Lateefa Mohamed AlMarzooqi*, Mouza AlMehairi, Ola Aldafrawy, Laleh Seddigh, Abdulla AlAbdulla, Alia Alfalasi, Alanood Alfarasi, Ziba Farhadian, Laila Alkaabi, Marwan Zidan
Dubai Health Authority
*Corresponding Author: Lateefa Mohamed AlMarzooqi, Dubai Health Authority.
Abstract
Background: Colorectal cancer (CRC) is a significant global health concern with high morbidity and mortality rates. Early detection through effective screening programs plays a crucial role in reducing the burden of this disease. However, challenges such as low adherence and disparities in access to screening exist regionally and globally.
Objectives: To evaluate the prevalence of colorectal cancer screening among UAE citizens aged 40 to 75 years who were at average risk for the disease and sought care at ambulatory healthcare facilities in the Emirate of Dubai between January 2019 and January 2020 and determine the prevalence of positive screening results and explore potential risk factors associated with positive screenings. Lastly, to investigate whether individuals with positive screening tests underwent subsequent diagnostic tests to confirm or rule out colorectal cancer.
Methods: The present study was a retrospective cross-sectional analysis targeting individuals who fulfilled predefined inclusion criteria.
Results: Out of a total of 36,126 eligible individuals for Colorectal Screening, 3145 (9 %) underwent screening using FIT/gFOBT during the study period. Among the screened individuals, 364 (11.6 %) had positive FIT/gFOBT results. However, only a fraction of them, specifically 111 (30.5 %), were referred to gastroenterology for a colonoscopy. Of the referred individuals, 61 (54.9 %) proceeded with a colonoscopy. Among the participants who underwent colonoscopy, 8 individuals (13.1 %) were diagnosed with colorectal cancer.
Conclusion: Given the vital role of colorectal cancer screening in early detection, our study's findings of a low 9 % participation rate highlight the need to enhance public awareness, streamline screening processes, and address barriers to improve screening rates.
Keywords: Colon, cancer, screening, prevalence, FOBT, United Arab Emirates, Dubai.
Introduction
Background
Colorectal cancer (CRC) is predicted to increase significantly, with an estimated 2.2 million new cases and 1.1 million cancer-related deaths projected by 2030, representing a 60 % rise in burden. CRC is the third most diagnosed cancer and the fourth leading cause of cancer-related deaths worldwide [1]. However, the burden of CRC is not evenly distributed, with most fatalities and cases concentrated in countries with high or very high human development indices (HDI) [1]. Notably, medium-to-high HDI countries in Eastern Europe, Asia, and South America are witnessing a rapid surge in CRC incidence and mortality rates [1].
The United Arab Emirates (UAE), known for its remarkable economic growth, is also witnessing an alarming increase in non- communicable diseases, including cancer, which became the UAE's third-leading cause of death in 2010. [3]
Despite efforts to implement screening and early detection strategies, the reported cases and mortality rates of CRC in the UAE have increased, indicating a shortfall in reaching the intended population [2]. CRC is now the third most prevalent malignancy in both sexes in the UAE due to the rising in its incidence. [3]
These projections are attributed to rapid population growth, aging, urbanization, increased exposure to cancer risk factors like smoking, high-calorie, low-nutrient diets, sedentary lifestyles, rising obesity rates, and environmental pollution. Consequently, these factors pressure the healthcare system tremendously [2,4].
A significant proportion of cancer cases can be prevented through lifestyle modifications, genetic testing of high-risk populations, and vaccination [2]. Many malignancies can be detected at early stages and effectively treated. It has been proven that early screening for colorectal cancer reduces mortality. [4] Even in cases where cancer is diagnosed at a later stage, interventions can be implemented to alleviate pain, control disease progression, improve quality of life, and support patients and their families [2].
Screening and early detection play a crucial role in improving cancer survival rates. Early detection is believed to enhance treatment outcomes and increase the chances of successful therapy.
Cancer substantially burdens societies worldwide, both in terms of epidemiology and financial impact. However, our current understanding of risk factors suggests that approximately one-third to one-half of cancers can be prevented, highlighting the importance of prevention, mainly primary prevention, as an effective strategy to address the complex challenges posed by cancer [5].
