Claudia Trojaniello*, Maria Grazia Vitale*, Vito Vanella, Ester Simeone, Benedetta Alfano, Luigi Scarpato, Paolo Antonio Ascierto#
Department Melanoma, Cancer Immunotherapy and Development Therapeutics, Istituto Nazionale Tumori IRCCS Fondazione Pascale, Napoli, Italy
*These authors contributed equally
#Corresponding Author: Paolo Antonio Ascierto, Department Melanoma, Cancer Immunotherapy and Development Therapeutics, Istituto Nazionale Tumori IRCCS Fondazione Pascale, Napoli, Italy
Abstract
Emerging evidence indicates that immune checkpoint inhibitors (ICIs) may be safe and effective in cancer patients who become infected with the novel 2019 severe acute respiratory syndrome coronavirus (SARS-CoV-2), the etiological agent responsible for the ongoing COVID-19 pandemic. However, limited data exist on the interplay between checkpoint inhibition and anti-tumor immune responses in the context of SARS-CoV-2 infection. Persistent viral infection and cancer both cause T cell exhaustion via the PD-1/PD-L1 axis and severe COVID-19 is associated with dysfunctional T cell responses and upregulation of exhaustion markers coupled with a systemic inflammatory milieu sometimes characterized as a "cytokine storm." The potential for SARS-CoV-2 to affect anti-tumor immune responses has been described previously in isolated case reports. Here we describe a case of an older man with advanced cutaneous squamous cell carcinoma who became infected with SARS-CoV-2 while receiving anti-PD-1 checkpoint blockade. The patient not only recovered from COVID-19 with no long-term adverse effects despite the presence of comorbidities but also a pronounced reduction in tumor burden was seen 2 months after ICIs were stopped due to infection. The case provides evidence for a virally mediated abscopal effect in patients with COVID-19 and cancer.
Keywords: COVID-19, skin neoplasms, immunotherapy
Introduction
COVID-19, the disease caused by the novel 2019 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), remains an ongoing global health emergency. Although highly effective vaccines have been developed since the virus first emerged in Wuhan, China, and subsequently spread to infect millions of people worldwide within 15 months, non-universal access and compliance, emerging variants, and recurrent local outbreaks necessitate ongoing social and physical distancing measures in several regions to slow the spread.
COVID-19 causes a multi-organ inflammatory syndrome characterized by acute respiratory distress, disseminated coagulation, and a "cytokine storm" [1]. Anti-inflammatory interventions such as corticosteroids and interleukin (IL)-6 modulation have reduced mortality in severe COVID-19. However, robust T-cell responses are also essential for viral clearance, and dysfunction in the lymphocyte compartment has been linked with worse outcomes [2-4]. T cell responses are known to be dysregulated in patients with cancer, and immune checkpoint inhibitor (ICIs) antibodies targeting the PD- 1/PD-L1 axis offer deep and durable responses to many patients with a variety of solid tumors. ICI therapy's known mechanisms and toxicities may include inflammatory pulmonary manifestations reminiscent of COVID-19 pneumonitis, causing speculation at the beginning of the pandemic that checkpoint blockade could cause worse outcomes in SARS-CoV-2-infected cancer patients. Emerging data suggest that developments in ICI-treated patients are no worse than individuals with comparable comorbidities receiving conventional anti-cancer treatment [5,6].
More data is needed to understand the safety and efficacy of ICI therapy in patients with cancer and COVID-19, especially the interplay between anti-viral and anti-tumor immunity. Here, we describe a case report of a patient with advanced cutaneous squamous cell carcinoma (CSCC) who became infected with SARS-CoV-2 while on the anti-PD-1 therapy cemiplimab. The patient recovered completely after hospitalization, interruption of ICI, and treatment with steroids. Furthermore, the patient's lesions continued to shrink after ICIs were halted, suggesting the possibility of a viral abscopal effect.
Case Presentation
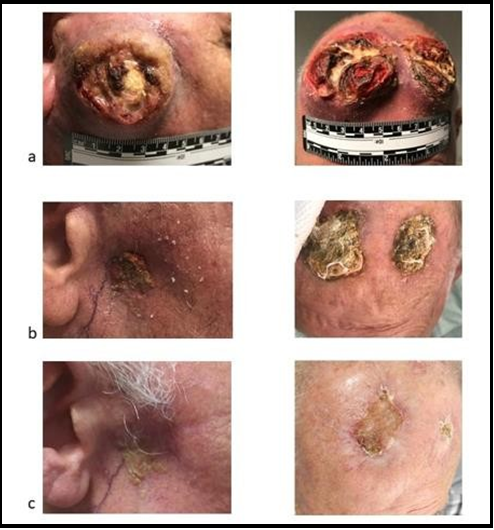
A male patient 82 years of age with a history of advanced CSCC was referred to our institution in June 2020 with rapid progression during radiotherapy. Previously, the patient had undergone local excision for a skin lesion on the frontal area of the head in 2019. After a local relapse of the excised lesion in April 2020, 50 Gy radiotherapy in 25 fractions was planned. When he arrived at our institute, an invasive disease lesion was present on the frontal lesion, in addition to an ulcerated lesion of 49 x 57 mm on the parotid gland with two right lateral cervical lymph nodes of 30 x 25 mm and 27 x 17 mm (see Figure 1).
