Bystron J*, Balner T, Kačorová J
The head of Dep. of Allergology and Clic. Immunology, Univerzity Hospital of Ostrava Unuverzity, Czech rep.
*Corresponding Author: Bystron J, The head of Dep. of Allergology and Clic. Immunology, Univerzity Hospital of Ostrava Unuverzity, Czech rep.
Abstract
Introduction: Original/review articles on the diagnosis, prevention and treatment of COVID-19 largely focus on the epidemiology, etiopathogenesis and management of severe cases resulting from SARS-CoV-2 infection. Less attention is paid to immunomodulatory drugs that can favourably inform human innate immunity at the initial phases of infection. Therefore, a pilot monocentric study evaluating the efficacy of IMUNOR® in the prevention and mitigation of COVID-19 has been conducted.
Material and methods: A monocentric pilot study (EudraCT 2020-005524-11) investigated 51 nurses at a university hospital during the third wave of the COVID-19 pandemic in the Czech Republic. The primary outcome measure was based on prevention success rates against SARS- CoV-2 infection in outpatient and inpatient nurses during a 1-month and 2-month period in a high-risk professional setting, respectively, with a 1-month follow-up. Secondary outcome measures focused on the severity of infection. The hospitalization rate in study participants and tolerability of IMUNOR® were also measured. A large-scale control group consisting of the remaining nurses in the same hospital was used.
Results: During the study, only two nurses on the IMUNOR® preventative regimen suffered a mild COVID-19 infection. No hospitalization was required and a 2-week home-based symptomatic treatment was sufficient. A statistically highly significant preventative effect of IMUNOR® (p<0·00001) was documented for a total of 70 per cent of days. In the remaining days, statistical significance could not be established, there being one incapacitated subject only at a time.
Discussion/conclusion: IMUNOR® appears to have a preventative potential against severe COVID-19 infection in high-risk professional populations.
Keywords: COVID-19, dialyzed leukocyte extract-DLE (IMUNOR®), prevention, innate immunity
1. Introduction and background
Original and review articles published to date in relation to the diagnosis, prevention and treatment of COVID-19 largely focus on the management of severe cases resulting from SARS-CoV-2 infection and on immunomodulation of respective stages in the immune reaction [1,2]. Less attention is paid to immunomodulatory drugs that can favourably inform human innate immunity at the initial phases of infection. Innate (non-specific) immunity, including properly functioning skin and mucous membrane barriers and first- line immunocompetent cells, is able to react to an invading viral agent and, providing the infectious load does not exceed certain limits and the immune system is not impaired, it can fight off the infection via common non-specific mechanisms.
The corresponding author of the present project builds upon a 20-year experience with the IMUNOR® dialyzed leukocyte extract (DLE) formula and pertinent literature related to its indication for recurrent (especially viral) infections of various bodily systems. The largest body of experience was accrued in asthma sufferers with exacerbations induced by viral infections [3,4]. The latter data correspond to existing reports published by authors outside the Czech Republic [5,6,7]. Previous research by Czech-speaking authors confirms IMUNOR®´s regenerative and activating potential toward hematopoietic and immunocompetent cells [8,9,10,11]. Besides, recent studies report the anti-inflammatory properties of DLE [12]. The preparation is well-tolerated and shows negligible adverse effects and interactions with other drugs.
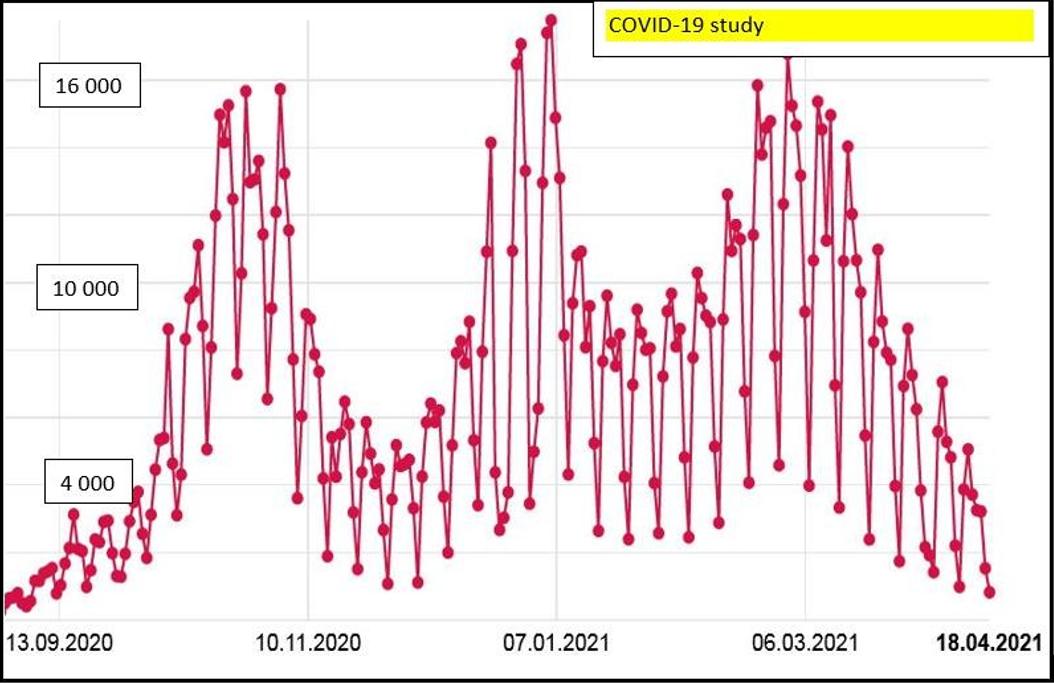
The present study was conducted from January 2021 through April 2021, when the third wave of the COVID-19 pandemic was in progress and the daily incidence reached over 10,000 new cases, i.e., more than 100 persons per 100,000 inhabitants (Chart 1).