Prevention programs play a crucial role in reducing cancer incidence and mortality rates, making them an integral part of the overall fight against the disease. For instance, colorectal, breast, and cervical cancer screening programs have contributed to alleviating the burden of these prevalent malignancies. Therefore, it is essential to prioritize both primary and secondary prevention strategies in the global battle against cancer [5].
The primary aim of this study was to assess the prevalence of colorectal cancer screening among UAE citizens aged 40 to 75 years who were at average risk for the disease and sought care at ambulatory healthcare facilities in the Emirate of Dubai from January 2019 to January 2020.
The secondary objectives of the study included determining the prevalence of positive screening results and investigating its possible risk factors, investigating whether individuals with positive screening tests underwent diagnostic tests, and examining the documented findings of the diagnostic tests.
Methodology
Our objective was to investigate the prevalence of colorectal cancer screening and the factors influencing it. We conducted a retrospective cross-sectional study focusing on individuals who met specific inclusion criteria. The eligible population consisted of UAE nationals between the ages of 40 and 75 years who underwent colorectal screening using the fecal occult blood test (FOBT) at the primary healthcare service of the Dubai Health Authority from January 2019 to January 2020.
The total number of eligible individuals from both genders was 36,126. We collected data specifically from 3,145 individuals who had undergone the FOBT screening during the specified period. Exclusion criteria for our study included individuals below 40 years old, those above 75 years old, and individuals already diagnosed with colorectal cancer.
The data for our study were collected from the electronic medical record system (Salama system) and extracted into an Excel sheet. This allowed us to identify and gather the necessary patient variables to address the research question and achieve the research objectives. The variables included age, gender, and BMI classification (less than 25 as normal or underweight, 25 -29.9 as overweight, and 30 or more as obese). Also, the data included whether the patient was previously diagnosed with diabetes, hypertension, Dyslipidemia, Inflammatory bowel disease, or if they are H. Pylori positive.