At presentation, the patient's Eastern Cooperative Oncology Group (ECOG) performance status score was 1. Baseline comorbidities were chronic obstructive pulmonary disease (COPD), managed with formoterol every 12 hours, and hypertension, for which the patient was treated with lercanidipine.
Systemic immunotherapy consisted of the anti-PD1 cemiplimab at a dosing schedule of 350 mg every 3 weeks. At the first assessment, significant size reductions were seen for the parotid lesion, the frontal lesions, and the lateral cervical lymph nodes (see Figure 1). Therapy was well-tolerated, and no immune-related adverse events (irAEs) were observed while on treatment. The patient received 8 cycles of cemiplimab before testing positive for COVID-19 during routine surveillance per institutional protocols. Upon diagnosis of COVID-19, cemiplimab was halted.
The patient was diagnosed with SARS-CoV-2 infection by nasopharyngeal swab test on 26 November 2020. At diagnosis, the patient had a moderate fever. White blood cell counts, hepatic, and renal function was all normal. Serum C-reactive protein was measured at 16.7 mg/L. Within 4 days, the patient developed bilateral pneumonitis and hypoxia with oxygen saturation of 93 % on room air and was hospitalized in a dedicated COVID center on 30 November 2020. Treatment for COVID-19 was initiated, consisting of dexamethasone 8 mg BID and supplemental oxygen at 2 L/min by nasal cannula. Rapid recovery occurred, with the patient no longer requiring respiratory support within 6 days. The patient was discharged from the hospital on 8 December 2020, despite persistent positivity for SARS-CoV-2 by nasopharyngeal PCR. Four subsequent PCR tests were performed after discharge, and the patient tested negative on 20 January 2021.
No long-term sequelae associated with COVID-19 were observed. The patient returned for a re-evaluation visit for the CSCC on 4 February 2021. At the visit, despite receiving no other systemic therapy for CSCC after cemiplimab was halted, lesion size and ulceration were further reduced (see Figure 1).
Figure 1: Images of cutaneous squamous cell carcinoma lesions at (a), showing reductions in size and ulceration from baseline while on-treatment with cemiplimab (b), and continued improvement after therapy was halted due to SARS-CoV-2 infection (c)
Discussions
We describe a case of a patient undergoing treatment with anti-PD-1 who derived continued clinical benefit with immunotherapy despite discontinuation of the ICI due to SARS-CoV-2 infection. The observation that anti-PD-1 therapy does not predispose patients to worse outcomes with COVID-19 is in line with the previous retrospective reports in patients with skin [6,7] and lung cancer [5], adding further evidence that checkpoint inhibitor therapy is likely safe and effective for patients with non-severe SARS-CoV-2 infections. Furthermore, this case highlights a potential interplay between anti-SARS-CoV-2 immunity and the anti-tumor response.
The mechanism responsible for severe COVID-19 likely involves the innate and adaptive arms of the immune system, with tissue damage stemming from both a monocyte- and macrophage-driven inflammatory cascade [1] and direct cytopathic effects [8], in part due to inadequate viral control. Cytotoxic T cells from patients with severe COVID-19 disease display a predominantly exhausted phenotype, characterized by high PD-1 expression [2,3].
The outcomes of this case indicate that pre-infection exposure to PD-1 blockade did not prevent a patient with CSCC from recovering from COVID-19 after steroid therapy. Although prolonged viral genome shedding via nasopharyngeal PCR was observed, the patient recovered completely after a short course of high-dose steroids despite baseline comorbidities that are known risk factors for severe diseases such as COPD [9]. Given the association between T cell exhaustion and painful illness, pilot trials have been launched investigating checkpoint blockade for otherwise healthy patients infected with SARS-CoV-2 (e.g., NCT04356508, NCT04268537, and NCT04413838). The mechanism-based reasoning suggesting that immunotherapy may enhance anti-SARS-CoV-2 immune responses is purely speculative. On the other side of the coin, however, this case and others [10-12] report cases of tumor regression secondary to SARS-CoV-2 infection.
Notably, the patient not only recovered from COVID-19 but also showed a dramatic reduction in lesion burden even after immunotherapy was halted, hinting that viral infection may have potentiated tumor immune control. Lesion shrinkage was observed 2 months after cemiplimab was stopped. Given the reported serum half-life for cemiplimab of roughly 20 days [13], tumor control's durability may be partially attributed to continuous receptor occupancy of the anti-PD-1 antibody. Yet the possibility of enhanced immune control through a virally mediated abscopal effect cannot be excluded.
