Barat Yusubov1, Mirkhalig Javadov2*, Khanbaba Huseynov3, Muradali Bakhshiyev4
1Department of Nephrology, Yeni Klinika Hospital, Baku, AZE
2Department of General Surgery and Transplantation, Yeni Klinika Hospital, Baku, AZE
3Department of Extracorporeal Detoxification and Hemodialysis, Scientific Surgical Center named after Academician M. Topchubashov, Baku, AZE
4Department of Internal Medicine, Azerbaijan Medical University, Baku, AZE
*Corresponding Author: Mirkhalig Javadov, Department of General Surgery and Transplantation, Yeni Klinika Hospital, Baku, AZE
Abstract
Background: In patients with end-stage renal disease (ESRD) on hemodialysis (HD), fibroblast growth factor 23 (FGF 23), intact parathyroid hormone (iPTH), and homocysteine (Hcy) are frequently elevated and have been individually linked to disordered mineral metabolism and cardiovascular risk. In addition to FGF 23, intact parathyroid hormone and homocysteine also play a role in the chronic kidney disease (CKD)- related mineral bone disease (MBD) process and cardiovascular disease (CVD) risk. Although elevated circulating FGF 23 has been consistently associated with higher risks of left ventricular hypertrophy (LVH) and mortality in CKD, it is still uncertain whether these relationships are causal.
Aim: To evaluate the correlation of FGF 23 with iPTH, Hcy, and routine biochemical parameters and the incidence of LVH in ESRD. These parameter values may guide us for new prognostic and treatment definition targets in HD patients with ESRD.
Method: This cross-sectional study was conducted in Azerbaijan. The Institutional Ethics Committee approved the study (research protocol code 012, number 012/24). ESRD patients who underwent dialysis for a minimum of 3 months were approached, and data was collected from 103 patients who agreed to participate in the study. These patients were divided into two groups: the main (n=75) group (with high FGF 23 and homocysteine levels) and the control (n=28) group (with no increase in homocysteine and FGF 23 levels). The detailed baseline information of biochemistry laboratory parameters included Hemogram (Hg), C-reactive protein (CRP), Glucose (Gluc), Creatinine (Crea), estimated glomerular filtration rate (eGFR), Potassium(K), Sodium (Na), Calcium (Ca), Phosphorus (P), Alanine aminotransferase (ALT) ,Aspartate aminotransferase (AST), iPTH, Hcy, and FGF 23. Cardiac evaluation of all HD patients was performed using Color Doppler Echocardiography and Electrocardiogram (ECG). Finally, the association was analyzed between the FGF 23 level and LVH. Descriptive statistics and frequency analysis were used to describe demographic and clinical features. The Chi-square (χ²) test was performed to compare groups based on their categorical variables. Mann–Whitney U test was employed to contrast clinical parameters between the main and control groups. All tests used statistical significance with p < 0.05. All statistical analyses were done using IBM SPSS Statistics version 25.0.
Results: In the sample, 65 were male (63.1%), and 38 were female (36.9%), with mean age, height, and weight of 64±13.64 years, 170 ± 6.9 cm, and 79 ± 13.07 kg. FGF 23 levels were positively correlated with phosphorus (r=0.78, p=0.01), creatinine (r=0.78, p=0.01), iPTH (r=0.61, P=0.01), and homocysteine (r=0.65, p=0.01). It was negatively correlated with GFR (r=-0.64, p=0.01). No statistically significant correlation was found with Ca values (r=-0.12, p>0.05). Regression analysis revealed that homocysteine levels were significantly associated with creatinine, urine protein, calcium, iPTH, FGF 23 (positive), and phosphorus, AST, and hematocrit (negative) (all p < 0.05). Serum FGF 23 levels were correlated with LVH in HD patients (P < 0.01).
Conclusion: High level of FGF 23 and Hcy is associated with abnormal renal function and mineral imbalance. There is a strong correlation of FGF 23 with increased Crea, P, and iPTH levels alongside reduced GFR. Relation was found between FGF 23 level and LVH incidence in ESRD HD patients. FGF 23 and homocysteine are potential prognostic markers of CKD-associated LVH and may represent future therapeutic targets, although interventional evidence is currently limited.
Keywords: Chronic Kidney Disease, Fibroblast Growth Factor 23, Homocysteine, Intact Parathyroid Hormone, Mineral Metabolism, Left Ventricular Hypertrophy
Introduction
Patients with end-stage renal disease (ESRD) undergoing hemodialysis (HD) face a higher risk of mortality due to chronic kidney disease-related mineral bone abnormalities (CKD-MBD) and cardiovascular disease (CVD) complications such as cardiorenal syndrome [1]. The reason behind increased CVD risk factors in CKD patients is pathologically changed phosphocalcic metabolism. CKD- MBD syndrome is characterized by an imbalance of mineral values and bone metabolism changes, including abnormal calcium (Ca), phosphorus (P) metabolism, and intact parathyroid hormone (iPTH) [2].
Fibroblast growth factor 23 (FGF 23) is one of the major phosphate metabolism regulators secreted from the osteocytes. High levels of FGF 23 are associated with mortality in ESRD patients with HD [3]. Bouma-de Krijger [4] reported no association between a single value of FGF 23 and all-cause mortality. However, increasing FGF 23 was associated with mortality risk. This is because FGF 23 inhibits PTH secretion and active vitamin D3 synthesis and consequently inhibits the reabsorption of P by the renal tubules, resulting in limiting intestinal P absorption [3, 4].