To ensure patient privacy and confidentiality, the data management process excluded personal information or identifiers that could reveal the patients' identities. All data was securely stored in a password-protected directory with restricted access only granted to the researchers. Before analysis, the data was cleaned, coded, and transferred to SPSS software for further processing.
For data analysis, numerical variables were presented as mean and standard deviation, while categorical data were presented as count and percentage. We utilized Microsoft Excel and SPSS software program version 21 for this purpose. The prevalence of colorectal cancer screening, along with its corresponding 95 % confidence intervals, were calculated to assess the risk factors of the positive screening results; we employed the t-test to compare the age and the chi-square test to compare the categorical variables between the groups of positive and the negative screening results. A p-value of less than 0.05 indicated a statistically significant effect.
Results
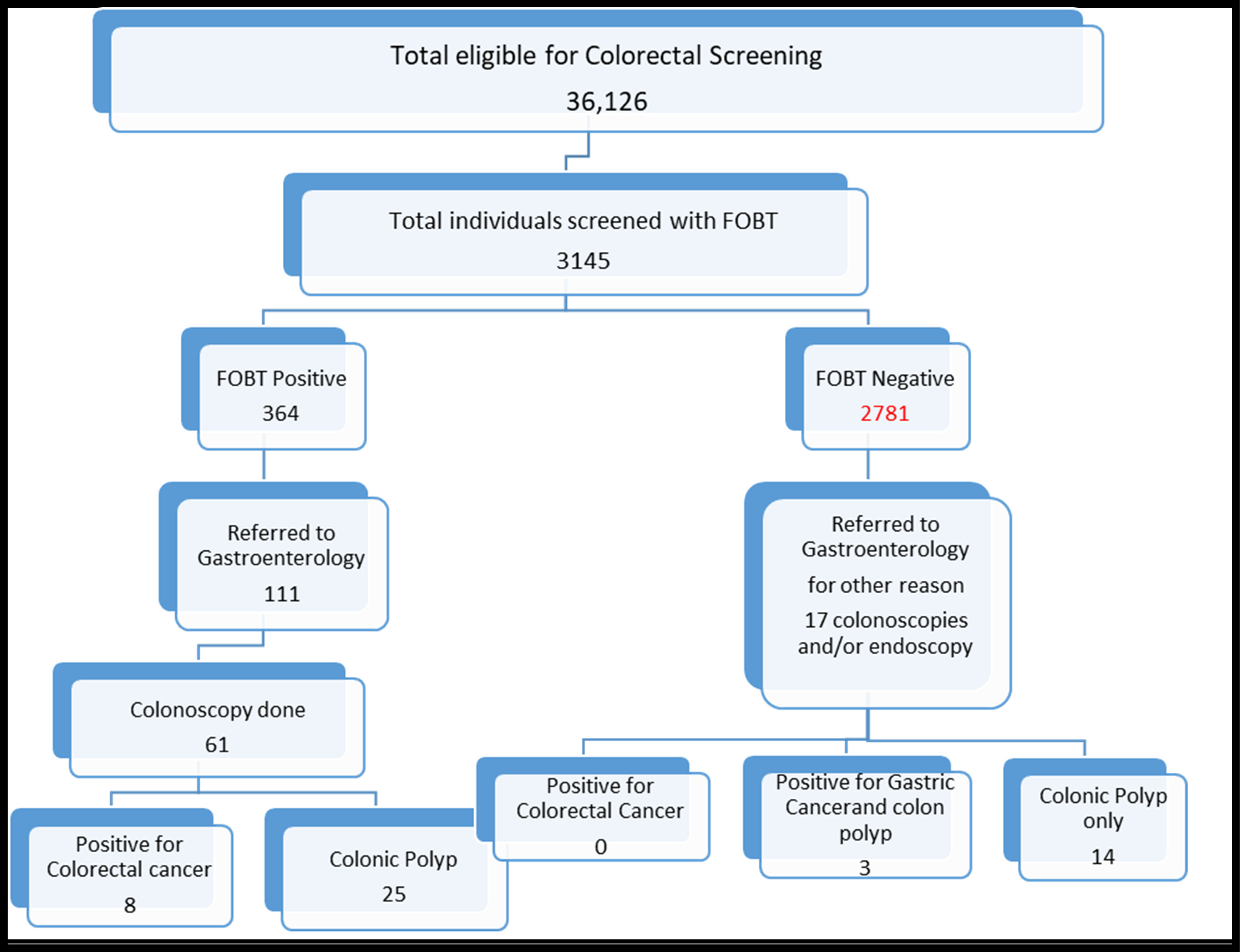
Figure. 1 flowchart for sample screened for colorectal cancer.
Out of a total of 36,126 eligible individuals for Colorectal Screening. During the study period, 3145 (9 %) qualified individuals were screened using FIT/gFOBT. Among those screened, 364 individuals (11.6 %) had positive FIT/gFOBT results. Of these, only a fraction, specifically 111 individuals (30.5 %), were referred to gastroenterology for a colonoscopy. Of the referred individuals, 61 (54.9 %) underwent a colonoscopy.
Among the participants who underwent colonoscopy, 8 individuals (13.1 %) were diagnosed with colorectal cancer, while 25 individuals (41 %) were diagnosed with colonic polyps. The remaining 28 individuals (45.9 %) were found to have the expected results.
In contrast, a small proportion of individuals (0.6 %) with a negative FOBT result were still referred to gastroenterology for constipation, chronic diarrhea, or chronic abdominal pain, necessitating colonoscopy, and endoscopy. Among these referrals, 3 individuals (0.1 %) were found to have certain conditions, while 14 individuals (0.5 %) were diagnosed with colonic polyps. However, no cases of colorectal cancer were detected in this group.
Our study's sample consisted of 1,840 females (58.5 %) and 1,305 males (41.5 %). The mean age of the participants was 56.5, with a standard deviation of ± 9.1. The age range varied from a minimum of 40 years to a maximum of 75 years. Among the participants, 51 individuals (1.6 %) had a family history of colorectal cancer, while 24 individuals (0.6 %) had been diagnosed with inflammatory bowel disease.
