock. Urgent chest x-ray showed cardiomegaly (Figure 2), and Electrocardiography (ECG) showed sinus rhythm with features of right axis deviation with right heart strain (Figure 3). After all these findings, patients were evaluated as septic versus cardiogenic shock. Pediatric cardiologists consulted immediately to roll out cardiac defects with a high possibility of CoA.
After the initial resuscitation patient's Systolic blood pressure improved and showed on the right upper extremity was 96 mmHg and on the right lower extremity was 40 mmHg. Initial laboratory tests showed leukocytosis with a white cell count of 21,000, low platelets 103, high creatinine 144, and high urea 16.8. The pediatric cardiologist confirmed the diagnosis of Certification of the aorta with ASD and left to right shunt at bedside echocardiography. The patient was electively intubated, and Prostaglandin infusion was started immediately after that. He became hypotensive, which required inotropic support, and was safely transported to the neonatal intensive care unit.
When he was stabilized, surgical intervention was performed. The patient tolerated the procedure very well, and a follow-up after two months after the approach showed an excellent outcome.
Figure 1a: Four champer views of the heart: showing RV is beggar than LV.
xRV: Right Ventricle, LV: Left Ventricle, RV: Right Atrium, RV: Left Atrium.
Figure 1b: Short axis parasternal view of the heart showing flattening of the interventricular septum towards the LV. RV: Right Ventricle, LV: Left Ventricle.
Figure 2: Portable anterior posterior chest x ray showing cardiomegaly.
Figure 3: The 12-lead electrocardiogram showing right axis deviation with sign of right heart strain.
Discussion
Coarctation of the aorta is a dangerous illness that frequently goes misdiagnosed. When newborns appear with shock, the most prevalent cause is an infection, which must always be ruled out, followed closely by left heart blockage (aortic stenosis, coarctation of the aorta). These heart lesions often exhibit minor and non-diagnostic murmurs. The electrocardiograms indicate right ventricular hypertrophy rather than the predicted left ventricular hypertrophy from a left heart obstructive lesion. A chest radiogram will reveal a dilated heart, pulmonary edema, or left-to-right shunt. Typically, the liver is enlarged. [26]
Recent investigations in Scandinavia discovered that at least half of these newborns were released without a diagnosis [27] and that most remained misdiagnosed five days after birth.[28] In California, Chang et al.[29] 27 percent of individuals with aortic coarctation died undetected at a median age of 17 days. Ward et al.[30] It was discovered that neonates with symptomatic aortic coarctation manifested between five and fourteen days after birth. This case showed how POCUS could help identify CHD when diagnostic clarity is lacking. Since 1990, the American College of Emergency Physicians (ACEP) has recommended using ultrasound by a trained emergency physician to be the scope of practice.[31] Also, the American Academy of Pediatrics published a formal program to improve practicing POCUS by Pediatric Emergency physicians (PEM).[32] Recently, in Pediatric patients, POCUS has been adopted in practice. Although the are many studies proving the ability to use POCUS by PEM physicians,[33] And literature showing after adequate training, PEM physicians can become competent in practicing POCUS.[34]
Many studies report the importance of using POCUS practically bedside echocardiography. In 2017 Austin T et al. [35] said a case of a child diagnosed with precordial tamponade in an emergency. Also, in 2018 Daniel Rosenfield et al. [36] reported that two patients diagnosed with POCUS had a ventricular septal defect.
CoA is one of the most missed diagnoses in the prenatal and postnatal period, which can lead to life-threatening cardiovascular if not discovered in the early neonatal period. And most of them do not have symptoms, and they look well with regular examination in the early days of life.[37] In our case, the child presented within the fifth day of life with a mixed picture of shock, which makes the diagnosis challenging, but by using POCUS, we were able to identify some cardiac abnormalities, which helped us make the diagnosis. Previous literature discussing some of the monsters was like our findings, like the dilation of the right ventricle more than the left one, and the report as a sign of prenatal diagnosis, although it could be a normal finding in the neonatal period. [38]
A clinical examination cannot easily detect aortic coarctation in the first two days after birth because the arterial duct is patent, allowing for lower body perfusion. In many countries, the date of maternal discharge following birth has been decreased from three days or more to 24 hours or even six hours postnatally. This has resulted in increased cases of undetected left heart obstructive disease, such as aortic coarctation, interrupted aortic arch, HLHS, or aortic stenosis.[39] This trend is likely to continue, with potentially fatal consequences for babies who are not diagnosed before being discharged from the hospital, unless additional postnatal screening tools, such as pulse oximetry of the pre-and post-ductal sites, and improved education of community healthcare workers in the signs of congenital heart disease, become available.[40]
Only a tiny percentage of kids born with coarctation are found during standard prenatal screening, increasing the risk of perinatal cardiovascular collapse and, in some cases, mortality, which occurs when the arterial duct shuts.[41] Examining the outflow tracts and transverse aortic and ductal arches using a simple ultrasound procedure of five transverse planes across the fetal body can enhance antenatal diagnosis.[42] Because diagnostic accuracy is difficult to achieve and the aortic arch may exhibit tubular hypoplasia without an area of blockage, some kids will need to be monitored in case coarctation occurs later in infancy.[43] In the case of an infant with coarctation, surgery is the preferred therapy; however, balloon dilatation and stenting may be beneficial if coarctation develops later in childhood.[44]
Our case report limitation is a "near miss." It was apparent in the possible antenatal diagnosis of such CHD using POCUS. Prenatal diagnosis of coarctation of the aorta improves survival and reduces morbidity.[45] We also encourage using POCUS as a diagnostic tool in the antenatal follow-up visits for pregnant females and the postnatal period in the pediatric emergency department.