FGF 23 also plays an important role in CVD pathological processes, including left ventricular hypertrophy, myocardial injury, coronary atherosclerosis, vascular calcification, etc. [5]. Vázquez-Sánchez [6] reported direct FGF 23 effects on the heart myocardium, and FGF 23 elevated plasma levels have been related to negative CVD results such as arrhythmias and heart failure, etc. The feedback of persistent hyperphosphatemia and deficiency of the cofactor Klotho explains the mechanism. The relation between FGF 23 levels and CVD, such as cardiorenal syndrome and MBD in hemodialysis patients, is unclear [7].
Renal hyperparathyroidism (rHPT) develops during the early stages of renal failure with high risks of bone fractures, CVD, and death. PTH and FGF 23 increase as kidney function declines when patients reach kidney failure and fail to exert their phosphaturic effects. This process leads to hyperphosphatemia and further elevations of both hormones. FGF 23 levels should be measured in clinical practice, targeting the control of it just as with PTH levels. The target range levels for intact PTH (iPTH) remain debatable. Furthermore, research is needed to determine optimal iPTH level ranges in ESRD HD patients [8, 9].
In addition to FGF 23, intact parathyroid hormone and homocysteine also play a role in the chronic kidney disease (CKD)-related mineral bone disease (MBD) process and cardiovascular disease (CVD) risk. Chen, Feng [10] showed high Homocysteine (Hcy) levels in CKD HD patients. Hyperhomocysteinemia (Hhcy) was recognized as an independent risk factor for developing CVD with an 85-90% rate, especially in combination with biochemical parameters such as creatinine, albumin, Calcium, and CRP. Thus, it is argued as a prognostic and predictive biomarker in ESRD, and it is recommended to monitor Hcy levels in all CKD HD patients.
Currently, no biomarker alone can be used to predict and follow up on the prognosis of CKD progression. GFR and creatinine are follow- up markers for kidney failure, along with early detection of CKD [2, 9]. There is a literature gap as there is limited research that simultaneously maps the biochemical interrelationships among FGF 23, iPTH, Homocysteine, CRP, lipids, and mineral metabolism markers in ESRD patients with chronic HD [10, 11, 12].
Patients with CKD experience substantially higher cardiovascular mortality than the general population, with LVH being the most common cardiac abnormality, affecting up to 75%. While the major cause of death in CKD patients is cardiovascular disease, and LVH in CKD has been reported as a negative prognostic factor. Although elevated circulating FGF 23 has been consistently associated with higher risks of left ventricular hypertrophy (LVH) and mortality in CKD, it is still uncertain whether these relationships are causal. However, there are some controversies regarding the hypothesis that FGF 23 is the direct stimulant to the heart contributing to the development of LVH [25, 26, 27].
Thus, this study aimed to evaluate the cross-sectional correlation of FGF 23 with iPTH, Hcy, and routine biochemical parameters in ESRD and the incidence of LVH in ESRD. These parameter values may guide us for new prognostic and treatment definition targets in HD patients with ESRD.
Materials And Methods
Study Design
This cross-sectional study was conducted between February and November 2024 at Yeni Klinika Hospital, Baku, Azerbaijan. The Institutional Ethics Committee approved the study (research protocol code 012, number 012/24).
Sample Size
ESRD patients who underwent dialysis for a minimum of 3 months were approached, and data were collected from 103 patients who agreed to participate in the study. These patients were divided into two groups: the main group (with high FGF 23 and homocysteine levels) and the control group (with no increase in Homocysteine and FGF 23 levels). FGF 23 higher than >500 pg/mL was considered to ensure that the main group contained participants with CKD (n=75) and there were enough participants in the control group (n=28). Similarly, plasma homocysteine concentrations were evaluated, with values greater than 15 µmol/L designated as high group. The planned examinations were performed for both groups. All relevant variables were collected prospectively by the researcher at the time of data collection. Records were reviewed for completeness during data entry, and any cases with missing values for key variables were excluded. As a result, the final dataset used for analysis had no missing data and 95% confidence intervals were reported to reflect the precision of estimated effect sizes.
Inclusion Criteria
1. Adult patients diagnosed with ESRD on maintenance HD
2. Absence of primary cardiovascular disease (CVD)
Exclusion Criteria
1. Presence of primary CVD or acute cardiovascular events
2. Acute renal failure
3. History of recent surgery (within the past 2 months)
4. Hemodialysis due to non-ESRD etiologies (e.g., autoimmune or oncological diseases)
Assessment Parameters
Demographic information, including age, gender, primary disease, and comorbid diseases, was collected. Data were collected for classic biochemistry laboratory parameters using standard procedures, including creatinine, urea, sodium, potassium, calcium, phosphorus, AST, ALT, and CRP, along with the target parameters such as FGF 23, iPTH, and homocysteine levels. Glomerular filtration rates (GFR) ml/min were calculated to assess renal function by using the CKD- EPI formula [21].