Regarding body mass index (BMI), 1,553 participants (49.9 %) were classified as obese, 1,154 (37.1 %) were overweight, and 405 (13 %) had a normal or underweight BMI.
Of the sample, 282 individuals (9 %) were smokers, while the remaining were nonsmokers or former smokers. Among the
participants, 553 (17.6 %) took Aspirin regularly. In terms of chronic diseases, 1,438 individuals (45.7 %) were diagnosed with hypertension, 1,309 (41.6 %) had diabetes mellitus, and 1,921 (61.1
%) had dyslipidemia. Additionally, 282 individuals (7.2 %) had a positive H. pylori test result (Table 1).
Furthermore, an analysis of the relationship between risk factors and FOBT results revealed a statistically significant relationship between age and positive FOBT results. The mean age of the positive FOBT group was 59.1 years ± 9.1, compared to 56.2 ± 9.1 in the negative FOBT group, with a p-value < 0.001. Also, more individuals reported taking Aspirin in the positive FOBT group (78 individuals, 21.5 %) compared to the negative FOBT group (17.1 %), with a p-value of
0.038. No statistically significant relationship was found between positive FOBT results and hypertension. The positive FOBT group had 182 individuals (50.1 %) with hypertension, while the negative FOBT group had 45.1 % with a p-value of 0.073. Moreover, no statistically significant relationship existed between obesity and positive FOBT results. The positive FOBT group had 47.4 % who were obese, 36.4 % who were overweight, and 13.2 % who had a normal or underweight BMI. In comparison, the negative FOBT group had 49.9 % obese, 36.6 % overweight, and 12.8 % with a standard or underweight BMI, with a p-value of 0.223. Similarly, there was no statistically significant difference between the two groups in the presence of inflammatory bowel disease. The positive FOBT group had 1.4 % with inflammatory bowel disease, while the negative FOBT group had 0.7 %, with a p-value of 0.153. Furthermore, no statistically significant relationships were observed between positive FOBT results and gender, diabetes, hypertension, dyslipidemia, family history of colorectal cancer, smoking, and H. pylori (Table 1).
Table 1: Comparison between the positive and negative FOBT groups
|
Variable |
Total 3145 (100 %) |
Positive FOBT 364 (11.5 %) |
Negative FOBT 2782 (88.5 %) |
P value |
|
Age (mean ± SD) |
56.5 ± 9.1 |
59.1 ± 9.1 |
56.2 ± 9.1 |
< 0.001 |
|
Male |
1305(41.5 %) |
153(42.1 %) |
1152(41.4 %) |
0.788 |
|
DM (yes) |
1309(41.6 %) |
161(44.4 %) |
1148(41.3 %) |
0.262 |
|
HTN (yes) |
1438(45.7 %) |
182(50.1 %) |
1256(45.1 %) |
0.073 |
|
Dyslipidemia (yes) |
1921(61.1 %) |
230(63.3 %) |
1691(60.8 %) |
0.344 |
|
FH colorectal cancer (yes) |
51(1.6 %) |
6(1.7 %) |
45(1.6 %) |
0.960 |
|
Inflammatory bowel disease (yes) |
24(0.6 %) |
5(1.4 %) |
19(0.7 %) |
0.153 |
|
Weight (BMI) Obesity Overweight Normal and underweight |
1553(49.9 %) 1154(37.1 %) 405(13 %) |
172(47.4 %) 132(36.4 %) 48(13.2 %) |
1381(49.5%) 1022(36.7 %) 357(12.8 %) |
0.223 |
|
Aspirin intake |
553(17.6 %) |
78(21.5 %) |
475(17.1 %) |
0.038 |
|
Smoking (yes) |
282(9 %) |
31(8.5 %) |
251(9 %) |
0.305 |
|
H pylori (positive) |
228(7.2 %) |
28(7.7 %) |
200(7.2 %) |
0.111 |
Discussion
Colorectal cancer screening has the potential to significantly reduce colorectal cancer mortality by enabling the detection and removal of precancerous polyps and early-stage cancers before they become malignant or spread [9]. If colorectal cancer screening were universally adopted, it could prevent up to 90 % of all colorectal cancer cases [7]. However, our study, which was conducted in the United Arab Emirates (UAE), revealed that only 9 % (3145 individuals out of a total of 36,126 unique individuals) who were eligible and visited primary care underwent colorectal cancer screening using FIT/gFOBT from 2019 to 2020. This rate is considerably low compared to other studies [12,16]. A similar study in Abu Dhabi in 2015-2016 showed that FIT/gFOBT screening was only performed for 23.5 % of eligible individuals [12]. Overseas studies, such as the one conducted in Utah, USA [6], also indicated unsatisfactory utilization of colorectal cancer screening tests [3,6].
