Konstantine Chakhunasvhili1,2*, Davit G. Chakhunashvili1,2, Eka Kvirkvelia2, Maia Chkhaidze2,3, Davit Rekhviashvili2, Zurab Kakabadze1
1Tbilisi State Medical University, Tbilisi, Georgia
2Children’s New Clinic After Irakli Tsitsishvili, Tbilisi, Georgia
3Geomedi University, Tbilisi, Georgia
*Corresponding Author: Konstantine Chakhunashvili, Tbilisi State Medical University, Tbilisi, Georgia & Children’s New Clinic After Irakli Tsitsishvili, Tbilisi, Georgia.
Abstract
Introduction: In 2019, a new strain of Coronavirus - Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-COV-2) emerged first in China, and then it spread worldwide wreaking havoc on numerous countries and numerous lives. In the pediatric population, disease progression is relatively milder, however, it is of vital importance to gather and analyze the existing data.
Methods: We reviewed and gathered data from all the hospitalized patients with Coronavirus Disease 2019 (COVID-19) diagnoses during the period between November 2020 and March 2021. Medical records of 604 children aged between 0-18 years were collected at Children's New Clinic after Irakli Tsitsishvili.
Results: The results showed the importance of Ferritin and D-dimer as inflammatory markers and a direct correlation between high numbers and severity of the disease. D-dimer, however, in comparison to the adult population, is more likely to be predictive of a severe inflammatory process, rather than a thrombotic event.
Conclusion: We can conclude that SARS-COV-2 causes significantly milder disease in children than in adults. However, the infection spreads much quicker among children because of low adherence to recommended protective actions habits and high morbidity can still cause issues since multiple complications can occur during and after COVID-19.
Keywords: MIS-C, AKI, SARS-COV-2, Bilateral Pneumonia, Severe COVID-19, D-Dimer in pediatric COVID-19, Ferritin in pediatric COVID-19, LDH in Pediatric COVID-19.
Introduction
In 2019, a new strain of Coronavirus SARS-COV-2 emerged first in China, and then it spread worldwide wreaking havoc on numerous countries and numerous lives [1-3]. Therefore, a pandemic has been declared by WHO [4]. In general, COVID-19 cases in pediatric patients have been rather mild compared to the adult population resulting in a lower hospitalization rate and close to zero death rate [5-8]. Although, it is of vital importance to gather and analyze the existing data from the hospitalized patients to further increase positive outcomes for this population. Thus, we will be reporting clinical, instrumental and laboratory data that have been acquired from 604 patients who tested positive for SARS-COV-2 and were hospitalized in Children’s New Clinic After Irakli Tsitsishvili, which is the biggest pediatric COVID center in Georgia.
Methodology
This study will contain a retrospective review of medical records for 604 pediatric patients with ages 0-18 years, who had a laboratory-confirmed diagnosis of COVID-19 and were hospitalized between November 2020 and March 2021. The study was conducted following the Helsinki Declaration, 1975, and Tbilisi State Medical University (TSMU) ethics committee approval (approval by meeting record 9, 13.01.2022). The data was gathered at Children’s New Clinic after Irakli Tsitsishvili.
Classification of Severity
The severity of the disease was classified into two groups: severe and non-severe [9]. Patients who were considered severe had either one or multiple of these conditions: dyspnea with desaturation (O2 < 92 %), acute respiratory distress (ARD), shock, encephalopathy, acute kidney injury (AKI), problems with coagulation, symptomatic myocardial involvement [10]. All the patients who didn’t develop these conditions were considered non-severe.
Laboratory Assays
The laboratory assay data were collected in the following fashion: baseline, which was collected and run upon admission of the patient; intermediary, which was collected and run in between discharge and admission; and endline, which was collected and run right before discharge. It should be noted that not all patients were subjected to intermediary and endline examinations, so the data could not be collected for those patients. The main laboratory assays that will be discussed here are Complete Blood Count (CBC), C-Reactive Protein (CRP), Alanine transaminase (ALT), Aspartate transaminase (AST), Ferritin, D-Dimer, Lactate dehydrogenase (LDH), and Procalcitonin (PCT).
Instrumental Examinations
Instrumental examinations, such as ultrasonography (US), radiography, and computed tomography (CT), were also collected from patients who underwent either one of these examinations.
Statistics
Microsoft Excel 2016 was used for basic statistics and the creation of figures specifically for this study.
Study Limitations
The limitations of this study are that it is a retrospective study and it reviews the medical record of only hospitalized patients, which means the data here cannot be used to generalize to the whole population. One more limitation is the period that the study was held, it should not be generalized to every patient since variant-specific properties might apply.