Cardiac evaluation of all HD patients was performed using Color Doppler Echocardiography and Electrocardiogram (ECG). Left ventricular hypertrophy (LVH) and diastolic function were classified according to the European Society of Cardiology criteria, defined as a left ventricular mass index (LVMI) >95 g/m² for females and >115 g/m² for males [17, 18]. LVMI was calculated using the formula proposed by Cosyns et al [28]. Finally, the association was analyzed between the FGF 23 level and LVH.
Biomarker Measurement And Reference Values
Blood samples were collected before HD and then centrifuged for serum fluid collection. Enzyme-linked immunosorbent assay (ELISA) was used for FGF 23 serum level measurement. The human FGF 23 ELISA kits utilized in this study were manufactured by Immutopics International (San Clemente, CA, USA; #60-6600). For FGF 23, normal value reference measurements were 10.84-23.72 pg/mL, which were determined by taking the mean value of FGF 23 (17.28 ± 6.44) from 28 healthy volunteers [2-4, 22]. Electrochemiluminescence immunoassay 5 (ECLIA, PTH Cobas®, Roche) was used to measure iPTH levels in the normal range of 15- 65 pg/ml [8, 9, 23]. The Fluorescence Polarization Immunoassay (FPIA) method was used for total Hcy concentration measurement. The normal reference range for total Hcy was 5-15 µmol/L [10, 13, 24]. The target reference range for Phosphorus (1.13-1.78 mmol/l), Calcium (2.1-2.4 mmol/l), and iPTH (150-300 pg/ml) in CKD patients were evaluated (K/DOQI guidelines) [13-16, 21].
Statistical Analysis
Descriptive statistics and frequency analysis were used to describe demographic and clinical features. The Chi-square (χ²) test was performed to compare groups based on their categorical variables. The Shapiro-Wilk test showed that the data did not follow a normal distribution, so non-parametric methods were chosen. Mann– Whitney U test was employed to contrast clinical parameters between the main and control groups. Spearman's rank correlation was chosen to assess the relationship between FGF 23 and numerous other chemical markers. All tests used statistical significance with p < 0.05. All statistical analyses were done using IBM SPSS Statistics version 25.0.
Results
The study involved 103 patients who were receiving maintenance hemodialysis because of ESRD. Patients baseline clinical and laboratory characteristics are shown (Table 1). In total of 103 HD patients, 65 were male (63.1%) and 38 were female (36.9%). The mean ± SD parameter result for age was 64±13.64. The detailed baseline information of biochemical parameters, including Hg, CRP, glucose, creatinine, GFR, K, Na, Ca, P, ALT, AST, iPTH, and homocysteine, FGF 23 were evaluated and presented in Table 1.
Table 1: Baseline clinical and laboratory characteristics (n=103)
|
Parameters |
Patient no = 103, n% |
|
|
|
|
|
|
|
Male |
65 (63.1) |
|
Gender (n% ) |
|
|
|
|
Female |
38 (36.9) |
|
|
|
Mean ± SD |
|
Age (year) |
64 ± 13.64 |
|
|
Height (cm) |
170 ± 6.9 |
|
|
Weight (kg) |
79 ±13.04 |
|
|
Vital parameters |
|
|
|
Systolic blood pressure (mm/Hg) |
140 ± 17.83 |
|
|
Diastolic blood pressure (mm/Hg) |
80 ± 6.13 |
|
|
Pulse (per minute) |
83 ± 11.3 |
|
|
Laboratory parameters |
|
|
|
HGB (g/dl) |
10.54 ± 4.18 |
|
|
HT (%) |
30.4 ± 6.36 |
|
|
CRP (mg/L) |
12.31 ± 18.67 |
|
|
eGFR (ml/min/1.73m2) |
11 ± 5.78 |
|
|
Creatinine (umol/l) |
599 ± 242.2 |
|
|
Glucose (mmol/l) |
6.2 ± 3.2 |
|
|
Urine protein (mg/dl) |
150 ± 85.8 |
|
|
Potassium (mmol/l) |
4.1 ± 0.57 |
|
|
Sodium (mEq/l) |
142 ± 3.32 |
|
|
Calcium (mmol/l) |
2.34 ± 3.17 |
|
|
Phosphorus (mmol/l) |
1.69 ± 1.94 |
|
|
ALT (U/l) |
22.1 ± 13.06 |
|
|
AST (U/l) |
22.4 ± 12.56 |
|
|
FGF 23 (pg/ml) |
876 ± 584.8 |
|
|
İPTH (pg/ml) |
153 ± 93.7 |
|
|
Homocysteine (umol/ml) |
|
20 ± 7.42 |
Descriptive statistics: Quantitative data presented as mean ± SD, categorical data presented as number (percentage), p-value <0.05 considered significant.
HGB, hemoglobin; HT, hematocrit; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; ALT, alanine transaminase; AST, aspartate aminotransferase; FGF 23, fibroblast growth factor 23; iPTH, intact parathyroid hormone.
The first group 75 patients (main group) with high FGF 23 and homocysteine levels with the second group 28 patients (comparison group) with no increase in FGF 23 and homocysteine levels.