Our study sample exhibited similarities to the selection described in Almansoori et al. (2021) regarding gender distribution and mean age. In their study, the male gender accounted for 38% of the sample, while it constituted 41.4 % in our research. Furthermore, the mean age in their model was 51.51 ± 9.3 years, whereas, in our study, it was 56.6 ± 9.1 years. Additionally, the prevalence of positive FOBT results in their study was 13.5 %, which closely aligns with our findings of 11.6 %.
The findings of our studies underscore the need to investigate and address the factors contributing to the low screening rate and improve screening practices. Among the screened individuals, only 11.5% (363 individuals) received positive results, with 42 % (153 individuals) being men and 57.8 % (210 individuals) being women. However, only 30% (111 individuals) of the individuals with positive results were referred to gastroenterology for further assessment.
Additionally, our study revealed that out of the total referred patients, only 61(55 %) individuals completed a colonoscopy as a further investigation for the positive FIT/gFOBT test. Among them, 8 individuals were diagnosed with colorectal cancer. Our study demonstrated a significant positive relationship between age and FIT/gFOBT results, with a p-value of 0.000 (< 0.001). A survey in China indicated that the age group of 70-75 had a higher risk of the disease than individuals between 50-60 [15]. However, a study conducted in Turkey reported no statistically significant association between age and positive FOBT results [14].
Our study did not find a significant relationship between higher BMI and positive fecal occult. However, this result could be attributed to the low number of screened individuals. Conversely, other studies [14,15] revealed a statistically significant relationship between higher BMI and fecal occult blood.
The Cheng Y-W, Li Y-C. 2022 study analyzing the relationship between socio-demographic characteristics and occult blood tests (FOBT) found that factors such as age, gender, exercise, use of tobacco and alcohol, occupational status, and income level did not show any statistically significant effects on FOBT positivity [14].
A study in China revealed that several variables, including gender, age, screening site location, type of medical unit performing the follow-up examination, family history, examination techniques, and follow-up time, are associated with positive FOBT results [15].
In our study, the compliance rate for colonoscopy was found to be 54.9%, which was lower compared to the rate reported by Purnomo et al. (2023) in Indonesia, which was 70.27 % [15]
An area where our study fell short was the inability to ascertain the underlying causes for the low rate of referrals for further investigations among individuals who tested positive. Furthermore, we needed to determine whether these patients sought other studies in alternative healthcare sectors.
These results emphasize the need for further investigation into the reasons behind the low screening percentage using the FIT/gFOBT test and future research to gather new data on screening practices. Implementing multifaceted education programs to enhance awareness and promote the practice of colorectal cancer screening among eligible individuals using FIT/gFOBT is recommended. This is supported by previous research demonstrating reduced colorectal cancer mortality through FOBT-based screening [13].
Conclusion
Screening plays a crucial role in the early detection of colorectal cancer. In our study, only 9 % of eligible individuals for Colorectal cancer screening did the screening; 11.6% had positive screening results. Our findings demonstrated that aspirin use, and older age are significantly associated with positive screening results. However, no significant relationships were observed between positive fecal occult blood test (FOBT) results and gender, inflammatory bowel disease, dyslipidemia, diabetes mellitus, and body mass index (BMI).