Chart 1: Daily incidence rates for the COVID-19 infection in the Czech Republic
During the latter period, a significant burden was imposed upon health care facilities and many hospitals succumbed to the overload of incoming SARS-CoV2 patients. Numerous hospital wards had to be reprofiled to meet the needs of this novel group of patients. Due to a lack of systematic vaccination in the period in question, a high percentage of health care workers fell ill. A major impact of the wave on nurses was witnessed, rendering them unable to work and either hospitalized with severe forms of infections, needing complex care, or resorting to sick leaves in household settings.
2. Materials and methods
2.1 Study group
2.1.1 Recruitment
For the purpose of the study, nurse volunteers were invited who neither had a history of COVID-19 nor had been vaccinated against SARS-CoV2. Subjects were enrolled on the INTRANET platform of the University Hospital in Ostrava from November 2020 through December 2020. All potential subjects eligible for the study were offered an opportunity to participate. Volunteers complying with all eligibility criteria and not subsumed under any exclusion criteria who signed an informed consent form were finally included. In subjects on long-term medication regimens for non-COVID-19 diseases whose condition was stable, no changes of the medication were performed. Ongoing therapy for diabetes, hypertension, thyroid disease etc. was maintained from baseline till the end of the present study with no interventions on our part in terms of dosage and schedule.
21.2 Eligibility criteria
The following eligibility criteria were applied:
1. Females or males aged 18 to 65 with no present clinical signs of a COVID-19 infection;
2. Females of fertile age who could become pregnant had to submit a negative urine or blood pregnancy test on initial contact or had to use appropriate contraceptive methods during the study period; females who could not become pregnant were allowed including those with a history of hysterectomy, bilateral oophorectomy or bilateral tubal ligation, as well as those at least 12 months post menopause or with an evidence of infertility of any type;
3. Capacity for co-operation and informed consent signature.
A baseline test for COVID-19 was not required, but the complete absence of clinical signs of a potential ongoing COVID-19 infection was binding. The rationale for this criterion was that a negative testing result does not eliminate candidates who might have had a history of asymptomatic or minimal-symptom form of the COVID-19 infection. Even a negative PCR test would not exclude possible reinfection or recurrence of a COVID-19-related disease.
2.1.3 Exclusion criteria
The following exclusion criteria were applied:
1. A history of other immunomodulatory treatment during a 30-day period prior to entering the study (e.g., inosine pranobex, bacterial lysate, immunoglucans, imunoglobulins etc.)
2. Presence of ongoing, insufficiently compensated cardiac, metabolic, endocrine, hepatic, renal, neurologic, oncological or psychiatric disease in patients whose participation in a clinical trial could pose a high risk based on the assessment made by the researching clinician;
3. Presence of a known hypersensitivity to the drug or its components;
4. Pregnancy and breastfeeding.
2.1.4 Demographic characteristics of the study group
54 subject were enrolled in the study group, comprising 3 medical professionals and 51 nurses. As the number of medical professionals was low, they were excluded from statistical processing.
Consequently, data available for 51 nurses were subjected to further analysis. 33 (64·7 per cent) nurses worked in an out-patient setting, while 18 (35·3 per cent) in in-patient settings. 50 (98·0 per cent) members of the group were female, 1 (2·0 per cent) was male.
The age characteristics of the group are overviewed in Table 1.