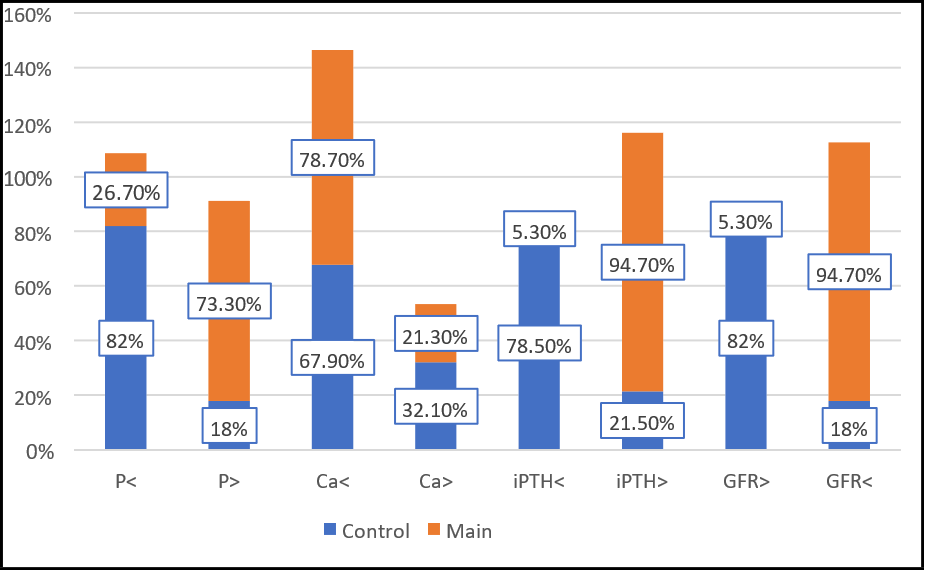
Chi-square (χ²) test was conducted for comparison of biochemistry parameter results of two groups. It was found that the distribution of male and female in the groups did not differ statistically (p=0.16) presented in Table 2. It was determined that phosphorus levels differed according to the groups in the study (p=0.01). Phosphorus levels were higher in the study group. Calcium levels did not differ significantly in the main and control groups (p=0.29). iPTH levels differed according to the groups (p=0.01) and iPTH levels were higher in the main group. In the study, it was determined that GFR levels differed according to the groups. GFR levels were lower in the main group .FGF 23 had a negatively correlation with GFR values (r=-0.64, P=0.01). FGF-23 and iPTH values showed positively correlation (r=0.61, P=0.01). FGF-23 and homocysteine values showed positively correlation (r=0.65, P=0.01). (Tables 3, 4).
Table 2: Examining the characteristics of gender distribution according to study groups (n=103)
|
Patient characteristics (n=103) |
Control (n=28) n (%) |
Main (n=75) n (%) |
p** |
|
Male |
15 (54%) |
50 (67%) |
0.16* |
|
Female |
13 (46%) |
25 (33%) |
*p-value <0.05 considered significant **Chi-square test was performed.
Table 3: Analysis of laboratory characteristics according to study groups (n=103)
|
Patient characteristics (n=103) |
Control (n=28) n (%) |
Main (n=75) n (%) |
p** |
|
Phosphorus < t.r.r. |
23 (82%) |
20 (26.7%) |
0.01* |
|
Phosphorus > t.r.r. |
5 (18%) |
55 (73.3%) |
|
|
Calcium < t.r.r. |
19 (67.9%) |
59 (78.7%) |
0.29 |
|
Calcium > t.r.r. |
9 (32.1%) |
16 (21.3%) |
|
|
iPTH < t.r.r. |
22 (78.5%) |
4 (5.3%) |
0.01* |
|
iPTH > t.r.r. |
6 (21.5%) |
71 (94.7%) |
|
|
GFR > 15ml/min/1.73m2 |
23 (82%) |
4 (5.3%) |
0.01* |
|
GFR < 15ml/min/1.73m2 |
5 (18%) |
71 (94.7%) |
Categorical data presented as number (percentage).
* p-value <0.05 considered significant **Chi-square test was performed.
Target reference range (t.r.r.) for phosphorus (1.13-1.78 mmol/l), target reference range for calcium (2.1-2.4 mmol/l), target reference range for intact PTH (150-300 pg/ml) (K/DOQI guidelines) [21-23].