Conclusion
In conclusion, CoA was detected postnatally in the case described. However, an early diagnosis can improve the preoperative state of infants arriving for surgery and prevent the tragedy of death at home in situations of an easily curable ailment. A Point of care Ultrasound aids in diagnosis, influencing clinical decision-making, selecting suitable action, and early contact with the relevant provider.
References
- Liu Y, Chen S, Zühlke L, Black GC, Choy MK, et al. (2019) Global birth prevalence of congenital heart defects 1970-2017: Updated systematic review and meta-analysis of 260 studies. International Journal of Epidemiology. 48(2): 455–463.
- Ma ZL, Yan J, Li SJ, Hua ZD, Yan FX, et al. (2017) Coarctation of the Aorta with Aortic Arch Hypoplasia: Midterm Outcomes of Aortic Arch Reconstruction with Autologous Pulmonary Artery Patch. Chinese medical journal. 130(23): 2802-2807.
- Ra-id Abdulla (2011) Heart Disease in children. Springer Science Business Media.
- Buyens A, Gyselaers W, Coumans A, Al Nasiry S, Willekes C, et al. (2012) Difficult prenatal diagnosis: fetal coarctation. Facts, views & vision in ObGyn. 4(4): 230–236.
- Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A (2008) Prevalence of congenital heart defects in metropolitan Atlanta, 1998-2005. J Pediatr. 153(6): 807-13.
- Hoffman JI, Kaplan S (2002) The incidence of congenital heart disease. J Am Coll Cardiol. 39(12): 1890-1900.
- Talner NS (1980) Report of the New England Regional Infant Cardiac Program. Pediatrics. 65(2 Pt 2): 375-461.
- Doshi AR, Chikkabyrappa S (2018) Coarctation of Aorta in Children. Cureus. 10(12): e3690.
- Gillam-Krakauer M, Mahajan K. Patent Ductus Arteriosus. [Updated 2021 Aug 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls.
- Walls, Ron M., et al. Rosen's Emergency Medicine: Concepts and Clinical Practice. Elsevier; 2018.
- Hoffman JI (2018) The challenge in diagnosing coarctation of the aorta. Cardiovasc J Afr. 29(4): 252-255.
- Patankar N, Fernandes N, Kumar K, Manja V, Lakshminrusimha S (2016) Does measurement of four-limb blood pressures at birth improve detection of aortic arch anomalies?. Journal of perinatology : official journal of the California Perinatal Association. 36(5): 376-80.
- Sun Z, Cheng TO, Li L, Zhang L, Wang X, et al. (2015) Diagnostic Value of Transthoracic Echocardiography in Patients with Coarctation of Aorta: The Chinese Experience in 53 Patients Studied between 2008 and 2012 in One Major Medical Center. PloS one. 10(6): e0127399.
- Simpson A, Sahn DJ, Valdes-Cruz LM, Chung KJ, Sherman FS, et al. (1988) Color Doppler flow mapping in patients with coarctation of the aorta: new observations and improved evaluation with color flow diameter and proximal acceleration as predictors of severity. Circulation. 77: 736–744.
- Doshi AR, Chikkabyrappa S (2018) Coarctation of Aorta in Children. Cureus. 10(12): e3690.
- Vieira RL, Hsu D, Nagler J, Chen L, Gallagher R, et al. (2013) Pediatric emergency medicine fellow training in ultrasound: consensus educational guidelines. Acad Emerg Med. 20(3): 300– 6.
- Shefrin AE, Warkentine F, Constantine E, Toney A, Uya A, et al. (2019) Consensus core point-of-care ultrasound applications for pediatric emergency medicine training. Aem Educ Train. 3(3): 251–258.
- Singh Y, Tissot C, Fraga MV, Yousef N, Cortes RG, et al. (2020) International evidence-based guidelines on point of care ultrasound (POCUS) for critically ill neonates and children issued by the POCUS working group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). Crit Care. 24(1): 65.
- Howard ZD, Noble VE, Marill KA, Sajed D, Rodrigues M, et al. (2013) Bedside ultrasound maximizes patient satisfaction. J Emerg Med. 46(1): 46-53.
- Jones AE, Tayal VS, Sullivan DM, Kline JA (2004) Randomized, controlled trial of immediate versus delayed goal-directed ultrasound to identify the cause of nontraumatic hypotension in emergency department patients. Crit Care Med. 32(8): 1703– 1708.