Results
General Cohort Information
During the period between November 2020 and March 2021, 604 patients were hospitalized at Children’s New Clinic (CNC). Out of 604, 290 were female and 314 were male. The majority of the patients (462/604, 76.5 %) were 5 years and younger. The most frequent presenting symptoms and signs were fever (450/604, 74.5 %), cough (300/604, 49.7 %), vomiting/nausea (120/604, 19.8 %), diarrhea (100/604, 16.5 %), catarrhal signs (76/604, 12.6 %), dyspnea (50/604, 8.3 %), headache (25/604, 4.1 %), abdominal pain (20/604, 3.3 %), myalgia (6/604, 1 %). No lethal outcome was observed (Table 1).
Hospital Stay Duration
The average hospital stay duration for all patients was 6.6 days. However, the mean hospital stay duration for patients younger than 2 years was 11.8 days. The longest hospital stay was 50 days and the shortest was 1 day. In total 325 (53.7 %) patients stayed five days or less in the hospital, out of the 299 (95.1 %) were considered non-severe, 16 (4.9 %) were severe. In the remaining 279 (46.3 %) children, the hospital stay was more than 5 days. Out of those patients, 213 (76.3 %) were considered to be non-severe, and 66 (23.7 %) were severe.
Severity of Disease
Out of 604 patients, 522 (86.4 %) were considered non-severe and 82 (13.6 %) were considered to be severe. The majority of the severe patients were older than 5 years, therefore, out of 462 children, who were younger than 5 years (Table 1), only 29 (6.2 %) were considered to be severe.
|
|
Number of Patients |
Percentage (%) |
|
Age < 5 years |
462 |
76.5 % (462/604) |
|
Age < 2 years |
185 |
30.6 % (185/604) |
|
Pneumonia |
33 |
9.9 % (33/334) |
|
Desaturation among patients with pneumonia |
10 |
30 % (10/33) |
|
Severe |
82 |
13.6 % (82/604) |
|
Pericardial Effusion |
7 |
8.5 % (7/82) |
|
Pleural Effusion |
8 |
9 % (8/82) |
|
Multisystem Inflammatory Syndrome in Children |
4 |
0.6 % (4/604) |
|
Acute Kidney Injury |
2 |
0.3 % (2/604) |
|
Symptomatic Thrombotic Event |
0 |
0 |
|
Death |
0 |
0 |
Instrumental Examinations
Radiographic examination of the chest was performed in 367 patients out of which 33 (8.9 %) patients had abnormal results and 334 (91.1 %) did not present changes. Pathologies, such as bilateral pneumonia (7/367, 1.9 %), pneumonia (23/367, 6.3 %), pneumothorax with pneumonia (3/367, 0.81 %) were detected. The maximum hospital stay duration was 50 days for 1 patient and the minimum stay duration was 3 days for 1 patient as well. 22 patients (67 %) had hospital duration of 14 days or shorter, however, 11 patients (33%) had hospital stay duration longer than 14 days.
7 cases of pericardial effusion were detected by ultrasound (US) examination. Out of those seven patients, 5 were not showing typical signs and symptoms of moderate or severe pericardial effusion, on the other hand, 2 patients had dyspnea, while no lung injury was detected.
1 patient had aggressive disease progression with significantly elevated CRP (120 mg/L) and White Blood Cells (WBC) (20.2 x 103) levels, although, no clinical signs or symptoms for pericardial effusion were detected. 8 cases of pleural effusion were detected, and most of them were linked to lung injuries.
Laboratory Assays
CBC
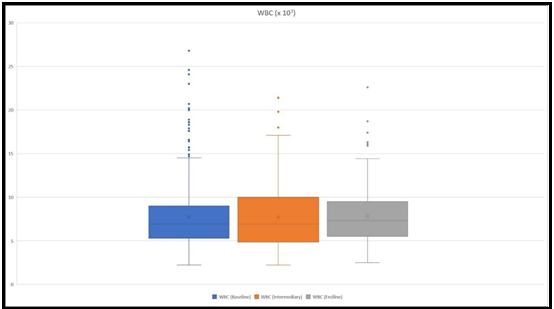
Blood samples for CBC were retrieved from all the patients at the baseline, 172 intermediary samples and 376 endline samples were collected. The mean WBC count at the baseline was 7.86 x 103, the mean WBC at intermediary collection was 7.8 x 103 and at the endline 8.09 x 103 (Figure 1). The mean band count was 5.7 % at the baseline, 4.3 % at the intermediary collection, and 2.9 % at the endline. The mean lymphocyte count was 44.5 % at the baseline, 46.11 % at intermediary collection, and 54.3 % at the endline. Patients who stayed at the hospital for more than five days and were considered severe had a mean WBC count of 9.1 x 103 at the baseline, 8.17 x 103 at an intermediary collection, and 7.8 x 103 at the endline. Whilst, their mean band count was 7.6 %, 3.5 %, and 2.3 %, respectively at the baseline, intermediary, and endline.