Table 1: Age characteristics of the study group
|
Age characteristics of the study group |
||||||||
|
Workplace |
n |
𝒙̅ |
𝒔𝒅 |
95% CI for mean value |
𝒎𝒆𝒅. |
𝒎𝒊𝒏. |
𝒎𝒂𝒙. |
|
|
𝒎𝒊𝒏 |
𝒎𝒂𝒙 |
|||||||
|
Out-patient |
33 |
47·8 |
12·083 |
43·6 |
52·1 |
47·0 |
25·0 |
70·0 |
|
In-patient |
18 |
44·0 |
10·163 |
39·0 |
49·0 |
43·5 |
24·0 |
64·0 |
|
Total |
51 |
46·5 |
11·490 |
43·3 |
49·7 |
46·0 |
24·0 |
70·0 |
The mean age of the study group participants was 46·5. No statistically significant difference was identified between the out- patient nurse and in-patient nurse subgroups.
2.1.5. Case history of enrolled subjects
Based on case histories of the subjects enrolled, the following data were analyzed:
Table 2: History of immune dysfunction before Visit 1
|
History of immune dysfunction before Visit 1 |
||||||
|
Workplace |
Yes |
No |
Total |
|||
|
n |
% |
n |
% |
n |
% |
|
|
Out-patient |
10 |
30·3 |
23 |
69·7 |
33 |
100 |
|
In-patient |
8 |
44·4 |
10 |
55·6 |
18 |
100 |
|
Total |
18 |
35·3 |
33 |
64·7 |
51 |
100 |
Pertinent analysis indicated that in approximately 30 per cent of out- patient nurses and 44 per cent od in-patient nurses, a history of immune dysfunction before Visit 1 could be attested (see the list of relevant disorders with immune involvement in Table 3).
Table 3: List of diagnoses included in the immune system dysfunction brackets (two or more disorders could concurrently be present in some cases)
|
History of immune dysfunction before Visit 1 – diagnoses |
|||
|
|
Workplace |
|
|
|
Diagnosis |
Out-patient |
In-patient |
Total |
|
allergic rhinitis |
3 |
2 |
5 |
|
allergy (other types) |
- |
3 |
3 |
|
bronchial asthma |
4 |
4 |
8 |
|
autoimmune thyroiditis |
1 |
- |
1 |
|
celiac disease |
1 |
- |
1 |
|
COPD |
1 |
- |
1 |
|
recurrent respiratory tract infections |
- |
1 |
1 |
|
rheumatoid arthritis |
- |
1 |
1 |
|
selective IgA deficit |
1 |
- |
1 |
|
Sjögren syndrome |
- |
1 |
1 |
|
suspected immune deficiency |
- |
1 |
1 |
|
ulcerative colitis |
1 |
- |
1 |
|
urticaria |
- |
1 |
1 |
|
post-viral fatigue syndrome |
- |
1 |
1 |
|
Total |
12 |
15 |
27 |
Table 4: History of chronic respiratory tract disease before Visit 1
|
History of chronic respiratory tract disease before Visit 1 |
||||||
|
Workplace |
Yes |
No |
Total |
|||
|
n |
% |
n |
% |
n |
% |
|
|
Out-patient |
4 |
12·1 |
29 |
87·9 |
33 |
100 |
|
In-patient |
4 |
22·2 |
14 |
77·8 |
18 |
100 |
|
Total |
8 |
15·7 |
43 |
84·3 |
51 |
100 |
Pertinent analysis indicated that in approximately 12 per cent of out- patient nurses and 22 per cent of in-patient nurses, a history of chronic respiratory tract disease before Visit 1 could be attested (Table 4). In all cases, the diagnosis was bronchial asthma.
Further medical history data relevant to the study group include the following: a history of hypertension before Visit 1 was confirmed in 6 (18·2 per cent) out-patient nurses and 1 (5·5 per cent) in-patient nurse. A history of other cardiovascular diseases before Visit 1 was established in 3 (9·1 percent) out-patient nurses and none of the in- patient nurses. A history of diabetes mellitus was confirmed in 1 (5·5 percent) in-patient nurses and none of the out-patient nurses. A history of obesity before Visit 1 was not confirmed in any nurse in either group. A history of smoking before Visit 1 was recorded in 1 nurse (3·0 per cent) in the out-patient group. Other nurses reported being non-smokers. Results of SARS-CoV-2 testing before Visit 1 were available for 1 nurse (5·5 per cent) in the in-patient group (a negative result of an RT-PCR test) and 6 nurses (18·2 per cent) in the out-patient group (four RT-PCR tests with three negative results and one positive result, respectively; two antigen tests with negative results). The subject who tested positive was symptom-free at the time of testing as well as at the time of study commencement.