Table 4: Analysis of the relationships between the changes in FGF-23 and other biochemistry laboratory parameters in the main group (n=75)
|
Main Group (n=75) |
|
|
FGF-23 |
P |
|
|
Crea |
r |
0.78* |
p=0.01 |
|
|
Ca |
r |
-0.12 |
p=0.29 |
|
|
P |
r |
0.78* |
p=0.01 |
|
|
iPTH |
r |
0.61* |
p=0.01 |
|
|
GFR |
r |
-0.64* |
p=0.01 |
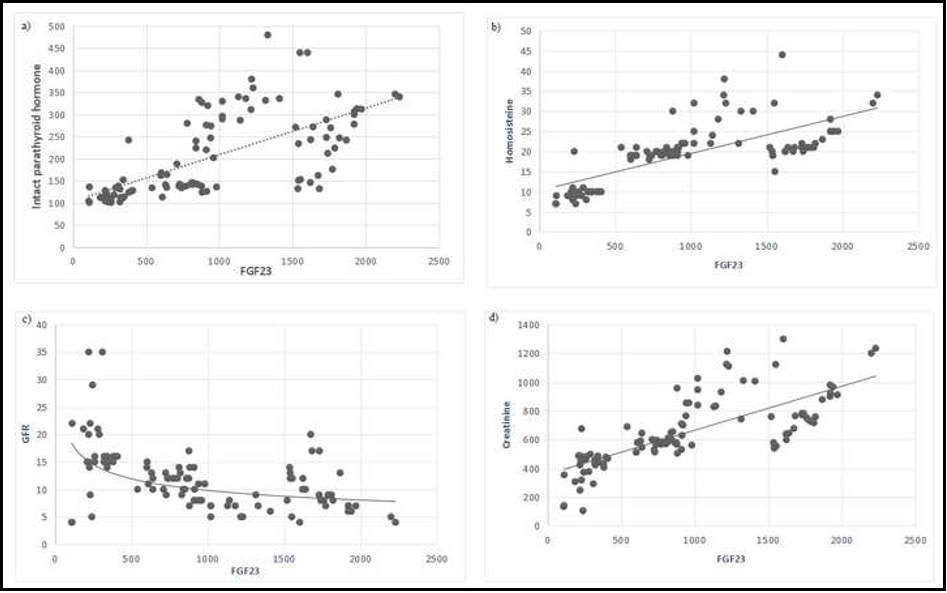
FGF 23 had a negatively correlation with GFR values and positively correlation with creatinine. Also, FGF 23 levels were positively associated with, intact parathyroid hormone levels and homocysteine levels in the main group. As a result, FGF 23, iPTH and homocysteine showed a linear increased tendency (Figure 1).
Figure 1: Correlation of serum levels of fibroblast growth factor 23 with intact parathyroid hormone (a), serum homocysteine rate (b), glomerular filtration (c), and (d) creatinine (Main group)
a) p-value <0.05 considered significant; r=0.61, p=0.01.
iPTH: intact parathyroid hormone (pg/ml); FGF-23: fibroblast growth factor 23 (pg/ml).
b) p-value <0.05 considered significant; r=0.65, p=0.01.
Hcy: homocysteine (umol/ml); FGF-23: fibroblast growth factor 23 (pg/ml).
c) p-value <0.05 considered significant; r=-0.64; p=0.01
GFR: glomerular filtration rate (ml/min/1.73m2); FGF 23: fibroblast growth factor 23: (pg/ml)
d) p-value <0.05 considered significant; r=0.78, p=0.01.
Creatinine (umol/l); FGF-23: fibroblast growth factor 23 (pg/ml).
Figure 2: Comparison of percentage of patients for each laboratory characteristics between groups
Regression analysis was performed while keeping Homocysteine (Model 1), iPTH (Model 2) and FGF 23 (model 3) as dependent variables. All three models were statistically significant, with ANOVA p-values less than 0.001, indicating good model fit. Model 1 had the highest explanatory power (R² = 0.846), followed by Model 2 (R² = 0.814) and Model 3 (R² = 0.806) (Table 5).
Table 5: Model Summaries
|
Model |
R |
R Square |
Adjusted R Square |
Std. Error of the Estimate |
ANOVAa |
|
1 |
.920a |
.846 |
.809 |
2.92328 |
.000b |
|
2 |
.902a |
.814 |
.768 |
49.13951 |
.000b |
|
3 |
.898a |
.806 |
.758 |
607.83522 |
.000b |
Regression analysis revealed that homocysteine levels were significantly associated with creatinine, urine protein, calcium, iPTH, FGF 23 (positive), and phosphorus, AST, and hematocrit (negative) (all p < 0.05) (Table 6).
Table 6: Impact of other study variables on Homocysteine
|
Model |
Unstandardized Coefficients |
Standardized Coefficients |
t |
Sig. |
||
|
B |
Std. Error |
Beta |
||||
|
1 |
(Constant) |
-9.015 |
27.126 |
|
-.332 |
.740 |
|
Height |
.045 |
.052 |
.047 |
.880 |
.381 |
|
|
weight |
.001 |
.028 |
.002 |
.031 |
.975 |
|
|
SBP |
-.042 |
.034 |
-.077 |
-1.253 |
.214 |
|
|
DBP |
.110 |
.100 |
.065 |
1.099 |
.275 |
|
|
Puls |
-.003 |
.047 |
-.003 |
-.066 |
.947 |
|
|
HGB |
.121 |
.081 |
.076 |
1.497 |
.138 |
|
|
HT |
-.143 |
.068 |
-.109 |
-2.088 |
.040 |
|
|
CRP |
.009 |
.030 |
.014 |
.286 |
.776 |
|
|
GFR |
-.059 |
.069 |
-.051 |
-.849 |
.398 |
|
|
Crea |
.015 |
.003 |
.434 |
4.859 |
.000 |
|
|
Glucose |
-.061 |
.160 |
-.019 |
-.380 |
.705 |
|
|
Urine_protein |
.020 |
.009 |
.103 |
2.156 |
.034 |
|
|
K |
1.697 |
1.140 |
.072 |
1.489 |
.140 |
|
|
Na |
-.011 |
.158 |
-.004 |
-.069 |
.945 |
|
|
Ca |
.259 |
.115 |
.111 |
2.248 |
.027 |
|
|
P |
-.926 |
.289 |
-.270 |
-3.208 |
.002 |
|
|
ALT |
1.066E-5 |
.029 |
.000 |
.000 |
1.000 |
|
|
AST |
-.083 |
.035 |
-.122 |
-2.405 |
.018 |
|
|
iPTH |
.016 |
.006 |
.251 |
2.601 |
.011 |
|
|
FGF-23 |
.002 |
.000 |
.352 |
3.900 |
.000 |
|
|
a. Dependent Variable: Hct |
||||||
In this study, a significant difference in FGF 23 mean value was found between LVH (1841.16 ± 1202.06 pg/mL) and non-LVH patients (295.14 ± 77.89 pg/mL) with the assumption that a higher level of FGF 23 leads to a greater incidence of LVH (P<0.01); (Table 7 and Figure 3).