Figure 1: The figure depicts WBC count ranges at the baseline, at an intermediary, and at the endline. The highest values were 26.8 x 103, 21.6 x 103, and 22.6 x 103, respectively at the baseline, intermediary, and at the endline.
CRP
All patients at the baseline were subjected to collecting samples for CRP measurement, 81 at the intermediary and 183 at the endline.
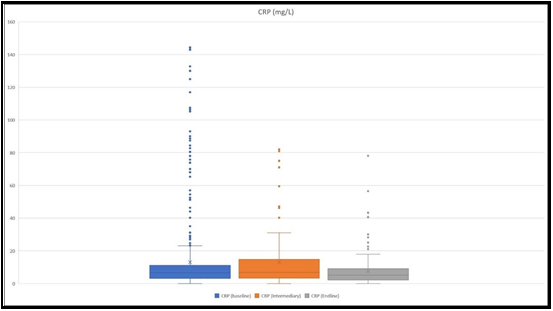
The mean results were as follows, 13.19 mg/L at the baseline, 13.78 mg/L at the intermediary collection, and 7.47 mg/L at the endline (Figure 2). A maximum CRP level of 143 mg/L was detected. At the baseline, CRP level above 50 mg/L was detected in 37 patients, out of which 13 (35 %) were considered severe. CRP level below 50 was detected in 567 patients, out of which only 61 (10.8 %) were considered severe.
In children who had CRP levels above 50 mg/L, 10 (27 %) had pneumonia, and half of them had bilateral lesions of the lung. Whilst, out of the children who had CRP levels below 50 mg/L pneumonia was detected in only 23 (4.01 %) and 4 had bilateral lung injury.
Figure 2: The figure depicts CRP level ranges at the baseline, at an intermediary, and at the endline. The highest values that we detected were 143 mg/L, 80.9 mg/L, and 78.08 mg/L, respectively at the baseline, intermediary, and at the endline.
Liver Function Tests (ALT, AST)
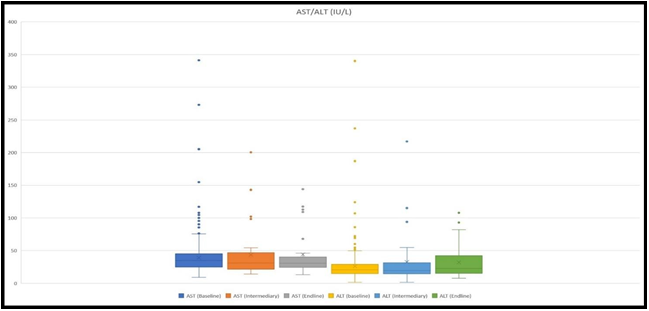
Sample for AST and ALT measurement was taken from 538 patients at the baseline, 38 at an intermediary, and 27 at the endline. Mean values for AST were 38.2 IU/L, 41.9 IU/L, and 44 IU/L, respectively at the baseline, intermediary, and endline. Whereas, the mean values for ALT were 25.1 IU/L, 30.9 IU/L, and 32.16 IU/L (Figure 3).
AST was 100 IU/L or higher in 14 patients. Their average hospital stay duration was 20 days (50 max., 3 min.). Out of the 9 (9/14, 64 %) were considered to be severe, 4 (4/14, 29 %) had pneumonia with a single case (1/4, 25%) of confirmed bilateral lung injury. At the same time, the number of severe patients, among those who had AST < 100 IU/L, was 74 (74/524, 14.1%) and 27 (27/524, 5.1 %) had pneumonia with 7 cases (7/27, 26 %) of the confirmed bilateral lesion. ALT was 100 IU/L or higher in 9 patients. Their average hospital stay duration was 29 days (50 max., 9 min.). Out of these children, 8 (8/9, 89 %) were considered to be severe, and 4 (4/9, 44 %) had pneumonia with a single (1 /4, 25 %) bilateral lesion to the lungs. Among those, who had ALT < 100 IU/L, only 62 (62/538, 11.5 %) were considered to be severe, 26 (26/438, 4.8 %) had pneumonia with 7 cases (7/26, 27 %) of bilateral lung injury.
Figure 3: The figure demonstrates ranges of AST and ALT levels at the baseline, at an intermediary, and at the endline. The highest values for AST were 341 IU/L, 200.5 IU/L, and 144 IU/L, whilst for ALT 340.1 IU/L, 217 IU/L, and 107.8 IU/L, respectively at the baseline, intermediary, and at the endline.