Recommendation
To enhance colorectal screening rates, it is essential to Provide regular and updated training to healthcare providers regarding the latest guidelines and evidence-based practices for colon cancer screening. Encourage providers to discuss screening options with eligible patients and provide clear recommendations based on individual risk factors. And implement comprehensive public education campaigns to raise awareness about the importance of colon cancer screening. Establish quality assurance programs to monitor and improve the accuracy and reliability of screening tests. Implement regular audits, performance feedback, and proficiency testing to ensure consistent, high-quality screening outcomes.
Additionally, community initiatives should be implemented to promote colorectal screening among the population. Furthermore, it is crucial to follow up with individuals who receive positive screening results closely. Conducting a comprehensive restudy program can help establish the correlation between risk factors and colorectal screening, especially considering the significant increase observed in 2022. Involving insurance companies to expand coverage for colorectal screening in the community population is another valuable step. By implementing these recommendations, we can strive towards higher participation rates, earlier detection of colon cancer, and improved outcomes for at-risk individuals. Efforts to enhance colon cancer screening will ultimately contribute to reducing the burden of this disease on individuals, families, and healthcare systems.
Disclosure statement: There is no conflict of interest to declare.
Funding: The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, et al. (2017) Global patterns and trends in colorectal cancer incidence and mortality. BMJ Gut. 66(4): 683.
- Al-Shamsi HO, Abyad AM, Rafii S (2022) A Proposal for a National Cancer Control Plan for the UAE: 2022–2026. Clin Pract.. American Journal of Public Health. 12(1): 118-132.
- Almansoori A, Alzaabi M, Alketbi L (2021) Colorectal Cancer screening in ambulatory healthcare service clinics in Abu Dhabi, United Arab Emirates in 2015–2016. BMC Cancer 21: 897.
- Issa IA, Noureddine M (2017) Colorectal cancer screening: An updated review of the available options. World J Gastroenterol. 23(28): 5086-5096.
- Valle I, Tramalloni D, Bragazzi NL (2015) Cancer prevention: state of the art and future prospects. J Prev Med Hyg. 56(1): E21- E27.
- Baker AN, Parsons M, Donnelly SM, Johnson L, Day J, et al. (2009) Improving colon cancer screening rates in primary care: a pilot study emphasising the role of the medical assistant. Qual Saf Health Care. 18(5): 355-9.
- Winawer S, Fletcher R, Rex D, Bond J, Burt R, et al. (2003) Gastrointestinal Consortium Panel. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 124(2): 544-60.
- U.S. Preventive Services Task Force (2002) Screening for colorectal cancer: recommendation and rationale. Ann Intern Med. 137(2): 129-31.
- Richardson L (2010) Vital Signs: colorectal cancer screening among adults aged 50--75 years, United States, 2008: Centers for Disease Control and Prevention.
- Centers for Disease Control and Prevention (CDC) (2001) Trends in screening for colorectal cancer--United States, 1997 and 1999. MMWR Morb Mortal Wkly Rep. 50(9): 162-6.
- Centers for Disease Control and Prevention (CDC) (2003) Colorectal cancer test use among persons aged > or = 50 years-- United States, 2001. MMWR Morb Mortal Wkly Rep. 52(10): 193-6.
- Hewitson P, Glasziou P, Watson E, Towler B, Irwig L (2008) Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 103(6): 1541-9.
- Rengucci C, De Maio G, Menghi M, et al. Diagnostic accuracy of fecal occult blood tests for detecting proximal versus distal colorectal neoplasia: a systematic review and meta-analysis. Clin Epidemiol. 2018;10:805-817.
- Cheng YW, Li YC (2022) Examining the Factors That Affect the Diagnosis of Patients with Positive Fecal Occult Blood Test Results. International Journal of Environmental Research and Public Health. 19(13): 7569.
- Purnomo HD, Permatadewi CO, Prasetyo A, Indiarso D, Hutami HT, et al. (2023) Colorectal cancer screening in Semarang,Indonesia: A multicenter primary health care based study. PLoS ONE. 18(1): e0279570.
- Al Abdouli L, Dalmook H, Akram Abdo M, Carrick FR, Abdul Rahman M (2018) Colorectal Cancer Risk Awareness and Screening Uptake among Adults in the United Arab Emirates. Asian Pac J Cancer Prev. 19(8): 2343–2349.