Table 7: Mean levels of FGF-23 in the LVH group
|
Variable |
LVH Category |
P Value |
|
|
|
YES |
NO |
|
|
FGF 23 (pg/mL) |
1841.16 ± 1202.06 |
295.14 ± 77.89 |
P<0.01 |
FGF-23: fibroblast growth factor 23 (pg/ml): (LVH) left ventricular hypertrophy
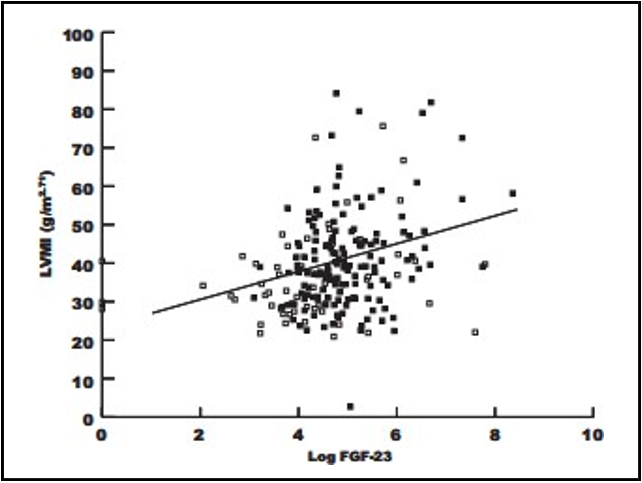
Figure 3: Correlation between log FGF-23 and LVMI (r=0.27, P<0.01).
Discussion
This single-center cross-sectional study showed the following results. We found that the mean FGF 23 level in the main group was significantly higher than control group (P=0.01). Also, FGF 23 levels were positively correlated with serum phosphorus, intact parathyroid hormone levels and homocysteine levels in the main group. This increase may be an important factor for resulting CKD-MBD in HD patients.
Electrolyte imbalances, such as hyperkalemia, hyperphosphatemia, and hypercalcemia, are usually the result of CKD-MBD, which have been associated with elevated levels of FGF 23 and PTH [16]. Nakagawa and Komaba [9] proposed that elevated levels of PTH and FGF 23 can cause multiple organ damage with advanced CKD. This study's results confirm this, as patients in the main group showed more serious disruptions in mineral metabolism (of phosphate and Calcium), PTH regulation, and renal function with high levels of FGF 23. Finding that FGF 23 and iPTH are positively related agrees with other research suggesting an influence of FGF 23 on the parathyroid glands [11]. Because FGF 23 is strongly related to P levels, it is clear that FGF 23 helps regulate phosphate balance, and Almquist, Isaksson [8] also noted its increased levels reflect the body's reaction to phosphate accumulation. In this study, FGF 23 and GFR revealed an inverse correlation, suggesting that rising FGF 23 levels as kidney function declines can contribute to additional kidney and heart complications. Zeng, Zha [2] investigated FGF 23 concentrations in 107 CKD patients and found an association with serum PTH and Ca levels in CKD HD patients. However, no correlation was found with P levels. Contrarily, this study found that patients with higher FGF 23 and Hcy demonstrated markedly increased Crea, P, and iPTH levels alongside reduced GFR. These results reinforce the pathophysiological relevance of FGF 23 and Hcy as markers of disease severity and potential contributors to CKD-related complications. This study also highlights the significant correlation between elevated levels of FGF 23 and Hcy with impaired renal function and disrupted mineral metabolism in patients with advanced CKD. Monitoring these parameters may provide valuable insights into disease progression and guide more individualized treatment strategies. Similarly, Kruglova MP et al. [17] showed Hcy as a predictive and prognostic marker for CKD HD patients. Hcy levels have been elevated in chronic renal failure patients.
Elevated PTH controlling synthesis of FGF 23 in bones and decreased renal Klotho expression increases serum P load, which also induces the production of FGF 23. Elevated FGF 23 plasma levels are related to impaired renal functions as represented by higher levels of creatinine and estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 [18]. This study also found a negative correlation of FGF 23 with eGFR.
FGF 23 concentration reduction can be one of the potential prognostic markers in everyday follow-up clinical practice of HD CKD patients to achieve a goal of dietary phosphate restriction and using phosphate binders for keeping lower FGF 23 concentrations. Non-calcium- containing phosphate binders, calcimimetics, and HDF are effective ways to decrease FGF 23 levels for patients with HD [19]. A recent study by Yang, Liu [20] proposed a KHA-200 hemoperfusion device in HD patients and showed that targeting blood urea nitrogen, Crea, uric acid, potassium, P, PTH, and Hcy, significantly improved the patient’s health. Therefore, other parameters focused in this study, including FGF 23, should be focused in future treatment research. Left ventricular hypertrophy (LVH) in patients with chronic kidney disease (CKD) contributes significantly to cardiovascular morbidity and mortality. Secondary hyperparathyroidism promotes fibrotic changes in both the vasculature and myocardium, leading to reduced cardiac contractility due to increased myocardial stiffness, impaired systolic and diastolic function, and disturbances in cardiac electrophysiological conduction [22, 23, 25].