Ferritin
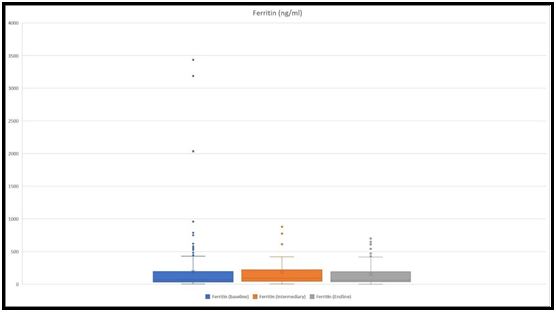
Ferritin levels were measured in 182 children at the baseline, 45 children at an intermediary, and 53 at the endline. Mean values were 158 ng/ml, 181 ng/ml, and 161 ng/ml, respectively at the baseline, intermediary, and ending (Figure 4).
Patients who had ferritin values of 500 ng/ml and higher at the baseline had a mean hospital stay duration of 11 days (max. 35, min. 2). 23 patients had such ferritin values, out of which 14 (61 %) were considered to be severe and 9 (39 %) were non-severe. 4 (4/24, 17%) had pneumonia and all of them had bilateral lesions.
On the other hand, 158 patients had ferritin values lower than 500 ng/ml at the baseline. Their mean hospital stay duration was 3 days (max. 5, min. 1). 27 (27/158, 17 %) were considered to be severe, 131 (131/158, 83 %) were non-severe. 13 (13/158, 8 %) had pneumonia and only 3 out of 13 had bilateral lung injury.
Figure 4: The figure shows ranges of ferritin levels at the baseline, at an intermediary, and at the endline. The highest values for ferritin were 3434 ng/ml, 879 ng/ml, and 650 ng/ml, respectively at the baseline, intermediary, and at the endline.
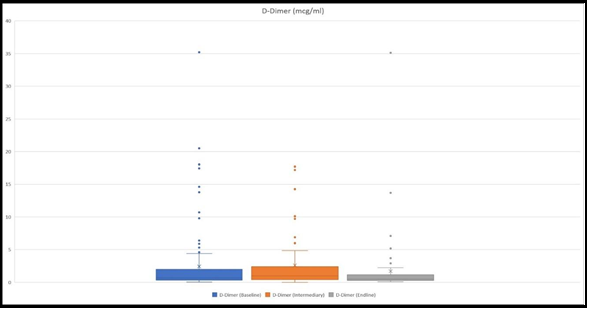
D-Dimer
D-Dimer was measured at the baseline in 125 children, 61 at an intermediary, and 70 at the endline. Mean values were 1.99 mcg/ml, 5.92 mcg/ml, and 1.64 mcg/ml, respectively at the baseline, intermediary, and ending (Figure 5).
Among the patients who had hospital stays more than 5 days, the mean D-Dimer value at the baseline was 2.41 mcg/ml, whilst those who stayed 5 days or less had a mean value of 1.57 mcg/ml.
In those patients who were considered to be severe and had their D- Dimer measured mean was 5.4 mcg/ml.
We also detected those 8 patients had D-dimer levels above 2 mcg/ml (13.78 mcg/ml, 18.03 mcg/ml, 2.01 mcg/ml, 2.3 mcg/ml, 2.4 mcg/ml, 3.17 mcg/ml, 3.3 mcg/ml, 4.13 mcg/ml), and were considered non- severe plus there was a serious discrepancy between these results and other laboratory results together with the clinical course of the disease. Therefore, the results were re-run and all of these patients had their D-dimer results lower than 0.5 mcg/ml.
There were also patients, whose clinical course and other laboratory results corresponded with the high D-Dimer levels. For example, one patient, whose D-Dimer was 20.5 mcg/ml, LDH was as high as 7696 U/L, serum creatinine was 2.17 mg/dl, and had a severe course of the disease with AKI and Multisystem Inflammatory Syndrome in Children (MIS-C). Another example was a patient who had D-Dimer 35.2 mcg/ml, ferritin as high as 3434 ng/ml with progressive kidney function deterioration, and in the end diagnosis of MIS-C was considered. There also were 5 patients whose D-dimer levels were also skyrocketing (1.85mcg/ml, 1.9 mcg/ml, 10.7 mcg/ml, 14.6 mcg/ml, 17.85 mcg/ml), all of them had signs for respiratory failure, and radiologically 3 of them had bilateral lung lesions. 2 patients with hypovolemic shock had significantly elevated D-Dimer levels (10.7 mcg/ml, 2.6 mcg/ml).