2.2 Control group
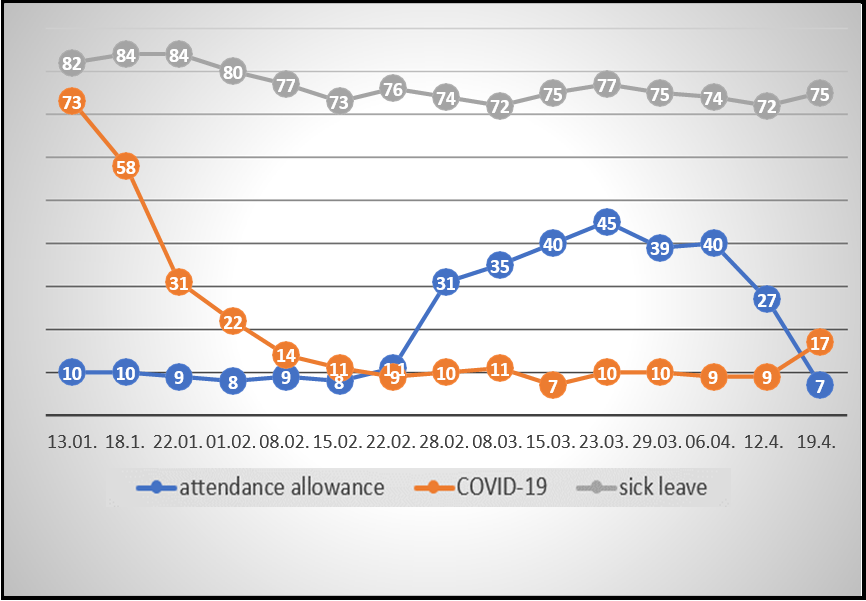
The control group consisted of all nurses working at the University Hospital who were not participants in the IMUNOR® study group, with a total of 1534 minus 51, i.e., n = 1483 persons as of January 4, 2021. Nurse employees on temporary sick leave or attendance leave for non-COVID-19 reasons were also included, albeit their risk of contracting pandemic infection might have been somewhat lower in the household. Morbidity in the control group was regularly checked by the University Hospital management and precise numbers of nurses with current temporal incapacity for work were reported to the Crisis Management Task Force once a week by head nurses of the respective departments and clinics (see Table 5).
2.3 Control File
The control set of nurses was also favored by the fact that in the period from January to April 2021, when the study was taking place, 1250 vaccination doses were applied in this control set (without distinguishing the type and number of individual nurses).
Table 5: Reports of nurses´ workplace absence at the University Hospital in Ostrava
|
Date |
4/I |
13/I |
18/I |
22/I |
1/II |
8/II |
15/II |
22/II |
28/II |
8/III |
15/III |
23/III |
29/III |
6/IV |
12/IV |
19/IV |
26/IV |
3/V |
|
AL |
2 |
10 |
10 |
9 |
8 |
9 |
8 |
11 |
31 |
35 |
40 |
45 |
39 |
40 |
27 |
7 |
1 |
1 |
|
Covid- 19+ |
99 |
73 |
58 |
31 |
22 |
14 |
11 |
9 |
10 |
11 |
7 |
10 |
10 |
9 |
9 |
17 |
16 |
16 |
|
NSI |
|
82 |
84 |
84 |
80 |
77 |
73 |
76 |
74 |
72 |
75 |
75 |
74 |
72 |
75 |
75 |
76 |
76 |
Comments: AL: workplace absence due to non-specific attendance leave (taking care of a family member)
Covid-19: incapacity for work due to confirmed COVID-19 infection
NSI: Non-specific incapacity for work/non-specific sick leave (other than COVID-19)
Total number of nurses at the University Hospital in Ostrava as of January 4, 2021: n = 1534
2.3 Goals and objectives
The purpose of the present study was to investigate the potential of an already registered preparation in fighting a highly contagious pandemic disease that most seriously impacts persons whose immune function is compromised (the elderly, patients with immunosuppression, exhausted patients and persons with co- morbidities). The main goal of our pilot investigation was to determine whether IMUNOR® administration exhibits preventative effects against or mitigating effects toward COVID-19 primary infection or recurrence.
In a broader concept of the anti-SARS-CoV-2 strategy, enhancement of bodily defense (i.e., immune) mechanisms should be included as an addition to direct anti-viral management strategies. First of all, modulation of the cytokine milieu whenever the so-called cytokine storm caused by SARS-CoV-2 should be considered to prevent organ damage (especially pulmonary damage) and to provide a shield protection during the lymphocytopenia phase to prevent superinfection. In this sense, the IMUNOR® preparation could be a promising candidate as part of a complex approach to the management and/or prevention of the COVID-19 infection.