Potential mechanisms for SARS-CoV-2-induced tumor control include activation of NK cells due to systemic hypercytokinemia including elevated IL-6 [1], cross-reactivity of virus-specific T cells with tumor antigens, and an abscopal effect whereby infection increases antigen presentation by myeloid cells in the stroma while also enhancing T cell activation through the release of danger- associated molecular patterns. Although the existence of a bonafide abscopal effect after radiotherapy remains controversial, the phenomenon of pathogen-triggered anti-tumor responses has been appreciated for more than one century—sarcoma regression after infection with Streptococcus was reported in 1871 [14], and intravesical instillation of the tuberculosis bacterium Bacillus Calmette-Guerin remains a mainstay of bladder cancer treatment to the present day. Before this case, two reports have described patients with lymphoma achieving complete remission after infection with SARS-CoV-2 [10,11], and another observed 25 % shrinkage of metastases in a patient with melanoma COVID-19 [12].
Conclusion
In summary, this report adds to the growing body of evidence that PD-1 blockade may be safe and effective for patients with cancer and COVID-19. Furthermore, the documentation of continuing tumor response even after cessation of therapy adds insight into the cross-talk between anti-viral and anti-tumor immunity, the mechanisms of which should be further explored to enhance the efficacy of both immunotherapy and COVID-19 treatments.
Statements
Acknowledgments: The authors thank the patient and his family. Additionally, the authors acknowledge Sam Million-Weaver, Ph.D., for medical writing assistance.
Statement of Ethics: Written informed consent was obtained from the patient to publish this case report and any accompanying images.
Conflict of Interest Statement
PAA—Contract research: BMS, Roche, Array, Sanofi; Consulting fee: BMS, Roche, Array, Novartis, Merck Serono, Pierre Fabre, Incyte, Medimmune, Sindax, Astrazeneca, Sun Pharma, Sanofi, Idera, Ultimovacs, Sandbox, Immunocore, 4sc, Alkermes, Italfarmaco, Nektar, Boehringer-Ingelheim, Eisai, Regeneron, Daiichi Sankyo, Pfizer, Oncosec, Nouscom, Takis, Lunaphore, Seagen; Travel support: MSD.
ES received honoraria from Bristol Myers Squibb, Novartis, and Merck Sharp & Dohme.
CT, MGV, VV, BA, and LS declare no conflict of interest.
Funding Sources: The authors have not declared a specific grant for this research from any funding agency in public, commercial or not-for-profit sectors.
Author Contributions
PAA, CT, and MGV led the development and conceptualization of this case report. VV and ES were responsible for the patient's treatment and follow-up. BA and LS collected all the data. All authors analyzed and interpreted the data, contributed to the content, writing, and revisions, and have read and approved the submitted manuscript
Data Availability Statement: Not applicable
References
- Arnaldez FI, O'Day SJ, Drake CG, Fox BA, Fu B, et al. (2020) The Society for Immunotherapy of Cancer perspective on regulation of interleukin-6 signaling in COVID-19-related systemic inflammatory response. Journal for Immuno Therapy of Cancer. 8(1): e000930.
- Mathew D, Giles JR, Baxter AE, Oldridge DA, Greenplate AR, et al. (2020) Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 369(6508): eabc8511.
- Diao B, Wang C, Tan Y, Chen X, Liu Y, et al. (2020) Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front Immunol. 11: 827.
- Li M, Guo W, Dong Y, Wang X, Dai D, et al. (2020) Elevated Exhaustion Levels of NK and CD8(+) T Cells as Indicators for Progression and Prognosis of COVID-19 Disease. Front Immunol. 11: 580237.
- Luo J, Rizvi H, Egger JV, Preeshagul IR, Wolchok JD, et al. (2020) Impact of PD-1 Blockade on Severity of COVID-19 in Patients with Lung Cancers. Cancer Discov. 10(8): 1121-1128.
- Trojaniello C, Vitale MG, Ascierto PA (2021) Checkpoint inhibitor therapy for skin cancer may be safe in patients with asymptomatic COVID-19. Ann Oncol. 32(5): 674-676.
- Isgrò MA, Vitale MG, Celentano E, Nocerino F, Porciello G, et al. (2021) Immunotherapy may protect cancer patients from SARS-CoV-2 infection: a single-center retrospective analysis. Journal of translational medicine. 19(1): 132.
- Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, et al. (2020) Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 20(10): 1135-1140.
- Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, et al. (2020) Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern Med. 180(10): 1345-1355.
- Challenor S, Tucker D (2021) SARS-CoV-2-induced remission of Hodgkin lymphoma. Br J Haematol. 192(3): 415.
- Sollini M, Gelardi F, Carlo-Stella C, Chiti A (2021) Complete remission of follicular lymphoma after SARS-CoV-2 infection: from the "flare phenomenon" to the "abscopal effect". Eur J Nucl Med Mol Imaging. 48(8): 2652-2654.
- Herrscher H, Sauer B, Truntzer P, Robert C (2021) Abscopal antitumor effect in a patient with melanoma and coronavirus disease 2019. European journal of cancer. 149: 91-93.
- Yang F, Paccaly AJ, Rippley RK, Davis JD, DiCioccio AT (2021) Population pharmacokinetic characteristics of cemiplimab in patients with advanced malignancies. Journal of Pharmacokinetics and Pharmacodynamics. 48(4): 479-494.
- Wiemann B, Starnes CO (1994) Coley's toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther. 64(3): 529-64.