Figure 5: The figure presents ranges of D-Dimer level at the baseline, at intermediary, and at the endline. The highest values were 35.2 mcg/ml, 17.17 mcg/ml, and 35.1 mcg/ml, respectively at the baseline, intermediary, and at the endline.
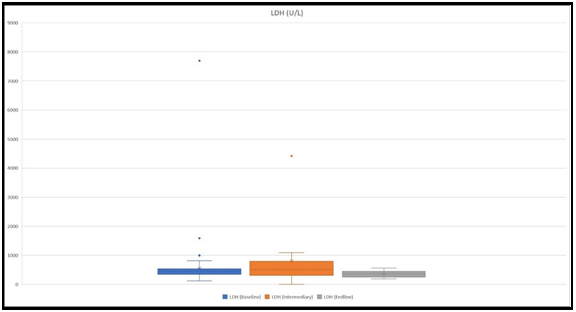
LDH
LDH levels were measured in 151 patients at the baseline, in 13 patients at an intermediary, and in 15 patients at the endline. Mean values that were received were 498 U/L, 804.6 U/L, and 354.6 U/L, respectively at the baseline, intermediary, and ending (Figure 6).
We detected two instances when LDH levels were higher than 1500 U/L at the baseline. Both of them had MIS-C.
Figure 6: The figure shows ranges of LDH levels at the baseline, at an intermediary, and at the endline. The highest values were 7696 U/L, 4414 U/L, and 354.6 mcg/ml, respectively at the baseline, intermediary, and at the endline.
Discussion
In this single-center retrospective study, we tried to analyze the vast amount of information that has been gathered at the Children’s New Clinic after Irakli Tsitsishvili. Since the pandemic is still ravaging Georgia, we will continue to monitor and try to detect variant-specific characteristics of COVID-19.
The current data about the severity of SARS-COV-2-induced disease suggests that the clinical course is much milder in children than in adults, the data that we gathered are also suggestive of that [11-16]. Information that was gathered suggests that children who were younger than 2 years had a longer hospital stay duration (6.6 days vs
11.8 days). Since the majority of the patients, who were considered to be severe were 5 years and older, we took a deeper look at the medical records for those below 2 years of age. A thorough analysis of their medical history showed us that inability of the caregiver to follow instructions for symptomatic treatment at home (mainly, adequate hydration) and the high risk of severe dehydration, hypovolemia was the reason for their prolonged hospital stay.
Radiographic data suggest that in the pediatric population lung involvement is significantly lower when compared to the adult population. As per our results, 33 cases of lung injury were confirmed out of 367 patients, which is 8.9 %. Whilst, in adults this number is drastically different and higher - it ranges anywhere between 47 % - 100 % [17-21].
Pericardial effusion was detected by ultrasonography in 7 patients, out of which 2 children had dyspnea when no lung injury was observed. Whilst it is already a well-established fact that myocardial, pericardial, and coronary artery involvement is quite frequent in MIS- C [22-24] our data together with other publications might be suggestive of the fact that mild pericardial involvement with or without myocardial involvement might be more frequent than currently detected and might even be found in patients who did not develop MIS-C [25,26].
We detected 4 patients with suggestive clinical and laboratory data who were considered to have MIS-C according to CDC criteria [27]. 2 of them had AKI, diarrhea, fever, gastrointestinal involvement (elevated AST/ALT) hypoalbuminemia, low platelet count, increased inflammatory markers (Ferritin, LDH, D-Dimer, Procalcitonin (PCT)), effusion was found in peritoneal, pericardial and pleural cavities, serum creatinine was increased in one patient (2.17 mg/dl), which had oliguria and hypertension, the other patients had progressively increased serum creatinine levels (last documented > 4 mg/dl), which eventually developed anuria and was transferred to a hospital with the capability of hemodialysis. 1 patient had a fever, vomiting, seizures, hypovolemia, and increased inflammatory markers (CRP, PCT, D-Dimer). The fourth one had a fever, vomiting, mucocutaneous involvement, effusion of the hip and knee joint, and significantly elevated inflammatory markers (CRP, D-Dimer, PCT). Blood cultures were negative for all of them. The minimum hospital stay was 15 days, maximum of 35.
CRP was highly predictive of severe disease when levels were above 50 mg/dl at the baseline, as opposed to levels below 50 mg/dl. Our data showed that in those children who had values > 50 mg/dl, 35 % (13/37) were considered severe and 27 % (10/37) had lung injuries, whilst 50 % (5/10) had bilateral injuries. Percentages were drastically different from those who had values below 50 mg/dl, 10.8 % (61/567) were severe, 4% (23/567) had pneumonia, and 17 % (4/23) had bilateral injuries to the lung. According to this data and other published data CRP can be a good tool in the assessment of the disease progression and suspecting serious complications such as MIS-C [28-30].