2.4 Outcome measures
2.4.1 Primary outcome measures
In harmony with the main goal of the study, the primary outcome measure was based on establishing a possible preventative effect of IMUNOR® in healthy volunteers (medical staff, nurses) in whom a transient secondary weakening of innate cellular immunity can be expected due to excessive workload and workplace factors, especially a close contact with SARS-CoV2-positive patients with clinically manifest disease.
Overall efficacy of IMUNOR® administration was assessed based on the comparison of clinically manifest subjects in the IMUNOR® preventative regimen group and in the cohort of treatment-naïve health care workers.
2.4.2 Secondary outcome measures
The following secondary outcome measures were established: a) efficacy of preventative administration of IMUNOR® in terms of the clinical severity of COVID-19 infection based on secondary clinical indicators, b) proportion of patients whose condition deteriorated to a moderately severe level resulting in hospitalization in the course of the study, c) proportion of patients whose condition deteriorated to a severe level necessitating the use of pulmonary ventilation support, d) length of hospitalization, and e) incidence of adverse events.
2.5 Characteristics of the preparation (drug) administered in the study
2.5.1 IMUNOR® composition
IMUNOR® (ImunomedicA, I-nc.) contains 10 mg of transferendi factor suillus in a glass vial. It is a freeze-dried ultrafiltrated low- molecular (below 10 000 D) porcine leukocyte extract. A single 10 mg dose is accrued by extraction of 109 leukocytes.
2.5.2 IMUNOR® administration
The dosage schedule in the present study consisted of a single IMUNOR® vial taken perorally in the morning on an empty stomach, once a week for four weeks (study phase I), or once a week during a total of eight weeks (transition to study phase II), corresponding to one, or two packages of the preparation, respectively. Total numbers of doses administered are overviewed in Table 6.
Table 6: Reported doses based on nurses´ workplace.
|
Number of doses based on nurses´ workplace |
||||||
|
Workplace |
Four doses |
Eight doses |
Total |
|||
|
n |
% |
n |
% |
n |
% |
|
|
Out-patient |
3 |
9·1 |
30 |
90·9 |
33 |
100 |
|
In-patient |
4 |
22·2 |
14 |
77·8 |
18 |
100 |
|
Total |
7 |
13·7 |
44 |
86·3 |
51 |
100 |
2.5.3 Subject Diary
After enrolment and informed consent, each study subject obtained a Subject Diary. Personal data, including workplace type, relevant medical history, ongoing therapies and all prospective changes in the health status in the course of the study were recorded in the diary, including any adverse effects possibly related to the drug under investigation.
The Diary was used as part of source documentation.
2.6 Study procedure
After approval from relevant authorities (State Institute for Drug Control, Ethics Committee of the University Hospital in Ostrava), the enrolment process was launched for volunteering employees at the University Hospital.
The initial meeting was scheduled for January 14, 2021, and attendees were instructed about the study goals, schedule, eligibility criteria, and exclusion criteria. Subjects compliant with the criteria were asked to study all requirements and those who signed informed consent forms were included in the study and given appropriate study drug packages.
A total of 56 health care workers volunteered. Two subjects were excluded based on appeal by the study monitor (2 nurses from the study group), hence, the final number of enrolled subjects equaled 54. As indicated, the subjects had been given detailed study information and had signed an informed consent; afterwards, IMUNOR® packages were distributed to them along with a Subject Diary. Since the number of medical professionals enrolled was very low (n = 3), the latter were excluded from statistical analysis and a homogenous group of nurses (n = 51) was analyzed, matching the control group in terms of age distribution, profession, physical and mental workload and the risk of contact with patients potentially suffering from coronavirus infection.
The original study plan aimed at recruiting 100 subject to be treated for a period of 1 month and followed up for another month. Since a massive vaccination campaign against COVID-19 infection was started at the University Hospital in January 2021 and the number of recruitable subjects dropped, the recruitment process was halted on February 2, 2021, with a final number of 54 subjects. The latter subjects received a monthly course of IMUNOR® therapy (1 vial per week for a total of four weeks). Based on Annex 1 of the study protocol, approved by the State Institute for Drug Control and the Ethics Committee at the University Hospital in Ostrava, the protocol was extended for 46 subjects to cover another 4 weeks of treatment (a total of 8 vials used during eight weeks with a 4-week follow-up period). All 100 four-vial packages granted by the drug manufacturer were thus invested in the study.