Hidaka et al. reported that some populational studies for CKD patients showed the association between elevated FGF 23 and high incidence of cardiovascular disease or all cause death, and some studies with an animal model of CKD suggested that FGF 23 itself induces LVH [25].
Karim et al.reported correlation between FGF 23 level and LVH incidence was related with increasing levels of FGF 23 were associated with LVH. Each 5% increase in heart muscle mass was accompanied by an increment in FGF 23 level [29]. Gutiérrez et al. [30] established that patients with FGF 23 levels above tertile three, >150 RU/mL, had 2.7 times greater risks for LVH than those with FGF 23 levels below this value.
Recent studies have provided evidence that a high plasma concentration of FGF 23 is associated with cardiac disease, including left ventricular hypertrophy (LVH), heart failure, atrial fibrillation, and cardiac death [31].
In this study, a significant difference in FGF 23 mean value was found between LVH (1841.16 ± 1202.06 pg/mL ) and non-LVH patients (295.14 ± 77.89 pg/mL ) with the assumption that a higher level of FGF 23 leads to a greater incidence of LVH (P<0.01). An association was found between FGF 23 level and LVH incidence in ESRD HD patients.
Only a few studies have assessed FGF 23, Hcy, and iPTH simultaneously in ESRD. This research urges that FGF 23, iPTH, and Hcy should always be tracked along with regular testing in HD CKD patients. FGF 23 and homocysteine are potential prognostic markers of CKD-associated LVH and may represent future therapeutic targets, although interventional evidence is currently limited. Thus, this study contributes significantly to this field of discussion.
Limitations
This work did not report on outcomes like heart issues, bone fractures, or death, which are typical problems linked to having too much FGF 23 and Hcy. The study did not control for factors such as phosphate binders, vitamin D analogs, and changes in nutritional status, so these might have influenced the results.
Other studies are required to see if FGF 23 and Hcy can truly cause damage and to check if their value predicts future outcomes for these patients. Further clinical trials should focus on using therapies to lower FGF 23 and Hcy in patients to see if they can slow kidney damage and improve patient health. Since these findings have been demonstrated with FGF 23 and Hcy, their inclusion in CKD management guidelines, with a focus on bone-mineral changes and cardiovascular risk, is advised.
Conclusions
High level of FGF 23 and Hcy is associated with abnormal renal function and mineral imbalance. There is a strong correlation of FGF 23 with increased Crea, P, and iPTH levels alongside reduced GFR. Relation was found between FGF 23 level and LVH incidence in ESRD HD patients. FGF 23 and homocysteine are potential prognostic markers of CKD-associated LVH and may represent future therapeutic targets, although interventional evidence is currently limited.
Author Contributions
All authors contributed equally to this work
Barat Yusubov: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Visualization, Writing – original draft, Writing – review & editing
Mirkhalig Javadov: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Visualization, Writing – original draft, Writing – review & editing
Khanbaba Huseynov: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Visualization, Writing – original draft, Writing – review & editing
Muradali Bakhshiyev: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Visualization, Writing – original draft, Writing – review & editing
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the New Clinic Public Legal Entity Medical Research Ethics Commission, which issued approval 012/24. Protocol Decision of the meeting of the Yeni Klinika Public Legal Entity Medical Research Ethics Commission. Adress: Heydar Aliyev ave. 142 Baku Azerbaijan Research protocol code: 012 Number: 012/24 Date: 11/12/2024.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data can be obtained upon request to the corresponding author.
Conflicts Of Interest
The authors declare no conflicts of interest.
References
- Aman KS, Ahaithmi AS (2021) Pattern of Cardio-Renal Syndrome Amongst End Stage Renal Disease Patients on Maintenance Hemodialysis. Yemeni J Med Health Res. 10(1&2).
- Zeng D, Zha A, Lei Y, Yu Z, Cao R, et al. (2023) Correlation of Serum FGF23 and Chronic Kidney Disease‐Mineral and Bone Abnormality Markers With Cardiac Structure Changes in Maintenance Hemodialysis Patients. Evid Based Complement Alternat Med. 2023(1): 6243771.
- Rodelo-Haad C, Rodríguez-Ortiz ME, Martin-Malo A, Pendon- Ruiz de Mier MV, Agüera ML, et al. (2018) Phosphate control in reducing FGF23 levels in hemodialysis patients. PLoS One. 13(8): e0201537.
- Bouma-de Krijger A, de Roij van Zuijdewijn CLM, Nubé MJ, Grooteman MPC, Vervloet MG (2021) Change in FGF23 concentration over time and its association with all-cause mortality in patients treated with haemodialysis or haemodiafiltration. Clin Kidney J. 14(3): 891–7.
- Memmos E, Papagianni A (2021) New insights into the role of FGF-23 and Klotho in cardiovascular disease in chronic kidney disease patients. Curr Vasc Pharmacol. 19(1): 55–62.