Increased AST and ALT levels equal to 100 IU/L and higher were highly predictive of severe disease according to the data. In the case of AST, children who had values > 100 IU/L had almost four times the chance of severe disease (64 % vs 14 %) and more than five times the chance of pneumonia (29 % vs 5 %). In the case of ALT, patients who had values > 100 IU/L had almost eight times the chance of severe disease (89 % vs 12 %) and almost ten times the chance of developing pneumonia (44 % vs 4.8 %). Similar findings have been found in other studies, reporting that levels below 100 IU/L were mostly detected in non-severe patients, however, values above that were frequently found in severe patients as well as in MIS-C cases [31-33].
Ferritin levels were also quite useful in predicting serious complications or severe disease if the levels were 500 ng/ml or higher. These levels were also clearly associated with longer mean hospital stay duration (11 days vs 3 days). The number of severe patients in this group of children was as high as 61 % (14/23), while in those who had < 500 ng/ml, this number was 17 % (27/158). There was also a significant difference in the rate of pneumonia (17 % vs 8 %), all of the patients (4/4) who had ferritin levels of 500 ng/ml or higher had bilateral lung lesions, while in the other group – 23 % (3/13) had bilateral injuries. These data are also following other articles that have shown - ferritin levels can be an important marker of severe disease both in the pediatric and adult population, although, in most of these reports the pediatric population with high ferritin levels tends to have hyperinflammatory processes, which was not the case in the data that we are reporting [34-38].
Our findings of D-Dimer indicated that if high levels (>2 mcg/ml) are detected and there are no other signs of a serious infection, it would be wiser not to panic, observe the patient and/or redo the testing to make sure that the received results are correct. On the other hand, the data suggest if there are signs of serious infection, complication, or other changes in inflammatory markers such as extremely high ferritin, or LDH, it should be taken into account so that we do not miss severe lung injury, AKI, MIS-C, etc. A thorough review of the medical records for the patients who had high D-Dimer suggested that in the pediatric population this was a sign of either a severe course of the disease or hyperinflammatory process rather than being prognostic of a hypercoagulative state, the latter is also suggested and reported by multiple authors as well [39-41].
We found that LDH was higher than 1500 U/L in two cases and both patients were considered severe, both of them had clinical and laboratory signs suggestive of MIS-C.
According to our data (Table 1) and other reported data, we can underline that severity of the disease among the pediatric population is significantly milder, although, it does not mean that either the caregiver or service provider should neglect the possible serious complications or severe course of the disease. Therefore, it would be wise to advise parents to vaccinate those, for whom the vaccine use has already been approved.
Conclusion
In the end, we can conclude that SARS-COV-2 causes significantly milder disease in children than in adults. However, the infection spreads quicker among children and high morbidity can still cause issues since multiple complications can occur during and after COVID-19. Also, it must be noted that high D-Dimer in this population should alert a pediatrician, if the clinical progression is also serious, to the possibility of a hyperinflammatory state as well as severe disease.
Author Contributions
All authors are responsible for the work described in this paper. All authors were involved in at least one of the following: [conception, design of work or acquisition, analysis, interpretation of data] and [drafting the manuscript and/or revising/reviewing the manuscript for important intellectual content]. All authors provided final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest: no conflicts of interest.
Authors and Affiliations:
Konstantine Chakhunashvili MD, PhD – Children’s New Clinic after Irakli Tsitsishvili, Ambulatory Service, Pediatrician, Tbilisi, Georgia;
Tbilisi State Medical University, Department of Pre-clinical and Experimental Anatomy, Visiting Professor, Tbilisi, Georgia. Georgia. Davit G. Chakhunashvili MD, PhD – Children’s New Clinic after Irakli Tsitsishvili, Ambulatory Service, Pediatrician; Tbilisi State Medical University, Department of Preclinical and Experimental Anatomy, Visiting Professor, Tbilisi, Georgia. Georgia.
Eka Kvirkvelia MD – Children’s New Clinic After Itakli Tsitsishvili, Ambulatory service, Junior Doctor Tbilisi, Georgia.
Maia Chkhaidze MD, PhD, – Children’s New Clinic after Irakli Tsitsishvili, Clinical Director, Tbilisi, Georgia; Geomedi University, Department of Pediatrics, Associate Professor, Tbilisi, Georgia.
David Rekhviashvili MD – Children’s New Clinic after Irakli Tsitsishvili, Director, Tbilisi, Georgia.
Zurab Kakabadze MD, PhD – Tbilisi State Medical University, Department of Pre-clinical and Experimental Anatomy, Professor/Head of the department, Tbilisi, Georgia.