The last study dose was administered on March 22, 2021. Enrolled subjects recorded their symptoms in the Subject Diary on a daily basis. On completion of the treatments period, diaries were submitted to the study team members.
Four weeks post the last treatment dose, subjects self-referred to the study team members to give a narrative of their health status in the previous 4 weeks; alternatively, part of the subject group were asked about the health status in the previous 4 weeks via a phone call. The findings were recorded in the Subject Diary and the last diary was filed in study documentation on April 30, 2021.
3. Findings/results
Key findings
A total of 54 subject finally participated in the study, namely, three medical professionals, 33 out-patient nurses and 18 in-patient nurses. The medical professional subgroup was not representative and was thus excluded from statistical analysis based on the decision of the study manager. Various causes for underrepresentation of medical professionals were identified, including rapid recruitment for vaccination schedules, lack of willingness to participate in clinical trials etc. Logically, the focus of statistical analysis was on the nurse subgroup (a total of 51 subjects) that matched the control group in terms of age, profession, risk ratios, and temporal variables. With the control group composed of nurses working in the same time span on the same types of workplaces (n = 1483), the case: control ratio amounts to 1:30.
Key findings in the present study in respective phases of IMUNOR® administration can be summarized in the following manner:
1. In 51 subjects in the study group, Phase I administration was initiated between January 14, 2021, and February 2, 2021. For respective subjects, the phase was finalized after four doses between February 12, 2021, and March 1, 2021. None of the subjects showed COVID-19 positivity.
2. In 43 subjects in the study group, Phase II was finalized after a total of eight doses between March 2, 2021, and March 22, 2021. The initiation period of this treatment was identical with the previous group. One subject (No. 12) contracted a mild form of the COVID-19 infection during Phase II (the second month of the extended 8-week IMUNOR® administration period) and continued in the one-dose-per week regimen.
3. In Phase III, all 51 subjects were followed-up for one month after the 4-dose or 8-dose schedule, respectively. One subject (No. 8) contracted a mild form of the COVID-19 infection four weeks post the IMUNOR® therapy period.
Extended follow-up after finalization of the Study Protocol witnessed another case report of a mild COVID-19 infection approximately six weeks after the 2-month treatment period comprising eight doses of IMUNOR®.
3.2 Statistical report
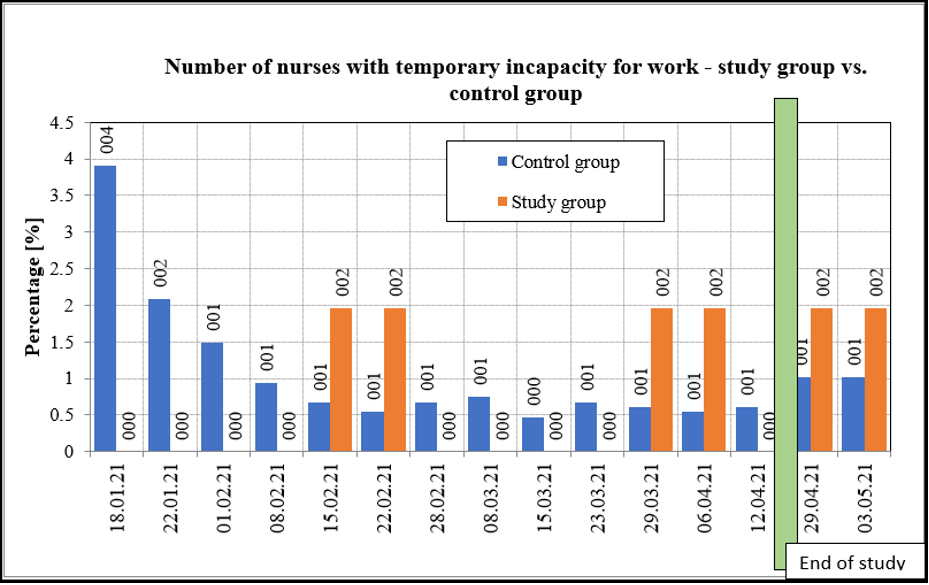
A detailed statistical overview comparing study group nurses versus control group nurses is provided in Table 7, Chart 2 and Chart 3.