- Melniker LA, Leibner E, McKenney MG, Lopez P, Briggs WM, et al. (2006) Randomized controlled clinical trial of point-of-care, limited ultrasonography for trauma in the emergency department: the first sonography outcomes assessment program trial. Ann Emerg Med. 48(3): 227–235.
- Kirkpatrick AW, Sirois M, Ball CG, Laupland KB, Goldstein L, et al. (2004) The hand-held ultrasound examination for penetrating abdominal trauma. Am J Surg. 187(5): 660–665.
- Moore CL, Copel JA (2011) Point-of-care ultrasonography. N Engl J Med. 364(8): 749–757.
- Liteplo AS, Marill KA, Villen T, Miller RM, Murray AF, et al. (2009) Emergency Thoracic Ultrasound in the Differentiation of the Etiology of Shortness of Breath (ETUDES): Sonographic B‐ lines and N‐terminal Pro‐brain‐type Natriuretic Peptide in Diagnosing Congestive Heart Failure. Acad Emerg Med. 16(3): 201-10.
- Wang Y, Liu C, Zhang Y, Wang M (2021) Prenatal diagnosis of coarctation of the aorta with a long and angled isthmus by two- and three-dimensional echocardiography: a case report. BMC Cardiovasc Disord. 21: 176.
- Hoffman JI (2018) The challenge in diagnosing coarctation of the aorta. Cardiovascular journal of Africa. 29(4): 252-255.
- Lannering K, Bartos M, Mellander M (2015) Late diagnosis of coarctation despite prenatal ultrasound and postnatalp oximetry. Pediatrics. 136: e406–412.
- Mellander M, Sunnegardh J (2006) Failure to diagnose critical heart malformations in newborns before discharge – an increasing problem? Acta Paediatr. 95(4): 407–413.
- Chang RK, Gurvitz M, Rodriguez S (2008) Missed diagnosis of critical congenital heart disease. Arch Pediatr Adolesc Med. 162(10): 969–974.
- Ward KE, Pryor RW, Matson JR, Razook JD, Thompson WM, et al. (1990) Delayed detection of coarctation in infancy: implications for timing of newborn follow-up. Pediatrics. 86(6): 972–976.
- Liteplo AS, Marill KA, Villen T, Miller RM, Murray AF, et al. (2009) Emergency Thoracic Ultrasound in the Differentiation of the Etiology of Shortness of Breath (ETUDES): sonographic B- lines and N-terminal pro-brain-type natriuretic peptide in diagnosing congestive heart failure. Acad Emerg Med. 16(3): 201–210.
- American College of Emergency Physicians. Council resolution on ultrasound. ACEP News.1990;9.
- Marin JR, Lewiss RE (2015) Point-of-care ultrasonography by pediatric emergency medicine physicians. Pediatrics. 135: e1113– e1122.
- Riera A, Hsiao AL, Langhan ML, Goodman TR, Chen L (2012) Diagnosis of intussusception by physician novice sonographers in the emergency department. Ann Emerg Med. 60(3): 264–268.
- Smith AT, Watnick C, Ferre RM (2017) Cardiac Tamponade Diagnosed by Point-of-Care Ultrasound. Pediatric Emergency Care. 33(2): 132–134.
- Rosenfield D, Fischer JW, Kwan CW (2018) Point-of-Care Ultrasound to Identify Congenital Heart Disease in the Pediatric Emergency Department. Pediatric Emergency Care. 34(3): 223– 225.
- Yun SW (2011) Congenital heart disease in the newborn requiring early intervention. Korean journal of pediatrics. 54(5): 183-91.
- Chang RK, Gurvitz M, Rodriguez S (2008) Missed diagnosis of critical congenital heart disease. Archives of pediatrics & adolescent medicine. 162(10): 969-74.
- Brown EM, Collins W, Leung T, Salmon AP (2002) Heart sounds made easy. edinburgh: Churchill Livingstone.
- Sola A, Golombek SG (2018) Early Detection with Pulse Oximetry of Hypoxemic Neonatal Conditions. Development of the IX Clinical Consensus Statement of the Ibero-American Society of Neonatology (SIBEN). International journal of neonatal screening. 4(1): 10.
- Buyens A, Gyselaers W, Coumans A, Al Nasiry S, Willekes C, et al. (2012) Difficult prenatal diagnosis: fetal coarctation. Facts, views & vision in ObGyn, 4(4): 230–236.
- Yeo L, Romero R (2017) Color and power Doppler combined with Fetal Intelligent Navigation Echocardiography (FINE) to evaluate the fetal heart.” Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 50(4): 476-491.
- Agasthi P, Pujari SH, Tseng A, Graziano JN, Marcotte F, et al. (2020) Management of adults with coarctation of aorta.” World journal of cardiology. 12(5): 167-191.
- Torok RD, Campbell MJ, Fleming GA, Hill KD (2015) Coarctation of the aorta: Management from infancy to adulthood. World J Cardiol. 7(11): 765-775.
- Franklin O, Burch M, Manning N, Sleeman K, Gould S, et al. (2002) Prenatal diagnosis of coarctation of the aorta improves survival and reduces morbidity. Heart. 87(1): 67-69.