References
- Wang F, Nie J, Wang H, Zhao Q, Xiong Y, et al. (2020) Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. J Infect Dis. 221(11): 1762–1769.
- Zhu N, Zhang D, Wang W, Li X, Yang B, et al. (2020) A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 382(8): 727–733.
- Ouyang Y, Yin J, Wang W, Shi H, Shi Y, et al. (2020) Downregulated Gene Expression Spectrum and Immune Responses Changed During the Disease Progression in Patients With COVID-19. Clin Infect Dis. 71(16): 2052–2060.
- Cucinotta D, Vanelli M (2020) WHO Declares COVID-19 a Pandemic. Acta Biomed. 91(1): 157–160.
- Badal S, Thapa Bajgain K, Badal S, Thapa R, Bajgain BB, et al. (2021) Prevalence, clinical characteristics, and outcomes of pediatric COVID-19: A systematic review and meta-analysis. J Clin Virol. 135: 104715.
- Kumar J, Meena J, Yadav A, Yadav J (2021) Radiological Findings of COVID-19 in Children: A Systematic Review and Meta-Analysis. J Trop Pediatr. 67(3): fmaa045.
- Singh A, Kainth D, Gaur S, Yadav DK, Anand S (2021) Characteristics of Systematic Reviews and Meta-analysis on COVID-19 in the Pediatric Population: A Bibliometric Review With Emphasis on Top 5 Cited Articles. Clin Pediatr. 60(9-10): 392–398.
- Wang Z, Zhou Q, Wang C, Shi Q, Lu S, et al. (2020) Clinical characteristics of children with COVID-19: a rapid review and meta-analysis. Ann Transl Med. 8(10): 620.
- Zheng P, Bao L, Yang W, Wang JJ (2020) Clinical symptoms between severe and non-severe COVID-19 pneumonia: A protocol for systematic review and meta-analysis. Medicine. 99(33): e21618.
- Graff K, Smith C, Silveira L, Jung S, Curran-Hays S, et al. (2021) Risk Factors for Severe COVID-19 in Children. Pediatr Infect Dis J. 40(4): e137–e145.
- Hendler JV, Miranda do Lago P, Müller GC, Santana JC, Piva JP, et al. (2021) Risk factors for severe COVID-19 infection in Brazilian children. Braz J Infect Dis. 25(6): 101650.
- Choi JH, Choi SH, Yun KW (2022) Risk Factors for Severe COVID-19 in Children: A Systematic Review and Meta- Analysis. J Korean Med Sci. 37(5): e35.
- Mania A, Faltin K, Mazur-Melewska K, Małecki P, Jończyk- Potoczna K, et al. (2021) Clinical Picture and Risk Factors of Severe Respiratory Symptoms in COVID-19 in Children. Viruses. 13(12): 2366.
- Ankermann T, Brinkmann F (2022) Comorbidities in Children with COVID-19 and MIS-C/PIMS-TS and Risk Factors for Hospitalization, Severe Disease, Intensive Care and Death. Klin Padiatr. 234(05): 257-266.
- Pérez-Sastré MA, Valdés J, Ortiz-Hernández L (2020) Clinical characteristics and severity of COVID-19 among Mexican adult. Gac Med Mex. 156(5): 373–381.
- Chen Y, Klein SL, Garibaldi BT, Li H, Wu C, et al. (2021) Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res Rev. 65: 101205.
- Wong HYF, Lam HYS, Fong AH, Leung ST, Chin TW, et al. (2020) Frequency and Distribution of Chest Radiographic Findings in Patients Positive for COVID-19. Radiology. 296(2): E72–E78.
- Han R, Huang L, Jiang H, Dong J, Peng H, et al. (2020) Early Clinical and CT Manifestations of Coronavirus Disease 2019 (COVID-19) Pneumonia. AJR Am J Roentgenol. 215(2): 338– 343.
- Liu KC, Xu P, Lv WF, Qiu XH, Yao JL, et al. (2020) CT manifestations of coronavirus disease-2019: A retrospective analysis of 73 cases by disease severity. Eur J Radiol. 126: 108941.
- Caruso D, Zerunian M, Polici M, Pucciarelli F, Polidori T, et al. (2020) Chest CT Features of COVID-19 in Rome, Italy. Radiology. 296(2): E79–E85.
- Bai HX, Hsieh B, Xiong Z, Halsey K, Choi JW, et al. (2020) Performance of Radiologists in Differentiating COVID-19 from Non-COVID-19 Viral Pneumonia at Chest CT. Radiology. 296(2): E46–E54.