Table 7: Statistical analysis of differences between the study group and the control group in terms of temporary incapacity for work
|
Statistical significance calculations for proportional differences between groups – temporary incapacity for work |
|||||
|
Group and date (day, month) |
Subjects with incapacity for work [ni] |
Total subjects [n] |
Percentage of incapacitated [%] |
The difference is statistically |
p-value |
|
Control group 18/01 |
58 |
1483 |
3·91 |
significant |
<0·00001 |
|
Study group 18/01 |
0 |
51 |
0·00 |
||
|
Control group 22/01 |
31 |
1483 |
2·09 |
significant |
<0·00001 |
|
Study group 22/01 |
0 |
51 |
0·00 |
||
|
Control group 01/02 |
22 |
1483 |
1·48 |
significant |
<0·00001 |
|
Study group 01/02 |
0 |
51 |
0·00 |
||
|
Control group 08/02 |
14 |
1483 |
0·94 |
significant |
<0·00001 |
|
Study group 08/02 |
0 |
51 |
0·00 |
||
|
Control group 15/02 |
10 |
1483 |
0·67 |
significant |
<0·00007 |
|
Study group 15/02 |
1 |
51 |
1·96 |
||
|
Control group 22/02 |
8 |
1483 |
0·54 |
significant |
<0·00001 |
|
Study group 22/02 |
1 |
51 |
1·96 |
||
|
Control group 28/02 |
10 |
1483 |
0·67 |
significant |
<0·00001 |
|
Study group 28/02 |
0 |
51 |
0·00 |
||
|
Control group 08/03 |
11 |
1483 |
0·74 |
significant |
<0·00001 |
|
Study group 08/03 |
0 |
51 |
0·00 |
|
|
|
Control group 15/03 |
7 |
1483 |
0·47 |
significant |
<0·00001 |
|
Study group 15/03 |
0 |
51 |
0·00 |
||
|
Control group 23/03 |
10 |
1483 |
0·67 |
significant |
<0·00001 |
|
Study group 23/03 |
0 |
51 |
0·00 |
||
|
Control group 29/03 |
9 |
1483 |
0·61 |
significant |
<0·00002 |
|
Study group 29/03 |
1 |
51 |
1·96 |
||
|
Control group 06/04 |
8 |
1483 |
0·54 |
significant |
<0·00001 |
|
Study group 06/04 |
1 |
51 |
1·96 |
||
|
Control group 12/04 |
9 |
1483 |
0·61 |
significant |
<0·00001 |
|
Study group 12/04 |
0 |
51 |
0·00 |
||
|
Control group 26/04 |
15 |
1483 |
1·01 |
significant |
<0·0063 |
|
Study group 26/04 |
1 |
51 |
1·96 |
||
|
Control group 03/05 |
15 |
1483 |
1·01 |
significant |
<0·0063 |
|
Study group 03/05 |
1 |
51 |
1·96 |
||
Statistical analysis indicates that the percentage of nurses with temporary incapacity for work on all respective dates is significantly different for the study group and the control group. Green fields indicate statistically significant difference in favor of the treated (study) group.
Chart 2: Number of nurses´ sick leaves at the University Hospital in Ostrava
Chart 3: Number of nurses with temporary incapacity for work
As far as the primary outcome measure is concerned, lower incidence rates of illness and fewer temporary incapacities for work due to COVID-19 infection were established for the group treated with IMUNOR®, compared to the control group. The result was statistically highly significant. Besides, lower prevalence of COVID- 19 infection was identified in the study group compared to the control group one month post the end of IMUNOR® administration. Again, the result was highly statistically significant. None of the IMUNOR®-treated subjects developed a moderately severe to severe form of the COVID-19 infection (up to one month post the end of IMUNOR® administration) that would necessitate hospitalization or complex care related to coronavirus infection. Based on case reports, the course of COVID-19 infection in study participants whose household members contracted the disease appeared markedly less severe than in the relatives or partners. The rare occurrence of COVID-19 infection in a study subject one month post IMUNOR® treatment showed a mild course with symptomatic treatment, home rest and temporary incapacity for work (sick leave) with no need of hospitalization (data based on a case report). No major adverse reactions were recorded in the IMUNOR® group and the preparation was well-tolerated. No suspension or pre-term termination of the treatment course was necessary.
4. Discussion and conclusion
Innate immune mechanisms constitute a major and sophisticated defense measure against many types of external and internal adversaries of the human organism, including viruses. Providing the immune system is in a good shape, it is capable of detecting danger and reacting to it properly. Immunomodulatory preparations (immunoglobulins, vitamins, bacterial lysates, immunoglucans etc.) can be used to foster regeneration and activation of the immune system. Both human and animal leukocyte dialysates have been demonstrated to possess activating and substituting properties of this kind. The mechanism of action of leukocyte dialysates such as IMUNOR® (also called ´transfer factors´ based on the Lawrence transfer factor whose properties include immune memory delegation) has not been fully explained in terms of particular structures responsible for its memory-delegating capacity [13, 14]. Nevertheless, it can be assumed that fragments of cell walls, organelles, receptors and cytokines contained in the transfer factor participate in the reproduction, maturation and activation of newly formed immune cells. It may be surmised that specific immune response pattern transfers are also involved.