- Vázquez-Sánchez S, Poveda J, Navarro-García JA, González- Lafuente L, Rodríguez-Sánchez E, et al. (2021) An overview of FGF-23 as a novel candidate biomarker of cardiovascular risk. Front Physiol. 12: 632260.
- Heijboer AC, Cavalier E (2023) The measurement and interpretation of fibroblast growth factor 23 (FGF23) concentrations. Calcif Tissue Int. 112(2): 258–70.
- Almquist M, Isaksson E, Clyne N (2020) The treatment of renal hyperparathyroidism. Endocr Relat Cancer. 27(1): R21–34.
- Nakagawa Y, Komaba H (2024) Roles of parathyroid hormone and fibroblast growth factor 23 in advanced chronic kidney disease. Endocrinol Metab (Seoul). 39(3): 407–15.
- Chen W, Feng J, Ji P, Liu Y, Wan H, et al. (2023) Association of hyperhomocysteinemia and chronic kidney disease in the general population: A systematic review and meta-analysis. BMC Nephrol. 24(1): 247.
- Komaba H (2023) Roles of PTH and FGF23 in kidney failure: a focus on nonclassical effects. Clin Exp Nephrol. 27(5): 395–401.
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, et al. (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med. 150(9): 604–12.
- Rout P, Jialal I (2023) Hyperphosphatemia. StatPearls. Treasure Island (FL): StatPearls.
- Zhou X, Guo Y, Luo Y (2021) The optimal range of serum intact parathyroid hormone for a lower risk of mortality in the incident hemodialysis patients. Ren Fail. 43(1): 599–605.
- Heybeli C, Tan SG, Kazancioğlu R, Smith L, Soysal P (2022) Prevalence of Electrolyte Impairments Among Outpatient Elderly Subjects. Bezmialem Science.
- Yamada S, Nakano T (2023) Role of chronic kidney disease (CKD)–mineral and bone disorder (MBD) in the pathogenesis of cardiovascular disease in CKD. J Atheroscler Thromb. 30(8): 835–50.
- Kruglova MP, Ivanov AV, Fedoseev AN, Virus ED, Stupin VA, et al. (2023) The diagnostic and prognostic roles played by homocysteine and other aminothiols in patients with chronic kidney disease. J Clin Med. 12(17): 5653.
- Kuwatsuru Y, Hirano T, Wakabayashi R, Ishisaki JY, Sokooshi H, et al. (2023) Changes in renal function over time in outpatients with eGFR ≥30 mL/min/1.73 m²: implication for timing of renal function testing before contrast-enhanced CT imaging. Jpn J Radiol. 41(9): 994–1006.
- Takkavatakarn K, Wuttiputhanun T, Phannajit J, Praditpornsilpa K, Eiam-Ong S, et al. (2022) Effectiveness of fibroblast growth factor 23 lowering modalities in chronic kidney disease: a systematic review and meta-analysis. Int Urol Nephrol. 54(2): 309-321.
- Yang Q, Liu G, Guo M, Yuan D, Huang J, et al. (2025) The clinical efficacy evaluation of the KHA-200 hemoperfusion device in the treatment of end-stage renal disease patients undergoing blood purification therapy. Kidney Dis (Basel). 11(1): 270–82.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group (2024) KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 105(4S): S117-S314.
- Kato H, Miyazaki H, Kimura T, Hoshino Y, Hidaka N, et al. (2023) Clinical performance of a new intact FGF23 immunoassay in healthy individuals and patients with chronic hypophosphatemia. Bone Rep. 18: 101659.
- Scicchitano P, Iacoviello M, Passantino A, Gesualdo M, Trotta F, et al. (2022) Plasma levels of intact parathyroid hormone and congestion burden in heart failure: clinical correlations and prognostic role. J Cardiovasc Dev Dis. 9(10): 334.
- Son P, Lewis L (2025) Hyperhomocysteinemia. Treasure Island (FL): StatPearls.
- Hidaka N, Inoue K, Kato H, Hoshino Y, Koga M, et al. (2023) FGF-23, Left Ventricular Hypertrophy, and Mortality in Patients With CKD: A Revisit With Mediation Analysis. JACC Adv. 3(1): 100747.
- Leifheit-Nestler M, Große Siemer R, Flasbart K, Richter B, Kirchhoff F, et al. (2015) Induction of cardiac FGF23/FGFR4 expression is associated with left ventricular hypertrophy in patients with chronic kidney disease. Nephrol Dial Transplant. 31(7): 1088-99.
- Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, et al. (2011) FGF23 induces left ventricular hypertrophy. J Clin Invest. 121(11): 4393-408.
- Lancellotti P, Zamorano JL, Habib G, Badano L (2016) Left ventricular systolic function. EACVI Textb. Echocardiogr. 2nd ed. Oxford University Press.
- Karim AMAA, Kasim H, Albaar A, Zatalia Ramadhan SR, Machmud N, et al. (2024) The correlation between serumfibroblast growth factor-23 levels and left ventricular hypertrophy in chronic kidney disease patients. J Renal Inj Prev. 13(1): e32227.
- Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, et al. (2009) Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 119(19): 2545-52.
- Nakano T, Kishimoto H, Tokumoto M (2023) Direct and indirect effects of fibroblast growth factor 23 on the heart. Front Endocrinol (Lausanne). 14: 1059179.