- Valverde I, Singh Y, Sanchez-de-Toledo J, Theocharis P, Chikermane A, et al. (2021) Acute Cardiovascular Manifestations in 286 Children With Multisystem Inflammatory Syndrome Associated With COVID-19 Infection in Europe. Circulation. 143(1): 21–32.
- Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, et al. (2020) Multisystem Inflammatory Syndrome in Children in New York State. N Engl J Med. 383(4): 347–358.
- Abrams JY, Oster ME, Godfred-Cato SE, Bryant B, Datta SD, et al. (2021) Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: a retrospective surveillance study. Lancet Child Adolesc Health. 5(5): 323–331.
- Seidel F, Kuehne T, Kelle S, Doeblin P, Zieschang V, et al. (2021) Cardiovascular magnetic resonance findings in non-hospitalized paediatric patients after recovery from COVID-19. ESC Heart Fail. 8(6): 5583–5588.
- Huang L, Zhao P, Tang D, Zhu T, Han R, et al. (2020) Cardiac Involvement in Patients Recovered From COVID-2019 Identified Using Magnetic Resonance Imaging. JACC Cardiovascular imaging. 13(11): 2330–2339.
- https://www.cdc.gov/mis/mis-c/hcp/index.html
- Yasuhara J, Kuno T, Takagi H, Sumitomo N (2020) Clinical characteristics of COVID-19 in children: A systematic review. Pediatr Pulmonol 55(10): 2565–2575.
- Kari JA, Shalaby MA, Albanna AS, Alahmadi TS, Sukkar SA, et al. (2021) Coronavirus disease in children: A multicentre study from the Kingdom of Saudi Arabia. J Infect Public Health. 14(4): 543–549.
- Zachariah P, Johnson CL, Halabi KC, Ahn D, Sen AI, et al. (2020) Epidemiology, Clinical Features, and Disease Severity in Patients With Coronavirus Disease 2019 (COVID-19) in a Children's Hospital in New York City, New York. JAMA Pediatr. 174(10): e202430.
- Lazova S, Alexandrova T, Gorelyova-Stefanova N, Atanasov K, Tzotcheva I, et al. (2021) Liver Involvement in Children with COVID-19 and Multisystem Inflammatory Syndrome: A Single- Center Bulgarian Observational Study. Microorganisms. 9(9): 1958.
- Zhou YH, Zheng KI, Targher G, Byrne CD, Zheng MH (2020) Abnormal liver enzymes in children and infants with COVID-19: A narrative review of case-series studies. Pediatr Obes. 15(12): e12723.
- Saleh NY, Aboelghar HM, Salem SS, Ibrahem RA, Khalil FO, et al. (2021) The severity and atypical presentations of COVID-19 infection in pediatrics. BMC Pediatr. 21(1): 144.
- Gómez-Pastora J, Weigand M, Kim J, Wu X, Strayer J, et al. (2020) Hyperferritinemia in critically ill COVID-19 patients - Is ferritin the product of inflammation or a pathogenic mediator? Clin Chim Acta. 509: 249–251.
- Hussein AM, Taha ZB, Gailan Malek A, Akram Rasul K, Qasim Hazim D, et al. (2021) D-Dimer and Serum Ferritin as an Independent Risk Factor for Severity in COVID-19 Patients. Mater Today Proc.
- Cheng L, Li H, Li L, Liu C, Yan S, et al. (2020) Ferritin in the coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J Clin Lab Anal. 34(10): e23618.
- Giannattasio A, D'Anna C, Muzzica S, Mauro A, Rosa M, et al. (2021) Is COVID-19 a hyperferritinemic syndrome in children? Clin Chem Lab Med 59(11): e409–e412.
- Zhao Y, Yin L, Patel J, Tang L, Huang Y (2021) The inflammatory markers of multisystem inflammatory syndrome in children (MIS-C) and adolescents associated with COVID-19: A meta-analysis. J Med Virol. 93(7): 4358–4369.
- Saleh M, Alkofide A, Alshammari A, Siddiqui K, Owaidah T (2021) Changes in Hematological, Clinical and Laboratory Parameters for Children with COVID-19: Single-Center Experience. J Blood Med. 12: 819–826.
- Aguilera-Alonso D, Murias S, Martínez-de-Azagra Garde A, Soriano-Arandes A, Pareja M, et al. (2021) Prevalence of thrombotic complications in children with SARS-CoV-2. Arch Dis Child. 106(11): 1129–1132.
- Logothetis CN, Weppelmann TA, Jordan A, Hanna C, Zhang S, et al. (2021) D-Dimer Testing for the Exclusion of Pulmonary Embolism Among Hospitalized Patients With COVID-19. JAMA Netw Open 4(10): e2128802.