In the present study, we suggest that preparations based on the above principle can be employed to enhance renewal and maturation of immunocompetent cells (cf. experimental works dealing with hemopoietic cells [8, 9, 10, 11]) and that a specific immune memory may be involved. Consultations with experts in veterinary medicine by the author indicate that porcine livestock (including that used to produce IMUNOR®) is immune against coronavirus infections. Despite the fact that alpha-coronaviruses are involved in porcine milieus, it may be presumed that a certain amount of cross-immunity exists toward beta-coronaviruses, including SARS-CoV-2, the causal agent of COVID-19 infection.
5. Recommendations and practical implications
Based on these preliminary results, an extension of the indication spectrum for IMUNOR® could be recommended to cover not only the treatment area but also the preventative area in patients with either clinically manifest or laboratory-confirmed impaired cell immunity.
The manufacturer could also take initiative to expand the prescription rights to include general practitioners (in indications stated above). It is warranted to design and conduct a larger study of IMUNOR® for the prevention of other major epidemic infections in specifically defined groups (families, workplace teams), including influenza and other major and/or severe acute viral infections.
Acknowledgment
We extend our thanks to Ing. Pavel Liška (affiliated to Zpracování statistických dat) for assisting in statistical data processing. Statistical processing was performed using the R software package, version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria, GNU GPL license).
Conflict of interest
The authors declare that they are not aware of any conflict of interest respecting the issue researched, the course of the study and the present publication. No financial funding by pharmaceutical company sources was involved. A total of 100 four-vial packages of IMUNOR® were provided free of charge by the manufacturer for pertinent institutional research purposes.
References
- Majumder J, Minko T (2021) Recent developments in therapeutic and diagnostic approaches to COVID-19. AAPS J. 23(1): 14.
- Lotfi M, Hamblin MR, Rezaeif N (2020) COVID-19: Transmission, prevention and potential therapeutic opportunities. Clin Chim Acta. 508: 254-66.
- Bystroň J. Transfer factors´ present place in clinical practice. Allergy 2015; 17(2): 137-41 (in Czech).
- Bystroň J, Petrů V, Kopecká K, Richterová I (2007) Influence of DLE-Imunor on clinical and basic immunological parameters in patients with recurrent and chronic infections. Allergy. 9(1): 61- 66.
- Wang JF, Park AJ, Rendini T, Levis WR (2017) Lawrence Transfer Factor: Transference of specific immune memory by dialyzable leukocyte extract from a CD8+ T cell line. J Drugs Dermatol. 16(12): 1198-1206.
- Myles IA, Zhao M, Nardone G, Olano LR, Reckhow JD, et al. (2017) CD8+ T Cells produce a dialyzable antigen-specific activator of dendritic cells. J Leukoc Biol. 101(1): 307-20.
- Acosta-Rios MP, Sauer-Ramírez E, Castro-Muñoz LJ, García- Solís M, Gómez-García C, et al. (2017) Effect of dialyzable leukocyte extract on chronic cervicitis in patients with HPV infection. J Med Life. 10(4): 237-43.
- Vacek A, Hofer M, Holá J, Weiterová L, Streitová D, et al. (2007) The Role of G-CSF and IL-6 in granulopoiesis stimulating blood serum activity on mule induced by orally administered ultrafiltered porcine leukocyte extract IMUNOR. J Int Immunopharmacol. 7(5): 656-61.
- Hofer M, Pospisil M, Znojil V, Holá J, Vacek A, et al. (2006) Meloxicam, a cyclooxygenase 2 inhibitor, promotes hematopoietic recovery in gamma-irradiated mice. Radiat Res. 166(3): 556-60.
- Hofer M, Vacek A, Holá J, Weiterová L, Streitová D (2006) Peroral IMUNOR, a low molecular weight immunomodulator prepared from degraded and ultrafiltered leukocytes, enhances recovery from cisplatin- or 5-fluorouracil-induced myelosuppression. Immunopharmaceutical immunotoxicol. 28(1): 1-11.
- Hofer M, Vacek A, Lojek A, Holá J, Streitová D (2007) Ultrafiltered porcine leukocyte extract (IMUNOR) reduces nitric oxide production and blood cell stimulating cytokine production in lipopolysaccharide-stimulated RAW 264, 7 macrophages. Int Immunopharmacol. 7(10): 1369-74.